Abstract
Objective
Congenital ventricular wall defects are very rare and include congenital ventricular aneurysms (CVAs) and diverticula (CVDs).
Method
We report a series of five fetuses: three with CVAs and two with CVDs referred due to fetal arrhythmia. In addition to routine fetal echocardiography, fetal magnetocardiography (fMCG) was used. The literature in CVA and CVD is reviewed.
Results
Incessant premature ventricular contractions (PVC), mainly bigeminy and trigeminy were found in three fetuses with CVAs and in one with CVD, who also had ventricular couplets. The other fetus with CVD, referred because of PVCs, had only sinus tachycardia. ST elevation was noted in two. Fetal movement had a variable impact on PVC’s. Postnatal evaluation demonstrated two persistent left ventricular aneurysms and one persistent right CVD; one CVD resolved at 35 weeks gestation. Two neonates had incessant PVCs. Both arrhythmias resolved spontaneously while being treated with propranolol.
Conclusion
FMCG is complementary to echocardiographic imaging. In fetuses with left ventricular wall defects, additional electrophysiological diagnosis can be made by fMCG, including the complexity of ventricular ectopy, arrhythmic response to fetal movement, presence of ST-T wave abnormalities, and atrial amplitude increases. Prenatal risk factor assessment using fMCG can additionally support post-natal treatment and follow-up.
Keywords: electrophysiology, fetal magnetocardiography (fMCG), ventricular aneurysm, fetus, premature ventricular contractions, ventricular diverticulum
Introduction
Congenital ventricular aneurysms and diverticula are uncommon cardiac malformations1. Mainly case reports exist in the literature. The first case was reported antenatally by Gembruch and colleagues in a 32 week gestational age (GA) fetus that developed ventricular extrasystoles2. Previous published data specified a prevalence of 0.5 in 100.000 born with an equal distribution in gender3. Because of the low prevalence of the disease, the two terms congenital ventricular diverticulum (CVD) and aneurysm (CVA) have often been used interchangeably.
CVAs have a loss of integrity of the myocardium and lack of one or more elements of the cardiac muscle. Mostly they are fibrotic and appear sac-like with paradoxical ballooning during ventricular systole. In contrast to these congenital defects, pseudoaneurysms have a portion which is walled off pericardium. Diverticula appear as dilation of the myocardium but with all three muscle layers retained. CVDs are dys- or akinetic and can spontaneously resolve. The etiology of CVAs and CVDs is unknown. An intrinsic abnormality in embryogenesis may lead to a focal defect of the muscular ventricular wall4. Aneurysms and diverticula can be acquired in the prenatal period from a viral infection5, inflammatory diseases, or coronary anomalies with stenosis6.
In the human adult, CVA and CVD are known to be associated with complex re-entrant ectopy and ventricular tachyarrhythmias, and impart a higher risk of sudden cardiac death. Of 41 fetal cases of CVA and CVD, eight of them (~20%) had cited arrhythmia (Table 1). We report a series of five fetuses presenting clinically with arrhythmias due to left ventricular wall defects, and we review the published literature.
Table 1. Literature review.
of fetal presentation of ventricular aneurysms and diverticuli
| Reference | GA (wks) | Location | Symptoms & Signs | Medication | Surgery cited | Outcome |
|---|---|---|---|---|---|---|
| Balakumar et al.10 | 24 | Aneurysm | Abnormal 4-chamber view, mitral & tricupsid regurgitation | None cited | No | N/A |
| Barberato et al.11 | 16 | Apical aneurysm | Pericardial effusion | None cited | No | IUFD (37 wks) |
| Bernasconi et al.12 | 24 | Submitral diverticulum | Pericardial effusion | None cited | No | IUFD (26 wks) |
| Brachlow et al.13 | 32 | Apical diverticulum | Normal cardiac function | None cited | No | Clinically well (3 yrs) |
| Carles et al.14 | 13 | Apical diverticulum | Pericardial effusion | None cited | No | TOP (14 wks) |
| Cavalle-Garrido et al.15 | 18 | Apical aneurysm | Abnormal 4- chamber view | Digoxin | No | Clinically well (2 yrs) |
| 19 | Sub-mitral aneurysm | Abnormal 4-chamber view, hydrops | None cited | No | IUFD (31 wks) | |
| 21 | Apical aneurysm | Abnormal 4-chamber view | None cited | No | Clinically well (2 yrs) | |
| 20 | Submitral diverticulum | Pericardial effusion, hydrops | None cited | No | IUFD (26 wks) | |
| Cesko et al.16 | 17 | Apical diverticulum | Pericardial effusion | None cited | No | TOP (22 wks) |
| Chaubal et al.17 | 21 | Apical aneurysm | Abnormal 4-chamber view | None cited | No | IUFD (27 wks) |
| Chiang et al.18 | 34 | Apical aneurysm | Cardiomegaly | None cited | No | Clinically well (1 yr) |
| El Kady et al.19 | 17 | Apical aneurysm | Pericardial effusion | Nifedipine and methyldopa-mother’s hypertension | No | IUFD (27 wks) |
| Fujita et al.20 | 26 | Apical aneurysm | Arrhythmia (ventricular extrasystoles) | None cited | No | Clinically well (2 yrs) |
| Gembruch et al.2 | 32 | Apical aneurysm | Arrhythmia (ventricular extrasystoles), dilated left ventricular | Propafenone for 4 months Antithrombotic prophylaxis with low-dose aspirin | No | Clinically well (2 yrs) |
| Espinoza et al.3 | 29 | Apical aneurysm | Low-lying placenta, cardiac failure | Digoxin | No | IUFD (31 wks) |
| Hornberger et al.22 | 25 | Apical aneurysm | Premature atrial contractions | Only abstract available | No | Clinically well (10 mo) |
| 28 | Apical aneurysm | Premature atrial contractions | Only abstract available | No | Clinically well (4 mo) | |
| Jacobson et al.23 | 33 | Free wall aneurysm | Pericardial effusion | None cited | Yes (8.5 mo) | Clinically well |
| Kitchiner et al.24 | 33 | Apical diverticulum | Cardiomegaly, mitral regurgitation | None cited | No | Clinically well |
| Lupoglazoff et al.25 | 28 | Apical aneurysm | Abnormal 4-chamber view | Only abstract available | No | Neonatal death |
| Marijon et al.26 | >26 | Apical aneurysm | Left ventricular dysfunction | None cited | No | Perinatal death (1 d) |
| >26 | Apical aneurysm | Normal cardiac function | None cited | No | Perinatal death (1 d) | |
| Matias et al.27 | 21 | Apical aneurysm | Pericardial effusion | Only abstract available | No | Perinatal death |
| 22 | Free wall aneurysm | Pericardial effusion | Only abstract available | No | TOP (23 wks) | |
| 26 | Apical aneurysm | Pericardial effusion, cardiomegaly | Only abstract available | No | IUFD (33 wks) | |
| McElhinnery et al.28 | 22 | Apical aneurysm | Hydrops | Only abstract available | No | TOP (23 wks) |
| 39 | Apical aneurysm | Asymptomatic | Only abstract available | No | Clinically well | |
| Nam et al.29 | 21 | Apical diverticulum | Cardiac malformation | None cited | No | TOP (22 wks) |
| Papagiannis et al.30 | 23 | Free wall aneurysm | Multiple congenital anomalies | Only abstract available | No | TOP (23 wks) |
| 32 | Apical aneurysm | Arrhythmia | Only abstract available | No | Clinically well (7 yr) | |
| Patel et al.31 | 25 | Apical aneurysm | Abnormal 4-chamber view | Digoxin and Captopril | No | Clinically well (3.5 mo) |
| Prefumo et al.34 | 12 | Free wall diverticulum | Pericardial effusion | None cited | No | Clinically well (17 mo) |
| Pipitone et al.32 | 25 | Apical aneurysm | Abnormal 4-chamber view, mitral regurgitation | None cited | No | Clinically well (1 yr) |
| Pradhan et al.33 | 28 | Apical diverticulum | Abnormal 4-chamber view, arrhythmia (ventricular extrasystoles) | Digoxin | No | Clinically well (1 yr) |
| Seo et al.35 | 21 | Apical aneurysm | Abnormal 4-chamber view, mitral regurgitation | None cited | Yes∘ | Clinically well (21 mo) |
| Sepulveda et al.36 | 19 | Apical aneurysm | Hydrops, Abnormal 4-chamber view | Only abstract available | No | TOP (19 wks) |
| Sharma et al.37 | 29 | Apical aneurysm | Pericardial effusion, cardiomegaly | None cited | Yes (3 d)Δ | Clinically well (4 mo) |
| Sherman et al.38 | 24 | Apical aneurysm | Pericardial effusion | Digoxin | No | IUFD (31 wks) |
| Tsujimoto et al.39 | 37 | Apical diverticulum | Arrhthmia (ventricular bigeminy) | None cited | No | Clinically well (2 yrs, 8 mo) |
| Weichert et al.1 | 32 | Free wall aneurysm | Arrhythmia (ventricular extrasystoles), cardiomegaly | None cited | No | Clinically well (2 yrs) |
modified Damus-Kaye-Stansel
resection of the aneurysm
Materials and Methods
Patients
The fMCG records of pregnant women with fetal ventricular wall defects referred to the Biomagnetism Laboratories at the Department of Medical Physics, University of Wisconsin-Madison from 2002 to 2012 were retrieved from our database.
Informed consent was obtained from each participant and the University of Wisconsin Institutional Review Board reviewed and approved the fMCG protocol.
The study included three subjects diagnosed with left ventricular wall aneurysm and two with diverticulum. We called them diverticula when there was as a continuous muscle on all edges. If they appeared to have interruption of the muscle element leaving only fibrous tissue, we called them an aneurism. The median gestational ages were 33 weeks (Range 22–34 weeks). The fMCG data were re-evaluated by two pediatric cardiologists for rhythm, cardiac time intervals, ST segment abnormalities, and signal amplitudes. Neonatal outcomes were reviewed.
Methods
fMCG is the magnetic analogue of the fetal ECG, but provides better signal quality and favorable signal transmission properties. A 37-channel monoaxial (Magnes, 4D Neuroimaging, Inc., San Diego, Calif., USA) and/or a 21-channel (Tristan Technologies, USA) vector superconducting quantum interference device (SQUID) was used to record the fMCG from the maternal abdomen. A SonoSite M-Turbo (Bothwell, Wash., USA) portable ultrasound scanner equipped with a 60-mm broadband (2–5 MHz) curved array transducer was first used to determine preliminary rhythm, and location of the fetal heart. The SQUID was placed directly above and in direct contact with the mother’s abdomen. Four to seven recordings of 10 minutes duration were obtained. Post processing required construction of an actocardiogram (simultaneous tracing of fetal activity and fetal heart rate), using fMCG data, to monitor and characterize fetal movement7. Spatial filtering was used to remove maternal interference. All fMCG recordings were reviewed by at least two pediatric cardiologists. Custom Matlab programs were used to measure cardiac time intervals and signal amplitudes. The precision of the electronic measurement calipers was approximately 0.001 sec. The P and QRS amplitude ratios (including both positive and negative components of each wave) were plotted against 68 health normal control fetuses. The ratios in the subjects at < 1 week of age were available in 3 infants during sinus rhythm and 1 during ventricular bigeminy. These were displayed electronically using Muse ECG storage and cardio-analytic system (GE Medical, Milwaukee, WI), and using the superimposition median view, the P:QRS ratio was again obtained using the Limb leads. These ratios, and the neonatal cardiac time intervals, are displayed in Table II. Linear regression was used to assess the dependence of the amplitude parameters on gestational age8.
Results
Fetal echo and fMCG results
All five fetuses presented to the Biomagnetism Laboratory due to irregular rhythm patterns (Table 2). They showed left ventricular wall defects confirmed by fetal and neonatal echocardiography. Three of them were located in the left ventricular apex, one in the right lateral ventricle, and one in the posterior LV submitral region. Left and right ventricular function was normal in all fetuses except in the region of the wall defect.
Table 2.
Pre and postnatal echo and fMCG findings in CVA and CVD
| Case | GA (weeks) | Location and Echo findings | fMCG findings | fCTIs (ms) | Neonatal Outcome nCTI’s (ms) | Follow-up |
|---|---|---|---|---|---|---|
| 1 | 33 | CVA, LV apex, moderate wall defect with adjacent hypokinesis | PVCs bigeminy, mild ST
elevation Fetal movement suppressed ectopy large amplitude P wave |
RR= 421 PR= 58 QRS= 61 QTc =447 P:QRS Ampl. 0.329:1 |
irregular PVCs, QRST discordance, ST elevation
Transient global LV dysfunction P:QRS Ampl ratio ECG −0 days, bigeminy = 0.211:1 ECG −7 days, Sinus rhythm P:QRS 0.078:1 RR=491 PR=98 QRS=66 QTc=481 |
2 ½ year: aneurysm
smaller arrhythmia resolved, propanolol 2mg/kg/day until 6 months of age |
| 2 | 28 | CVD, LV Submitral | PVCs, couplets minimum change in ectopy with movement large amplitude P wave |
RR= 488 PR= 99 QRS= 44 QTc =391 P:QRS Ampl. 0.252:1 |
PVCs spontaneously resolved ECG at 0 days Sinus rhythm, RR=451 PR=156 QRS=54 QTc=429 P:QRS Ampl ratio 0.17:1 |
3 months: abnormal segment of the left
ventricular wall no arrhythmia |
| 3 | 34 | CVA, LV apex, normal cavity size and function | PVCs trigeminy ectopy aggravated by fetal movement large amplitude P wave |
RR= 423 PR= 124 QRS= 59 QTc =390 P:QRS Ampl. 0.247:1 |
Sinus rhythm, no arrhythmia ECG at 0
days RR=540 PR=110 QRS=62 QTc=427 P:QRS Amp ratio 0.066:1 |
2 ½ months: unchanged
aneurysm no arrhythmia |
| 4 | 34 | CVD, LV apex, large wall defect | sinus tachycardia, mild ST - elevation | RR= 309 PR= 102 QRS= 42 QTc =349 P:QRS Ampl. 0.063:1 |
sinus rhythm, normal conduction intervals, frequent monomorphic PVCs ST elevation | 15 months: aneurysm
smaller arrhythmia resolved, propanolol 2 mg/kg/day until 9 months of age |
| 5 | 22 | CVA, RV lateral, large wall defect | PVCs bigeminy Fetal movement suppressed ectopy |
RR= 478 PR= 96 QRS= 77 QTc= 446 P:QRS Ampl. 0.182:1 |
Sinus rhythm, Few PVC’s | 3 months: aneurism
smaller Arrhythmia resolved at birth. No medication. |
Abbreviations: Amplitude (Ampl), fetal cardiac time intervals (fCTIs), congenital ventricular diverticulum (CVD), congenital ventricular aneurysm (CVA), premature ventricular contractions (PVCs), left ventricle (LV)
Electrophysiologic patterns were complex, and varied considerably. In addition to rare sinus rhythm, four fetuses presented with incessant premature ventricular contractions. One had couplets and bigeminy, two had ventricular bigeminy as the predominant pattern, and one had ventricular trigeminy. Coupling intervals (PVC onset to prior QRS onset) were not stable. One fetus with CVD and history of ventricular ectopy by echocardiography had only sinus tachycardia (Figure 1). Three of four fetuses exhibited a change in PVC response during fetal movement. In two fetuses the ectopy was suppressed temporarily by fetal activity and in one it was exacerbated (Figure 2).
Figure 1. fMCG findings (Case 1–5).
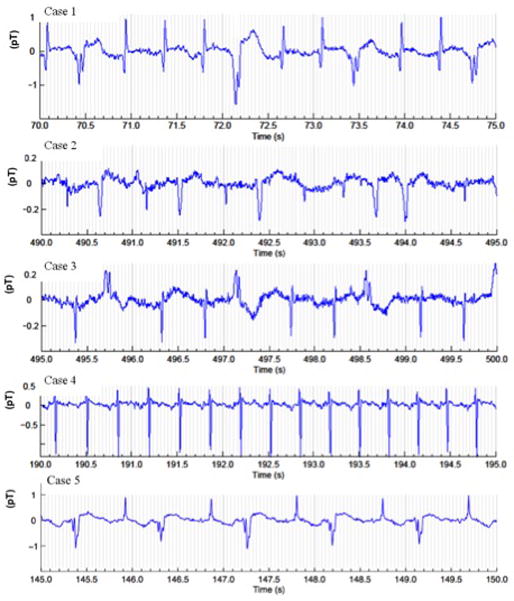
Four fetuses presented incessant premature ventricular contractions. Trigeminy is demonstrated in Cases 1 and 3. Ventricular couplets are noted in Case 2, and bigeminy is demonstrated in Cases 2 and 5. Case 4 had sinus tachycardia. Case 1 and 4 demonstrate ST elevation.
Figure 2. PVC response during fetal movement (Cases 1–3, 5).
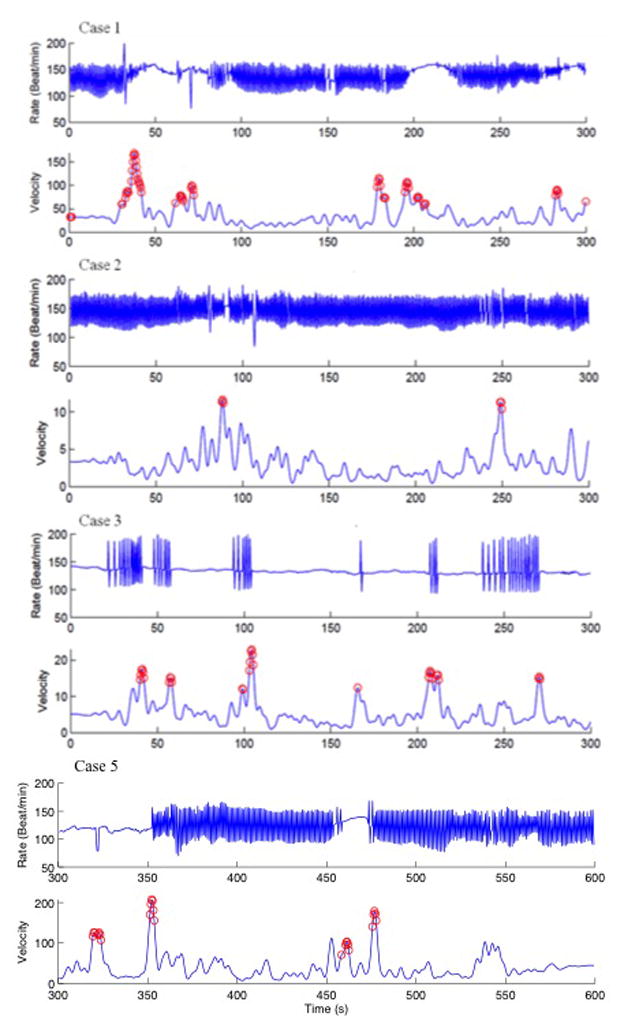
For each case, the top panel shows the heart rate trend (Rate, beats/min) over five minutes, and the bottom panel (velocity) shows the fetal actogram, with maximum slope activity marked by open circles. In Cases 1 and 5, fetal movement abolished PVCs, Case 2 showed no effect, while in case 3 fetal movement induced PVCs.
Four fetuses had normal cardiac time intervals, and the fetus with an extensive right ventricle (RV) aneurysm had QRS prolongation. QTc intervals were impacted by QRS duration, but were not prolonged. Two fetuses had mild ST- elevation. The main statistical data are presented in Table 2. The most striking observation was that P: QRS amplitude ratio in the left-sided aneurysms ranged between 0.22–0.31 (normal value P: QRS ratio = 0.1), indicating early signs of atrial hypertrophy or dilatation, which was not evident by echocardiography. The fetus with RV aneurism had a less dramatic P:QRS amplitude ratio increase of 0.18.
Neonatal echo and electrophysiologic outcome
Postnatal evaluation by echocardiography demonstrated three persistent left ventricular aneurysms and one CVD. One CVD resolved at 35 weeks gestation. Two neonates had incessant PVCs after birth and were treated with propranolol (2–3 mg/day) for 3 and 9 months (Figure 3) and the remainder were not treated. Of the two with ST elevation pre-natally, one had persistent ST elevation postnatally. Neonatal P:QRS ratio’s were available in 3 of 5 subjects, and were not as large as fetal ratios. In one neonate (case 1) the P:QRS during ventricular bigeminy was larger than 1 week later during sinus rhythm. In no case, however was the postnatal P:QRS ratio larger than the fetal ratio.
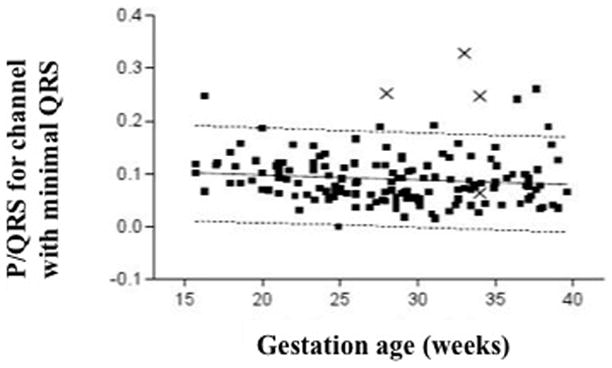
Figure 3. P:QRS ratio.
All 4 cases with PVCs (shown as X’s) compared to healthy controls (black boxes).
Intermediate-term follow - up
Follow- up examinations were available in all infants at age ranges from 3–35 months. One fetus showed LV CVD resolution in utero. In two infants, left ventricular wall defects were stable, whereas in the other two infants (RV-1, LVapex-1) the defects became smaller. Therefore surgical resection was not necessary in any patient. Arrhythmias resolved in all subjects, confirmed by Holter monitoring. One infant with an LV apical aneurism and persistent ventricular arrhythmia had moderate global LV hypokinesis at birth which improved (except in the region of the aneurism) over 3 weeks. No neonatal deaths have occurred.
Discussion
The main findings in this study are that left or right ventricular wall defects in utero are associated with a high incidence of arrhythmia (5 in 5 cases), specifically premature ventricular contractions. All subjects had PVCs—four of them in utero and one postnatally. The one with PVCs postnatally, had a history of echo-documented PVCs and fMCG-documented sinus tachycardia in utero. One could speculate that the tachycardia had suppressed the PVCs in utero. In addition to the PVCs, we found couplets, bi/trigeminy, atrial amplitude increases and ST elevation. QRS prolongation was noted in one fetus. Ventricular wall defects in our series were not associated with QT prolongation.
The combined use of fetal MCG and echocardiography detected not only a higher frequency of ectopy and other conduction abnormalities (tachycardia, ST-T changes, bundle branch block, and atrial hypertrophy), but also the size, morphology, and contraction characteristics of the CVAs and CVDs. Both methods complement one another in prenatal cardiac monitoring.
The clinical outcome in our series was far better than has been reported in the literature (Table 1), which is largely comprised of case studies. No patients experienced intrauterine fetal demise, cardiac arrest, or sudden infant death, and in several patients, fetal CVAs and CVDs regressed over time. While only non-malignant arrhythmias were detected in this series, we speculate that arrhythmic deaths can be one of several mechanisms of fetal demise. Complications of ventricular wall defects such as hydrops, large pericardial effusions, and poor ventricular function, may all increase the likelihood of unstable arrhythmias when an arrhythmic substrate exists. Lethal arrhythmias may go undetected in echo/Doppler imaging due to its intermittency, whereas monitoring by fMCG allows continuous capture of all QRS complexes, similar to cardiac telemetry. In consequence, if the arrhythmia can be characterized, premature delivery can be avoided and if neonatal cardiac output can be maintained, surgical resection may not be necessary. Different treatment strategies for fetal arrhythmias, according to the precise electrophysiological findings, are recommended by Donofrio and colleagues in the new Scientific Statement of the American Heart Association “Diagnosis and Treatment of Fetal Cardiac Disease”9.
Li and colleagues have established P and QRS amplitude values in normal subjects by fetal magnetocardiography8. Increased amplitudes were found in subjects with fetal arrhythmias. This implied that arrhythmias in utero can be accompanied by hypertrophy, similar to the MCG and ECG findings, and that these may include atrial hypertrophy. Atrial hypertrophy is a critical adaptation of the fetus during low cardiac output states such as those associated with severe acute bradycardia8. It commonly accompanies incessant arrhythmias with AV dissociation where the atrium contracts against a closed AV valve. In this study we found four of five patients with elevated P and QRS amplitudes. A lack of increase was found in the fetus with sinus tachycardia. P:QRS amplitude might initially be increased, and the incessant tachycardia might be the resolution as another sign of recovery of the heart, since this fetus’s CVD resolved in utero. Similar to the findings of Li and colleagues8, fMCG evidence of atrial hypertrophy was not associated with visible atrial hypertrophy or distension during sinus rhythm. The neonatal ECGs in three (cases 1, 2, and 3), for whom measurements could be measured using superimposition views, were not associated with significant P:QRS amplitude increase, except in one fetus (Fetus 2) where the P:QRS amplitude ratio during ventricular bigeminy significantly increased compared with sinus rhythm (though not to ratios seen prenatally in that fetus). These findings suggest, that the flow dynamics of transitional circulation, or of acute hemodynamic changes during bigeminy and other conduction disturbances, likely impact the atrial signal size relative to the ventricle during normally conducted beats in the neonate.
Ventricular aneurysms appear to be heterogenous, and this may account for the variable post-natal findings and outcomes. In support of this, the electrophysiologic characteristics also appeared to be heterogenous. ST elevation was observed in only two of five, sinus tachycardia in one. In two fetuses, the ectopy appeared to suppress with fetal movement Changes in autonomic activity based on the gestation of the fetus might also influence fetal presentation. The timing of resolution of the arrhythmias also varied with birth being an important transition point.
Conclusion
In summary, we have shown for the first time that precise electrophysiological diagnosis of CVA’s and CVDs can be made in utero by using fMCG. This procedure should be considered, in addition to fetal echocardiography, in fetuses with ventricular aneurysms or diverticula to characterize the extent of ventricular arrhythmias, predict prognosis, and assess potential need for antiarrhythmic treatment post-natally. CVAs and CVDs appear to have a favorable prognosis and conservative prenatal management is advised. The P:QRS amplitude abnormalities, QRS duration, and ST-T wave abnormalities may be useful in further risk-stratification.
Figure 4.
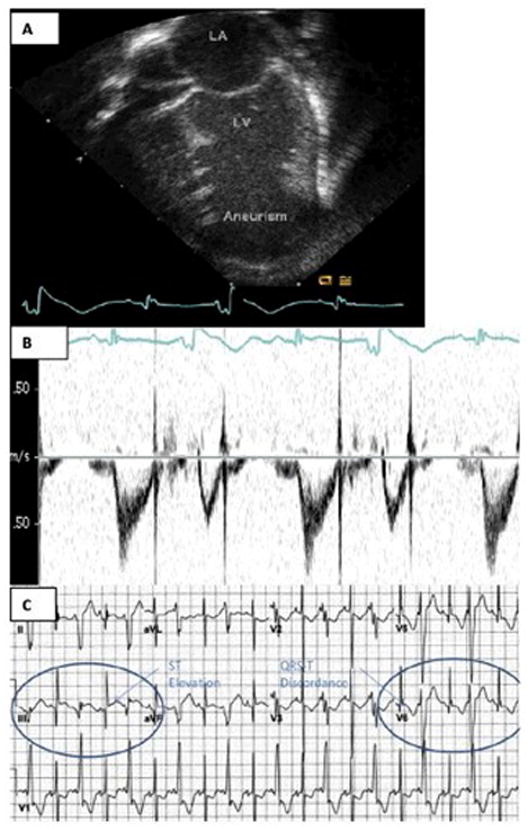
Postnatal findings of a left ventricular aneurysm (Case 1): a) Neonatal echocardiography b) Neonatal Doppler c) ECG. Areas of ST elevation of the sinus QRS (Lead II) and reciprocal T wave inversion (V5) are shown within the ovals.
Bulleted Statement.
In the human adult, CVA and CVD are known to be associated with complex re-entrant ectopy and ventricular tachyarrhythmias, and impart a higher risk of sudden cardiac death. In this study we could show, that in fetuses with left ventricular wall defects, additional electrophysiological diagnosis can be made by fetal magnetocardiography, including the complexity of ventricular ectopy, arrhythmic response to fetal movement, presence of ST-T wave abnormalities, and atrial amplitude increases.
Acknowledgments
Source of funding: National Institutes of Health grant R01 HL63174, Friede-Springer-HerzStiftung
List of abbreviations
- fMCG
fetal magnetocardiography
- SQUID
superconducting quantum interference device
- CVD
congenital ventricular diverticulum
- CVA
congenital ventricular aneurysm
- PVCs
premature ventricular contractions
- fCTIs
fetal cardiac time intervals
- GA
gestational age
Footnotes
Conflict of interest: The authors declare no conflict of interest
Reference list
- 1.Weichert J, Chiriac A, Axt-Fliedner R. Fetal diagnosis of left ventricular aneurysm of the free wall and the interventricular septum: report of two cases and review of the literature. J Matern Fetal Neonatal Med. 23(12):1510–1515. doi: 10.3109/14767051003678127. [DOI] [PubMed] [Google Scholar]
- 2.Gembruch U, Steil E, Redel DA, et al. Prenatal diagnosis of a left ventricular aneurysm. Prenat Diagn. 1990;10(3):203–209. doi: 10.1002/pd.1970100312. [DOI] [PubMed] [Google Scholar]
- 3.Espinoza J, Kalache K, Goncalves LF, et al. Prenatal diagnosis of membranous ventricular septal aneurysms and their association with absence of atrioventricular valve ‘offsetting’. Ultrasound Obstet Gynecol. 2004;24(7):787–792. doi: 10.1002/uog.1769. [DOI] [PubMed] [Google Scholar]
- 4.Treistman B, Cooley DA, Lufschanowski R, et al. Diverticulum or aneurysm of left ventricle. Am J Cardiol. 1973;32(1):119–123. doi: 10.1016/s0002-9149(73)80097-9. [DOI] [PubMed] [Google Scholar]
- 5.Brachlow A, Sable C, Smith S, et al. Fetal diagnosis and postnatal follow-up of an asymptomatic congenital left ventricular diverticulum. Pediatr Cardiol. 2002;23(6):658–660. doi: 10.1007/s00246-002-9002-4. [DOI] [PubMed] [Google Scholar]
- 6.Grotenhuis HB, Backx A, Nijveld A. Resection of a cardiac aneurysm in an infant with anomalous origin of the left coronary artery from the pulmonary trunk. Cardiol Young. 2004;14(1):106–108. doi: 10.1017/s1047951104001222. [DOI] [PubMed] [Google Scholar]
- 7.Lutter WJ, Wakai RT. Indices and detectors for fetal MCG actography. IEEE Trans Biomed Eng. 2011;58(6):1874–1880. doi: 10.1109/TBME.2011.2131141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Strasburger JF, Cuneo BF, et al. Giant fetal magnetocardiogram P waves in congenital atrioventricular block: a marker of cardiovascular compensation? Circulation. 2004;110(15):2097–2101. doi: 10.1161/01.CIR.0000144302.30928.AA. [DOI] [PubMed] [Google Scholar]
- 9.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, Lacey S, Lee W, Michelfelder EC, Sr, Rempel GR, Silverman NH, Spray TL, Strasburger JF, Tworetzky W, Rychik J. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the american heart association. Circulation. 2014 doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 10.Balakumar K. Prenatal diagnosis of left ventricular aneurysm. Indian J Radiol Imaging. 2009;19(1):84–86. doi: 10.4103/0971-3026.45353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberato MF, Barberato SH, Binotto CN, et al. Prenatal diagnosis of left ventricular aneurysm and diverticulum. Arq Bras Cardiol. 2009;93(2):e36–38. doi: 10.1590/s0066-782x2009000800025. [DOI] [PubMed] [Google Scholar]
- 12.Bernasconi A, Delezoide AL, Menez F, et al. Prenatal rupture of a left ventricular diverticulum: case report and review of the literature. Prenat Diagn. 2004;24(7):504–507. doi: 10.1002/pd.912. [DOI] [PubMed] [Google Scholar]
- 13.Brachlow A, Sable C, Smith S, et al. Fetal diagnosis and postnatal follow-up of an asymptomatic congenital left ventricular diverticulum. Pediatr Cardiol. 2002;23(6):658–660. doi: 10.1007/s00246-002-9002-4. [DOI] [PubMed] [Google Scholar]
- 14.Carles D, Maugey-Laulom B, Habboud H, et al. Early prenatal diagnosis of ventricular diverticulum complicated by serous pericardial effusion. Prenat Diagn. 1995;15(8):778–780. doi: 10.1002/pd.1970150817. [DOI] [PubMed] [Google Scholar]
- 15.Cavalle-Garrido T, Cloutier A, Harder J, et al. Evolution of fetal ventricular aneurysms and diverticula of the heart: an echocardiographic study. Am J Perinatol. 1997;14(7):393–400. doi: 10.1055/s-2007-994167. [DOI] [PubMed] [Google Scholar]
- 16.Cesko I, Hajdu J, Csapo ZD, et al. Fetal hydropericardium associated with left ventricular diverticulum. Prenat Diagn. 1998;18(7):721–724. [PubMed] [Google Scholar]
- 17.Chaubal N, Dighe M, Shah M, et al. Congenital left ventricular aneurysm: prenatal sonographic diagnosis. J Ultrasound Med. 2004;23(1):125–128. doi: 10.7863/jum.2004.23.1.125. [DOI] [PubMed] [Google Scholar]
- 18.Chiang YC, Yang CK, Shih JC, et al. Prenatal diagnosis of congenital left ventricular aneurysm by four-dimensional ultrasonography with spatio-temporal image correlation (STIC) Ultrasound Obstet Gynecol. 2006;28(3):345–347. doi: 10.1002/uog.3803. [DOI] [PubMed] [Google Scholar]
- 19.El Kady D, Gerscovich EO, Moon-Grady A, et al. Congenital cardiac left ventricular aneurysm with pericardial effusion: early prenatal diagnosis and intervention. J Ultrasound Med. 2005;24(7):1011–1015. doi: 10.7863/jum.2005.24.7.1011. [DOI] [PubMed] [Google Scholar]
- 20.Fujita Y, Hidaka N, Yumoto Y, et al. Measurement of the fetal isovolumetric contraction time in the fetus with a left ventricular aneurysm. J Obstet Gynaecol Res. 38(3):586–588. doi: 10.1111/j.1447-0756.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 21.Goncalves LF, Simms J, et al. Aneurysm, left ventricle. Fetus. 1992;1:7–10. [Google Scholar]
- 22.Hornberger LK, Dalvi B, Benacerraf BR. Prenatal sonographic detection of cardiac aneurysms and diverticula. J Ultrasound Med. 1994;13(12):967–970. doi: 10.7863/jum.1994.13.12.967. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson RL, Perez A, Meyer RA, et al. Prenatal diagnosis of fetal left ventricular aneurysm: a case report and review. Obstet Gynecol. 1991;78:525–528. [PubMed] [Google Scholar]
- 24.Kitchiner D, Leung MP, Arnold R. Isolated congenital left ventricular diverticulum: echocardiographic features in a fetus. Am Heart J. 1990;119(6):1435–1437. doi: 10.1016/s0002-8703(05)80204-2. [DOI] [PubMed] [Google Scholar]
- 25.Lupoglazoff JM, Ricard G, Luton D, et al. Antenatal diagnosis of congenital left ventricular aneurysm. Arch Mal Coeur Vaiss. 2003;96(5):529–533. [PubMed] [Google Scholar]
- 26.Marijon E, Ou P, Fermont L, et al. Diagnosis and outcome in congenital ventricular diverticulum and aneurysm. J Thorac Cardiovasc Surg. 2006;131(2):433–437. doi: 10.1016/j.jtcvs.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 27.Matias A, Fredouille C, Nesmann C, et al. Prenatal diagnosis of left ventricular aneurysm: a report of three cases and a review. Cardiol Young. 1999;9(2):175–184. doi: 10.1017/s1047951100008404. [DOI] [PubMed] [Google Scholar]
- 28.McElhinney DB, Silverman NH. Left ventricular aneurysm in the fetus: a diagnosis with a mixed prognosis. Cardiol Young. 1999;9(2):123–126. doi: 10.1017/s1047951100008313. [DOI] [PubMed] [Google Scholar]
- 29.Nam KH, Kwon JY, Son GH, et al. Prenatally diagnosed left ventricular diverticulum with thoracoabdominal wall defect: a case and review of the literature. J Perinatol. 2010;30(11):760–762. doi: 10.1038/jp.2010.106. [DOI] [PubMed] [Google Scholar]
- 30.Papagiannis J, Van Praagh R, Schwint O, et al. Congenital left ventricular aneurysm: clinical, imaging, pathologic, and surgical findings in seven new cases. Am Heart J. 2001;141(3):491–499. doi: 10.1067/mhj.2001.113076. [DOI] [PubMed] [Google Scholar]
- 31.Patel CR, Judge NE, Muise KL, et al. Prenatal myocardial infarction suspected by fetal echocardiography. J Am Soc Echocardiogr. 1996;9(5):721–723. doi: 10.1016/s0894-7317(96)90071-1. [DOI] [PubMed] [Google Scholar]
- 32.Pipitone S, Sperandeo V, Mongiovi M, et al. Prenatal diagnosis of ventricular aneurysm: a report of two cases and a review. Prenat Diagn. 2002;22(2):131–136. doi: 10.1002/pd.259. [DOI] [PubMed] [Google Scholar]
- 33.Pradhan M, Dalal A, Kapoor A, et al. Fetal left ventricular diverticulum presenting as dysrhythmia: diagnosis and management. Fetal Diagn Ther. 2008;23(1):10–14. doi: 10.1159/000109219. [DOI] [PubMed] [Google Scholar]
- 34.Prefumo F, Bhide A, Thilaganathan B, et al. Fetal congenital cardiac diverticulum with pericardial effusion: two cases with different presentations in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2005;25(4):405–408. doi: 10.1002/uog.1855. [DOI] [PubMed] [Google Scholar]
- 35.Seo DM, Won HS, Ko JK, et al. Modified damus-kaye-stansel/dor procedure for a newborn with severe left ventricular aneurysm. Korean Circ J. 2011;41(8):494–496. doi: 10.4070/kcj.2011.41.8.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sepulveda W, Drysdale K, Kyle PM, et al. Congenital left ventricular aneurysm causing hydrops fetalis: prenatal diagnosis with color Doppler ultrasonography. J Ultrasound Med. 1996;15(4):327–331. doi: 10.7863/jum.1996.15.4.327. [DOI] [PubMed] [Google Scholar]
- 37.Sharma JR, Oforl-Amanfo G, Marboe C, et al. Congenital left ventricular aneurysm with pericardial effusion: prenatal diagnosis, surgical management and follow-up. Pediatr Cardiol. 2002;23(4):458–461. doi: 10.1007/s00246-002-1528-y. [DOI] [PubMed] [Google Scholar]
- 38.Sherman SJ, Leenhouts KH, Utter GO, et al. Prenatal diagnosis of left ventricular aneurysm in the late second trimester: a case report. Ultrasound Obstet Gynecol. 1996;7(6):456–457. doi: 10.1046/j.1469-0705.1996.07060456.x. [DOI] [PubMed] [Google Scholar]
- 39.Tsujimoto H, Takeshita S, Kawamura J, et al. Isolated congenital left ventricular diverticulum with perinatal dysrhythmia: a case report and review of the literature. Pediatr Cardiol. 2000;21(2):175–179. doi: 10.1007/s002469910032. [DOI] [PubMed] [Google Scholar]


