Abstract
The Val158Met (rs4680) single nucleotide polymorphism (SNP) of the catechol-O-methyltransferase gene (COMT) influences executive function and prefrontal function through its effect on dopamine (DA) metabolism. Both HIV and the Val allele of the Val158Met SNP are associated with compromised executive function and inefficient prefrontal function. The present study used behavioral and neuroimaging techniques to determine independent and interactive associations between HIV serostatus and COMT genotype on working memory and prefrontal function in women. For the behavioral study, 54 HIV-infected and 33 HIV-uninfected women completed the 0-, 1- and 2-back conditions of the verbal N-back, a working memory test. For the imaging study, 36 women (23 HIV-infected, 13 HIV-uninfected) underwent functional magnetic resonance imaging (fMRI) assessments while completing the N-back task. HIV-infected women demonstrated significantly worse N-back performance compared to HIV-uninfected women (p<0.05). A significant serostatus by genotype interaction (p<0.01) revealed that, among Val/Val, but not Met allele carriers, HIV-infected women performed significantly worse than HIV-uninfected controls across N-back conditions (p<0.01). Analogous to behavioral findings, a serostatus by genotype interaction revealed that HIV-infected, Val/Val carriers showed significantly greater prefrontal activation compared to HIV-uninfected, Val/Val carriers (p<0.01). Conversely, HIV-uninfected, Met allele carriers demonstrated significantly greater prefrontal activation compared to HIV-infected, Met allele carriers. Findings suggest that the combination of HIV infection and the Val/Val COMT genotype leads to working memory deficits and altered prefrontal function in HIV-infected individuals.
Keywords: HIV, COMT genotype, single nucleotide polymorphism (SNP), working memory, prefrontal function, dopamine
I. Introduction
HIV targets the central nervous system within days after infection, which ultimately leads to neurological, behavioral, and cognitive complications (Brew et al., 1988; Grant et al., 1989; McArthur, 1994). Even after the introduction of combined antiretroviral therapy (cART), mild neurocognitive deficits persist in approximately 45% of individuals with HIV/AIDS particularly in the domains of executive function, learning, and memory (Heaton et al., 2011). Compared to HIV-infected men, HIV-infected women may be at increased risk for cognitive decline due to poverty, low education, substance abuse, and barriers to health care services prevalent in predominantly minority, urban-dwelling populations (Basso & Bornstein, 2000, Bornstein et al., 1993; Maki & Martin, 2009). Genetic factors that put HIV-infected individuals at risk for neurocognitive deficits are not well known, especially in women. This study examines whether a genetic polymorphism in the catechol-O-methyltransferase gene (COMT) is associated with deficits in neurocognitive functions dependent on integrity of prefrontal circuitry such as executive function in women with HIV.
The COMT enzyme is responsible for the metabolic degradation of catecholamines, primarily dopamine (DA) (Lachman et al., 1996), with a high degree of activity in the prefrontal cortex (PFC) (Garris et al., 1993). The Val158Met (rs4680) single nucleotide polymorphism (SNP) of the COMT gene influences the activity and thermal stability of the COMT enzyme (Lachman et al., 1996), whereby Met/Met homozygotes have approximately one quarter of the enzyme activity compared to the Val/Val genotype (Bilder et al., 2004). This reduction in COMT activity results in slower DA breakdown and increased synaptic DA availability, particularly in the PFC (Chen et al., 2004; Lotta et al., 1995). Therefore, the Met/Met genotype shows the greatest level of DA signaling in the PFC and the Val/Val genotype shows the least.
The COMT Val158Met polymorphism influences PFC-mediated cognitive domains that are dependent on DA, particularly executive functions such as working memory (Tunbridge et al., 2006; Farrell et al., 2012). The relationship between PFC DA levels and executive function is typically characterized by an inverted U-shaped curve where levels at the peak of the curve are optimal for executive function. Conversely, DA levels to the left or right of the peak lead to poorer performance on these tasks (Cai & Arnsten, 1997; Granon et al., 2000; Zahrt et al., 1997; Cools & D’Esposito, 2011; Egan et al., 2001). For example, in both healthy adults and individuals with schizophrenia, Val/Val participants demonstrate poorer performance on executive function tasks, including working memory (Egan et al., 2001; Goldberg et al., 2003; Minzenberg et al., 2006), suggesting that for those groups, the lower levels of prefrontal DA associated with the Val/Val genotype results in suboptimal levels that fall to the left of the peak. In neuroimaging studies, the Val allele was associated with increased prefrontal activation during working memory tasks in individuals with schizophrenia (Egan et al., 2001; Ho et al., 2005) and healthy adults (Bertolino et al., 2006; Heinz & Smolka, 2006; Jacobs & D’Esposito, 2011; Meyer-Lindenberg et al., 2006). The increased PFC activation in low DA, Val/Val participants is interpreted as representing decreased brain processing efficiency (Heinz & Smolka, 2006; Jacobs & D’Esposito, 2011; Meyer-Lindenberg et al., 2006).
To our knowledge, no study to date has examined the interactive associations between COMT genotype and HIV serostatus on working memory and its neural correlates. The HIV virus is indirectly neurotoxic to DA neurons by way of released, neurotoxic proteins (Bennett et al., 1995; Itoh et al., 2000; Reyes et al., 1991). HIV-infected cells infiltrate the DA-rich basal ganglia (Navia et al., 1986) and secrete toxic viral proteins including Tat and gp120 that result in DA dysregulation and excitotoxicity leading to cell death (Koutsilieri et al., 2001). In vivo and in vitro studies have shown that dopaminergic neurons are functionally and structurally susceptible to the effects of Tat and gp120 including oxidative stress, functional alterations in cell receptors and apoptosis (Agrawal et al., 2010; Aksenova et al., 2006; Ferris et al., 2009). For example, in vitro studies have found that DA neurons treated with the HIV Tat protein show a significant decrease in binding of the DA transporter (Aksenova et al., 2006) and inhibited expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in the DA synthesis pathway (Zuali et al., 2000).
The DA dysfunction in HIV contributes to working memory deficits (Woods et al., 2009; Kumar et al., 2011). HIV is associated with deficits on multiple working memory tasks (Farinpour et al., 2000; Martin et al., 2001; Sun et al., 2010), including the N-back (Hinkin et al., 2002). In fMRI studies using the N-back test, symptomatic and asymptomatic HIV-infected men showed significantly greater brain activation in bilateral PFC compared to uninfected men. Similar to Val/Val carriers, the greater activation was interpreted as decreased processing efficiency (Chang et al., 2001; Ernst et al., 2002). Here, we examine the effect of COMT genotype on N-back performance and on patterns of brain activation during the N-back, in a sample of HIV-infected and uninfected women. We hypothesized that HIV serostatus and the Val/Val genotype would be associated with poorer working memory performance and greater PFC activation during the N-back. Based on the assumption that HIV-infected individuals with the Val/Val genotype have suboptimal DA levels that fall to the left of the peak, we also hypothesized that the Val/Val genotype would compound the negative effect of HIV on working memory such that HIV-infected Val/Val carriers would show the worst accuracy and the greatest PFC activation overall.
2. Methods & Materials
2.1 Participants
Participants were recruited from the Chicago Women’s Interagency HIV Study (WIHS) Consortium.
Behavioral Study: Inclusionary criteria included consent to genetic testing and English as a first language. We enrolled 140 women (97 HIV-infected and 44 HIV-uninfected), 34 of whom were excluded because of: self-reported use of a stimulant drug in the past six months (n=18); antipsychotic medication use (n=4); serious head injury (n=4); stroke or cerebrovascular disease (n=3); diabetes (n=2); self-reported dementia (n=1); AIDS-defining illness (i.e., history of a CD4 count <200 cells/mm3) (n=1); and fewer than eight years of formal education (n=1) because of the complexity of the memory task. Nine women (8% HIV-infected, 9% HIV-uninfected) were further excluded due to below chance (less than 25% accuracy) or outlier (>3 SDs from mean) behavioral performance on the N-back. Ninety-seven participants were included in the final sample. Imaging Study: Inclusionary and exclusionary criteria were the same as the behavioral study with the addition of age ≥ 60 years, metal in the body, claustrophobia, weight ≥ 250 lbs (due to scanner dimensions), and a positive pregnancy or toxicology test (DrugCheck 5 panel cup by Express Diagnostics International). Toxicology tests were used only in the fMRI study to exclude individuals who used illicit substances within 24 hours to 10 days of testing. The detection time is based on the varying half-life of illicit drugs including cocaine, amphetamine/metamphetamine, opiate, phencyclamine, and THC in urine). Bilinguals were excluded because the brain systems subserving tasks that rely on verbal stimuli differ between native English speakers and bilinguals (Proverbio et al., 2002). Forty-six women were enrolled in the imaging study. Ten women were excluded from analysis for the following reasons: positive toxicology screen for illicit drug use (n=2); movement in excess of two voxels (6.25 mm) (n=6); and below chance or outlier performance on the N-back (n=2). The final sample included 36 (23 HIV-infected) participants, 22 of whom previously participated in the behavioral study.
2.2 Measures
N-back (Postle et al., 2000). The N-back is a widely-used, parametrically-designed working memory task, which incrementally varies working memory load. There are three conditions, each consisting of 40 trials: the 0-, 1- and 2-back. In each condition, a letter is presented every 1.8 seconds for 200 milliseconds on a computer screen. In the 0-back, the participant is instructed to press the “yes” button every time a previously designated target letter is presented. The 0-back is used as the control condition because it does not involve a memory load and is a measure of attention and stimulus response time. In the 1-back, the participant is instructed to press “yes” when the letter presented matches the previously presented letter. In the 2-back, the participant is instructed to press “yes” when the letter presented matches the letter presented two trials earlier. The primary outcome was percent accuracy per condition, and the secondary outcome was average reaction time per condition.
2.3 Procedure
Behavioral Study
The N-back was incorporated into Chicago WIHS Consortium Core visits from May to November 2008. During the biannual WIHS visit, participants were asked about socio-demographic and health-related variables including medication use, drug and alcohol use, and depressive symptoms as measured by the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977). CD4 absolute count (T-helper cell count) and plasma HIV RNA viral load were measured for HIV-infected women. The Wide Range Achievement Reading Test-Revised (WRAT-R) (Jastak et al., 1984), a measure of educational attainment, was also obtained.
Imaging Study
Participants completed a 2.5-hour study visit at the UIC Center for Magnetic Resonance (MR) Research, and provided a urine sample for toxicology (DrugCheck 5 panel cup by Express Diagnostics) and pregnancy testing (hCG Urine Pregnancy Test Strip). Before scanning, participants received detailed instructions on the N-back and practiced a computerized N-back task. The in-scanner N-back was similar to the behavioral study except that the trials per condition were truncated from 40 to 35 to allow for a manageable scanning time.
Blood oxygen level-dependent (BOLD) imaging was performed on a General Electric 3.0 Tesla scanner. Thirty-seven images were acquired in an oblique, axial plane. The fMRI N-back test was administered using a block design with the following acquisition parameters: TR=2000ms, TE=25 ms, flip angle 90°, NEX=1, acquisition matrix=64×64, FOV=20 cm2, slices=37, slice thickness=3mm, skip=1 mm, Bandwith=62.5 kHz, volumes=270, acquisition time=8:56. Structural MRI was performed with a three-dimensional inversion recovery prepared spoiled gradient recalled echo acquisition.
2.4 Genotyping
Genotyping for Val158Met (rs4680) was done as part of the genome wide association study in WIHS at Illumina Assay Services in San Diego, CA using validated commercially available assays from Applied Biosystems (www.appliedbiosystems.com).
2.5 Data analysis
Behavioral study
Based on previous COMT and working memory studies (Minzenberg et al., 2006; Bertolino et al., 2006; Jacobs & D’Esposito et al., 2011) we assessed relationships between genotype and neurocognitive outcomes assuming a dominant genetic model (i.e. Val/Val vs. Met allele carriers). We examined the main and interactive associations of HIV serostatus and COMT genotype on demographic and clinical characteristics with ANOVAs for continuous variables and Chi-squares (Χ2) for categorical variables. Any variable that significantly differed between genotype or serostatus groups and significantly related to the N-back performance was included in the analysis as a covariate. To examine the independent and interactive associations of serostatus, COMT genotype, and N-back condition, a mixed factor ANOVA was conducted with N-back condition (0-, 1-, and 2-back) as the within-subjects factor and HIV serostatus (HIV+ vs. HIV−) and COMT genotype (Val/Val vs. Met allele carriers) as the between-subjects factors. To address potential bias associated with population stratification, we also conducted analyses within an African-American sample. All p values were two-sided (p<0.05). Analyses were performed using SPSS (version 18.0 for Windows; SPSS, Chicago, IL).
fMRI study
Preprocessing and analysis of the neuroimaging data was completed using statistical parametric mapping (SPM8, Welcome Department of Imaging Neuroscience, London, UK). MRI data were converted to ANALYZE format. Preprocessing steps included motion correction (realignment), coregistration between functional images and the EPI template, spatial normalization, and spatial smoothing smoothed with an 8-mm Gaussian kernel. A first-level analysis step was conducted to determine regionally activated brain volumes in individual participants using a general linear model and convolved with the hemodynamic (BOLD) response function. To examine activation associated with working memory, brain activation during the control condition (0-back) was subtracted from that during each of the experimental conditions (1- and 2-back).
A second-level full factorial model examined the effects of COMT genotype (Val/Val; Met allele carriers), serostatus (HIV+; HIV−) and condition (1-back; 2-back) as predictors. A threshold of p<0.01 (uncorrected, minimum cluster size ≥ 15) was used for 2- and 3-way interactions. A threshold of p<0.05 (uncorrected, minimum cluster size k=15) was used to further probe significant interactions. We conducted a region of interest analysis based on brain regions reported as significantly active during the N-back in previous fMRI studies including the bilateral PFC and posterior parietal cortex, anterior cingulate, caudate and putamen (Egan et al., 2001; Bertolino et al., 2006; Heinz & Smolka, 2006; Jacobs & D’Esposito, 2011; Meyer-Lindenberg et al., 2006). Anatomical localization of significant activations was determined using the xjView toolbox (http://www.alivelearn.net/xjview). Unlike the behavioral study, we did not repeat analyses in an African-American subsample because that would remove only two of 33 fMRI participants.
The DNA sequence corresponding to the SNP analyzed was derived from the National Center for Biotechnology Information (NCBI) SNP Database (dbSNP) using the accession number, rs4680.
3. Results
Behavioral Study
Table 1 shows demographic and clinical information by serostatus and COMT genotype. Participants ranged in age from 25 to 71 years (M=41, SD=10) and 81% were African American. Compared with HIV-seronegative women, HIV-seropositive women were older, obtained lower scores on the WRAT-R, and had a higher prevalence of hepatitis C and past illicit drug use (p’s<0.05). Compared with Met allele carriers, Val/Val carriers were less likely to have recently (past six months) used marijuana (p<0.05). Among Met allele carriers, HIV-seronegative women were more likely to have graduated high school, be premenopausal, and engage in hazardous drinking and recent marijuana use compared to HIV-seropositive women (p’s>0.05). Among the demographic/clinical variables that significantly differed between serostatus or genotype groups, illicit drug use was the only variable to significantly relate to N-back percent accuracy (p’s<0.05). All analyses, therefore, controlled for past illicit drug use and the interactive effects of past illicit drug use and genotype.
Table 1.
Behavioral study: Demographics for participants as a function of HIV serostatus (HIV+, HIV−), COMT Val158Met genotype group (Val/Val, Met allele carriers) and the two combined.
| HIV Serostatus (N = 97) |
COMT Genotype (N = 97) | Statistical Results (p-value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Background Characteristics |
HIV+ (n = 67) M (SD) |
HIV− (n = 30) M (SD) |
Met allele carriers (n=55) |
Val/Val (n = 42) | Main Effects | Interaction | |||
| HIV+ (n = 44) M (SD) |
HIV− (n = 11) M (SD) |
HIV+ (n = 23) M (SD) |
HIV− (n = 19) M (SD) |
Serostatus | Genotype | Serostatus × Genotype |
|||
| Age at Visit | 43.2 (9.7) | 35.6 (9.5) | 42.6 (9.7) | 33.6 (8.5) | 44.4 (9.9) | 36.7 (10.0) | 0.01 | ns | ns |
| Graduated high school | 67% | 83% | 58% | 91% | 86% | 79% | ns | ns | 0.05 |
| WRAT Score | 84.9 (2.2) | 92.1 (3.3) | 83.8 (19.4) | 92.1 (15.4) | 85.9 (17.2) | 92.2 (13.4) | ns | ns | ns |
| CES-D >16 | 43% | 24% | 45.2% | 18.2% | 36.8% | 27.8% | ns | ns | ns |
| Race/Ethnicity | |||||||||
| African-American (non-Hispanic) | 82% | 80% | 82% | 91% | 83% | 74% | ns | ns | ns |
| White (non-Hispanic) | 6% | 0% | 7% | 0% | 4% | 0% | - | - | - |
| Hispanic | 9% | 20% | 7% | 9% | 13% | 26% | - | - | - |
| Other | 3% | 0% | 4% | 0% | 0% | 0% | - | - | - |
| Hepatitis C Virus | 36% | 10% | 33% | 9% | 41% | 11% | 0.01 | ns | ns |
| Recently Used Crack/Cocaine/Heroin | 0% | 0% | 0% | 0% | 0% | 0% | ns | ns | ns |
| Ever Used Crack/Cocaine/Heroin | 64% | 40% | 64% | 45% | 65% | 37% | 0.03 | ns | ns |
| Currently Smoking | 45% | 40% | 48% | 54% | 39% | 32% | ns | ns | ns |
| Heavy Alcohol Use | 4% | 13% | 4% | 36% | 4% | 0% | ns | ns | <0.01 |
| Recently Used Marijuana | 9% | 23% | 13% | 45% | 0% | 10% | ns | 0.03 | 0.02 |
| Menopause Stage | |||||||||
| Premenopausal | 43% | 65% | 34% | 80% | 60% | 54% | ns | ns | 0.03 |
| Perimenopausal | 24% | 18% | 27% | 10% | 18% | 23% | - | - | - |
| Postmenopausal | 33% | 17% | 39% | 10% | 23% | 23% | - | - | - |
| CD4 Level | |||||||||
| > 500 | 59% | - | 54% | - | 67% | - | - | ns | - |
| > 200 & ≤ 500 | 35% | - | 46% | - | 17% | - | - | ns | - |
| < 200 | 6% | - | 0% | - | 16% | - | - | ns | - |
| CD4 nadir | 336 (165) | - | 317 (151) | - | 287 (127) | - | - | ns | - |
| Viral Load | |||||||||
| Undetectable | 60% | - | 61% | - | 57% | - | - | ns | - |
| < 10,000 | 25% | - | 23% | - | 29% | - | - | ns | - |
| ≥ 10,000 | 15% | - | 15% | - | 14% | - | - | ns | - |
| Medication Use | ns | ||||||||
| cART | 85% | - | 85% | - | 85% | - | - | ns | - |
| Non-cART | 0% | - | 0% | - | 0% | - | - | ns | - |
| ART naïve | 11% | - | 15% | - | 0% | - | - | ns | - |
| Medication compliance (>95%) | 82% | - | 82% | - | 83% | - | - | ns | - |
Note. ns = not significant, p<0.05. WRAT =Wide Range Achievement Test; CES-D = depressive symptoms measured by Center for Epidemiologic Studies Depression Scale with >16 cut-off; “Recent” refers to within 6 months of the most recent WIHS visit. Heavy alcohol use reflects >7 drinks per week or more than 4 drinks in one sitting. CD4 Level = T-Helper cell count per mm3 of blood; CD4 nadir = lowest recorded CD4 level to date; cART =combined antiretroviral therapy; ART = antiretroviral therapy
As found in the WIHS more generally (Sundermann et al., 2013), more HIV-infected women carried the Met allele (67%) than HIV-uninfected women (37%), p=0.13. Therefore, the cognitively disadvantageous genotype was more prevalent among the HIV-uninfected (cognitively advantaged) group, an effect that might mask the negative effect of the Val/Val genotype and the positive effect of seronegativity. We, therefore, evaluated the interactive effect of genotype and serostatus by stratifying by genotype and examining the effect of serostatus within each genotype separately. Genotype distributions were in Hardy-Weinberg equilibrium for the full sample, Χ2=0.6, p>0.05 and for African Americans, the predominant race group, Χ2=0.08, p>0.05.
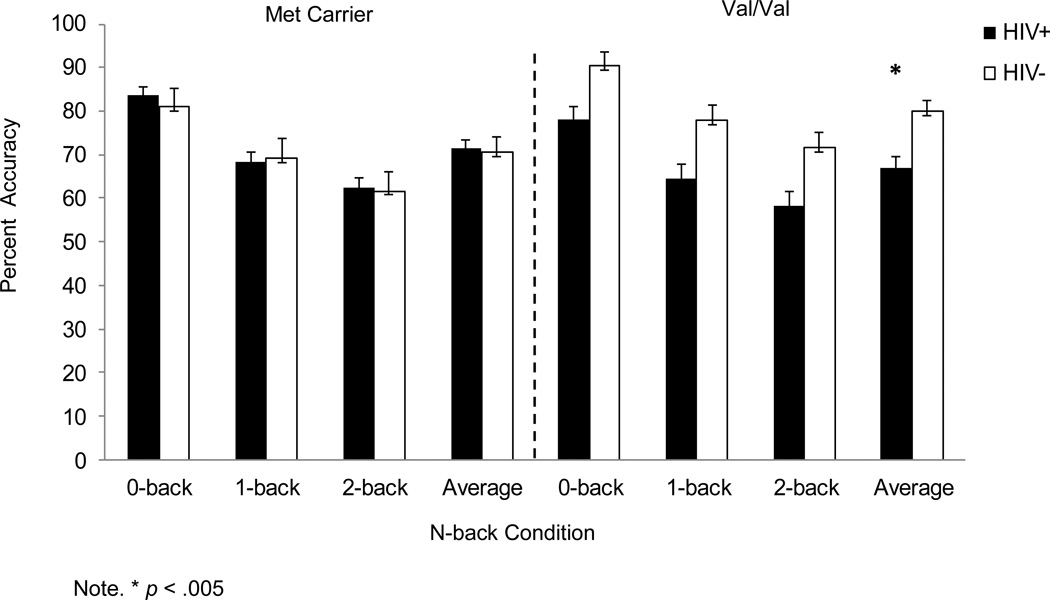
Accuracy, but not reaction time, changed with increasing difficulty in the N-back, p<0.05 (Figure 1). The covariate, past drug use, had a significant, negative effect on N-back performance in the overall sample (p<0.01); however, the interactive effect of past drug use and genotype was not significant (p=0.76). As hypothesized, HIV-infected women had lower accuracy across N-back conditions (M=69.3, SE=1.5) compared to HIV-uninfected women (M=75.3, SE=2.2) (p<0.05). Contrary to our hypothesis, there was no main effect of genotype. There was, however, a significant serostatus by genotype interaction (p<0.01) such that, among Val/Val carriers, but not among Met carriers, accuracy was significantly lower in HIV-infected women (M=67.2, SE=2.5) versus uninfected controls (M=80.3, SE=2.8) (p<0.001). No other interactions were significant (p>0.05). Results did not change when analysis was restricted to African Americans (HIV-infected=55, HIV-uninfected=24).
Figure 1.
Adjusted mean N-back percent accuracy (per trial and average total) as a function of serostatus for Met carriers (left panel) and Val/Val carriers (right panel).
Imaging Study
The imaging sample included 36 women (23 HIV+, 13 HIV−) with an average age of 43.0 (SD=8.5), with 95% African American and 79% high school graduates. Among HIV-infected women, 6% had a current CD4+ lymphocyte count < 200 cells/µl, 60% had an undetectable plasma HIV RNA level, and 85% received cART treatment. Serostatus and genotype groups did not differ on any demographic or clinical characteristic. The imaging sample was similar to the behavioral sample except that those in the imaging study, were less likely to show depression (15% vs. 37%) and more likely to be African American (94% versus 81%). Among HIV-infected women, those in the imaging study were more likely to graduate high school (80% versus 67%) and had lower rates of Hepatitis C (21% vs. 36%).
Genotype distributions were in Hardy-Weinberg equilibrium for the entire sample (Χ2=0.07, p>0.05), and for African-Americans, the predominant race group (Χ2=0.29, p>0.05). As in the behavioral study and in studies of HIV-infected women more generally (Sundermann et al., 2013), the distribution of COMT genotype differed by HIV serostatus (Χ2=7.9, p=0.005). Again, the proportion of carriers of the typically beneficial allele (Met) was significantly higher in HIV-infected women (65%) compared to HIV-uninfected women (38%), and the proportion of carriers of the Val allele was significantly higher in HIV-uninfected (62%) women compared to the HIV-infected woman (35%). This confounder reduces the likelihood of detecting the overall effects of HIV and genotype. Therefore, we again stratified by genotype and examined the effect of serostatus to probe the interaction between serostatus and HIV.
With increasing difficulty across N-back conditions (0-, 1-, 2-back), percent accuracy decreased (p’s<0.001) and reaction time increased (p’s<0.001). In contrast to the behavioral study, serostatus and COMT genotype had no independent or interactive associations on N-back percent accuracy or reaction time (p’s>0.05).
There was a significant main effect of N-back condition on brain activation across serostatus groups; as working memory load increased from the 1- to 2-back conditions, BOLD signal increased in the right posterior parietal lobe (BA7), right dorsolateral PFC (DLPFC; BA9), left superior frontal gyrus (BA8), bilateral middle frontal gyrus (BA46, BA8, BA11) and left inferior frontal gyrus (BA11). Table 2 provides the Talairach coordinates and statistical values for each brain region showing significantly greater activation in the 2-back versus the 1-back.
Table 2.
Results from the fMRI study: Brain region, Brodmann area (BA), Talairach coordinates, cluster size (k) and statistical information for clusters that show significantly greater activation in the 2-back versus the 1-back.
| Brain Region | BA | Talairach Coordinates (x, y, z) |
Cluster size (k) | Z score |
|---|---|---|---|---|
| R Posterior Parietal Cortex | 7 | 38, −60, 28 | 18 | 3.22 |
| 7 | 34, −64, 36 | 1122 | 4.38 | |
| 7 | −22, −66, 52 | 1209 | 4.72 | |
| R Dorsolateral PFC | 9 | 54, 30, 36 | 726 | 4.05 |
| 9 | 42, 20, 54 | 26 | 3.35 | |
| L Middle Frontal Gyrus | 46 | −46, 20, 26 | 203 | 3.36 |
| 8 | −2, 24, 50 | 119 | 3.82 | |
| R Middle Frontal Gyrus | 8 | 4, 24, 50 | 65 | 3.24 |
| 11 | 22, 44, −18 | 39 | 3.20 | |
| 11 | 46, 44, −14 | 30 | 2.80 | |
| L Superior Frontal Gyrus | 8 | −30, 30, 54 | 22 | 2.83 |
| 8 | −38, 14, 56 | 50 | 3.51 | |
| L Inferior Frontal Gyrus | 11 | −44, 42, −14 | 48 | 3.40 |
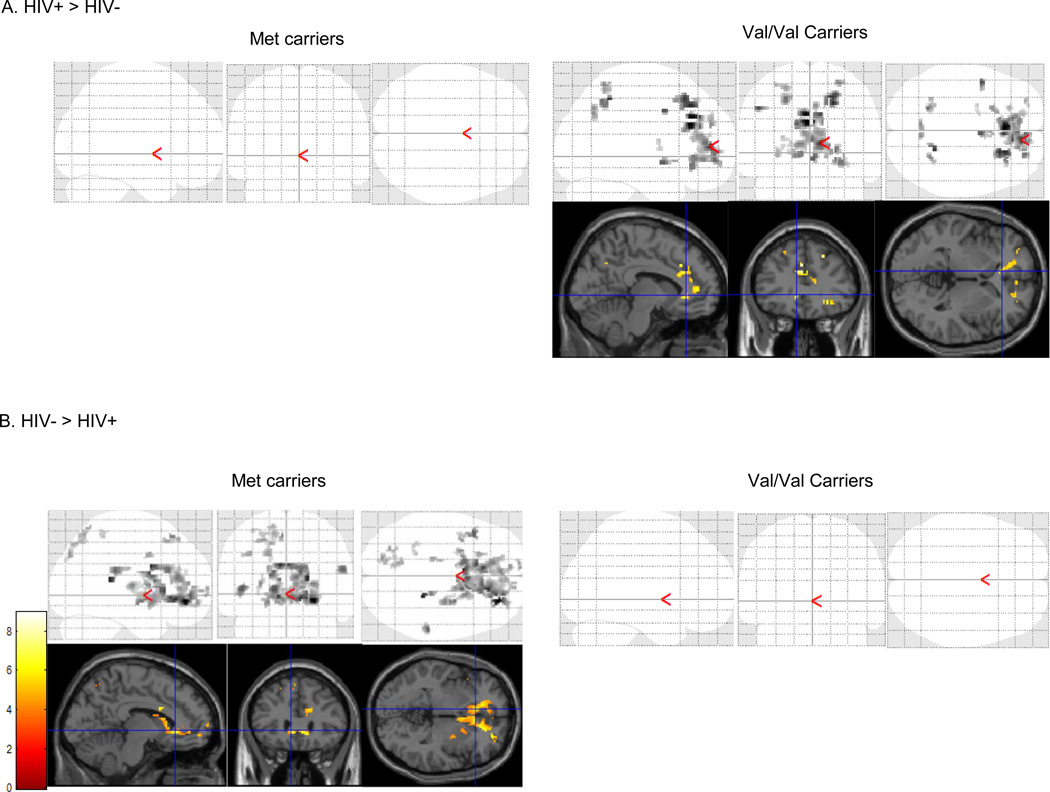
As shown in Table 3, serostatus interacted with genotype to alter BOLD signal in the left anterior cingulate (BA32), right anterior cingulate (BA32), right DLPFC (BA10) and right middle frontal gyrus (BA11). Among Val/Val carriers, HIV-infected women showed greater activation in the left anterior cingulate (BA24), right anterior cingulate (BA32), right DLPFC (BA10), and right medial frontal gyrus (BA11). Conversely, among Met allele carriers, HIV-uninfected controls showed significantly greater activation in the right medial frontal gyrus (BA11) and the left anterior cingulate (BA32) compared to the HIV-infected individuals (Figure 2).
Table 3.
Results from the fMRI study: Brain region, Brodmann area (BA), Talairach coordinates, cluster size (k) and statistical information for significantly active brain regions associated with the 2-way, serostatus by genotype, and 3-way, serostatus×genotype×N-back condition, interactions and the follow-up contrasts
| Brain Region | BA | Talairach Coordinates (x, y, z) |
Cluster size (k) |
Z score |
Contrast |
|---|---|---|---|---|---|
| Serostatus × Genotype Interaction | |||||
| L Anterior Cingulate | s32 | −6, 8, 24 | 85 | 3.19 | Val/Val: HIV+ > HIV− |
| R DLPFC | 10 | 16, 38, 34 | 43 | 2.83 | Val/Val: HIV+ > HIV− |
| R Medial Frontal Gyrus | 11 | 24, 30, −12 | 30 | 2.77 | Met carriers: HIV− > HIV+ |
| 11 | 26, 48, −6 | 71 | 3.35 | Val/Val: HIV+ > HIV− | |
| R Anterior Cingulate | 32 | 8, 34, 20 | 211 | 2.71 | Val/Val: HIV+>HIV− |
| L Caudate | −12, 14, 18 | 52 | 3.33 | Val/Val: HIV+>HIV− | |
| Serostatus × Genotype × Condition Interaction | |||||
| R Anterior Cingulate | 32 | 8, 22, 30 | 174 | 4.12 | Val/Val: HIV+>HIV− on 2-back |
| L Anterior Cingulate | 32 | −6, 12, 40 | 15 | 3.35 | Val/Val: HIV+>HIV− on 2-back |
Figure 2.
Brain activation during the N-back in Val/Val carriers vs. Met allele carriers that is (A) greater in HIV-infected compared to HIV-uninfected women (B) and greater in HIV-uninfected compared to HIV-infected women.
Table 3 also shows results of the significant serostatus × genotype × condition interaction characterized by increased activation in the right anterior cingulate (BA32, k=174, z=4.12) and left medial frontal gyrus (BA9, k=15, z=3.35). These significant clusters were driven by HIV-infected Val/Val carriers in the 2-back condition.
4. Discussion
This two-part investigation examined performance and functional neuroimaging outcomes on the N-back as a function of COMT genotype and serostatus in women. As predicted based on findings from predominantly male, HIV+ cohorts (Farinpour et al., 2000; Martin et al., 2001; Sun et al., 2010; Hinkin et al., 2002; Stout et al., 1995), HIV-infected women showed impaired N-back performance compared to HIV-uninfected controls. Contrary to our hypotheses, the Val/Val genotype did not relate to poorer N-back performance when all study participants (cases and controls) were examined together. HIV-infected women in this subsample were more likely to have a beneficial Met allele than HIV-uninfected women. The same Met allele frequency difference was previously found in comparisons between HIV-infected and uninfected women in the general WIHS population (Sundermann et al., 2013) demonstrating that this Met allele imbalance between serostatus groups generalizes to a population of multi-racial, low-income women and is not simply a sampling bias within this subsample. The higher proportion of Met allele carriers among HIV-infected women might be due to the positive association between Met allele and propensity for risk-taking (Bousman et al., 2010a; Amstadter et al., 2012; van den Bos et al., 2008). After stratifying by genotype, we found poorer working memory performance in HIV-infected Val/Val carriers versus HIV-uninfected Val/Val carriers but no effect of serostatus among Met carriers. These findings occurred across N-back conditions, suggesting that, together, HIV serostatus and Val/Val genotype negatively affects attentional processes, short-term memory, and more complex updating and temporal indexing components of working memory. Among Val/Val carriers, activation was greater in the anterior cingulate and medial frontal gyrus among HIV-infected compared to uninfected women. The statistical results suggest that the COMT genotype has a moderating effect on the relationship between HIV serostatus and prefrontal function such that the negative effects of HIV on executive function might become evident only in the context of lower baseline dopaminergic function (Val/Val genotype). An inverted U-shaped curve characterizes the relationship between executive function performance and PFC DA levels (Cai & Arnsten, 1997; Granon et al., 2000; Zahrt et al., 1997; Egan et al., 2001). It is plausible that HIV-infected women with the Val/Val genotype may fall to the left of this curve to a point representing lowered PFC DA levels and compromised performance. Alternatively, the effect of HIV on working memory might not be robust enough to be detected clinically in asymptomatic individuals who have the advantageous Met allele and the associated increase in PFC DA levels.
Two previous studies investigated the effect of COMT genotype on executive function in HIV, but involved predominantly male cohorts and did not include HIV-uninfected controls (Bousman et al., 2010b; Levine et al., 2012). Consistent with our findings, a study of 192 HIV-infected, non-methamphetamine users found an association between the Met/Met genotype and better executive function (Bousman et al., 2010b). Conversely, a study of 184 HIV-infected individuals (87% male) in more advanced stages of HIV (mean CD4=219) found no effect of COMT genotype on the Letter-Number Sequencing test (LNS) and the Paced Auditory Serial Addition Test (Levine et al., 2012). The discrepancy between that study and the others might be due to the inclusion of individuals with more advanced disease where the effect of disease might mask any COMT effect (Stout et al., 1995; York et al., 2001), the use of the LNS which might be less sensitive than the N-back to COMT (Bousman et al., 2010b) or to low female representation (13%).
Our neuroimaging findings revealed a HIV serostatus by genotype interaction such that Val/Val carriers showed increased brain activation during the N-back in DLPFC, anterior cingulate cortex, and medial PFC. These brain areas are reliably activated during working memory tasks in studies of HIV or COMT (Egan et al., 2001; Minzenberg et al., 2006; Heinz & Smolka, 2006; Jacobs & D’Esposito, 2011; Meyer-Lindenberg et al., 2006). DLPFC is most reliably activated in neuroimaging studies of COMT and working memory, due to its role in multiple working memory processes including the monitoring and manipulation of information held in working memory (Owen, 1997; Petrides, 1994), response selection (Rowe et al., 2000), and strategy use (Bor et al., 2003; Bor et al., 2004). The anterior cingulate is interconnected with the PFC (Posner & DiGirolamo, 1998) and richly innervated by midbrain DA projections (Seamans & Yang, 2004). Activation in anterior cingulate is observed in tasks involving attention, error detection and decision making, suggesting that this region helps to coordinate multiple cognitive processes associated with a challenging task (Posner & DiGirolamo, 1998; Chein & Schneider, 2005; Kondo et al., 2004; Petersen et al., 1989). Evidence suggests that the bilateral medial PFC regulates uncertainty (Elliott et al., 1999; Fukui et al., 2005; Volz et al., 2003; Volz et al., 2004) and is activated in response to guessing demands two-choice guessing task (Elliott et al., 1999). The N-back might invoke a substantial degree of uncertainty, particularly the 2-back, because participants are instructed to respond to each trial even if they are unsure of the answer (mean 2-back %accuracy=63%; chance performance=25% accuracy).
The brain regions with increased PFC activation in relation to working memory load were similar to the brain regions that showed greater activation in HIV-infected versus uninfected Val/Val carriers (right middle frontal gyrus, right DLPFC, right posterior parietal cortex). The increased activation in HIV-infected, Val/Val carriers might represent greater effort and compensatory recruitment of a larger network of brain areas in order to maintain behavioral competency. Supporting this interpretation, activation was greatest among HIV-infected, Val/Val carriers in the 2-back versus 1-back condition. Although some brain regions showed greater activation in HIV-uninfected versus infected Met allele carriers, they did not overlap with brain areas related to working memory load.
Our study has limitations. First, the sample size in the behavioral study is similar to other studies investigating COMT and cognition, but our imaging sample included only 11 HIV-uninfected, Met allele carriers. Second, toxicology screens were used in the neuroimaging, but not the behavioral study. Although reported rates of current substance use are low among this group of HIV-infected women, we cannot exclude the possibility that covert drug use confounded the behavioral results. Past, illicit drug use was prevalent in our sample, differed by serostatus in the behavioral study and had a significant, negative effect on N-back performance. Although we cannot exclude the possibility of past drug use impacting results, we minimized this impact by adjusting for past drug use and demonstrating no significant interactive effects of past drug use and genotype indicating that the effect of drug use is consistent across genotypes. Thirdly, our sample included women only. While not a limitation per se, results cannot be generalized to men, particularly in light of the role of COMT in metabolizing catecholestrogens and estrogen’s agonistic effect on DA (Becker, 2000; Thompson & Moss, 1994; Xiao & Becker, 1994). Fourth, menopause status differed across serostatus and genotype groups but did not relate to n-back performance and did not influence the findings when used as a covariate.
As a major strength, this is the first investigation of the interactive effects of COMT genotype and HIV serostatus on working memory and PFC function and the largest neuroimaging study of HIV-infected women to date. Together, HIV serostatus and the Val/Val COMT genotype, which has been associated with suboptimal DA levels, were associated with working memory deficits and inefficient PFC function. COMT genotype might represent a biomarker to identify HIV-infected individuals at risk for working memory deficits.
Acknowledgements
Erin Sundermann, Pauline M. Maki, Jeffrey R. Bishop, Deborah M. Little: study concept and design. Erin Sundermann, Vanessa Meyer, Deborah M. Little, Kathleen Weber: data acquisition. Jeffrey R. Bishop: Genotyping. Erin Sundermann, Vanessa Meyer, Deborah M. Little, Pauline M. Maki: MRI data-analysis. Leah H. Rubin, Erin Sundermann, Pauline M. Maki, Jeffrey R. Bishop: statistical analysis, interpretation. Erin Sundermann: initial manuscript preparation. All authors provided critical revision of manuscript for important intellectual content and approved the final manuscript.
Erin Sundermann’s participation was funded by the National Institute of Mental Health (NIMH 1F31MH083537-01), the Mt. Sinai Institute for NeuroAIDS Disparities (2009-01946), and the Alice Dan Dissertation Research Award. V. Meyer’s effort on this project was supported by the National Institute on Drug Abuse (1F31DA028573). Leah Rubin’s participation was supported by grant number 1K01MH098798-01 from the NIMH and K12HD055892 from the National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Office of Research on Women's Health (ORWH). Dr. Bishop’s participation was funded by grant number K08MH083888 from the NIMH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, University of Illinois at Chicago, Baylor Scott & White Health, Texas A&M University Health Sciences College of Medicine, Rush University Medical Center or The Core Center at Stroger Hospital of Cook County.
The Women’s Interagency HIV Study (WIHS) is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, the National Institute on Deafness and Other Communication Disorders, and the NIMH. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Study investigators received training, mentorship, and guidance in the execution and interpretation of this study from the Chicago Developmental Center for AIDS Research (D-CFAR), an NIH funded program (P30 AI08251), which is supported by the following NIH institutes and centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NCCAM).
Footnotes
Results of this study were presented as a poster at the 2013 Human Brain Mapping Annual Meeting in Seattle, WA.
Conflicts of Interest
All authors, Erin E. Sundermann, Jeffrey R. Bishop, Leah H. Rubin, Deborah M. Little, Vanessa J. Meyer, Eileen Martin, Kathleen Weber, Mardge Cohen and Pauline Maki, declare that they have no conflicts of interest.
References
- Amstadter AB, Macpherson L, Wang F, Banducci AN, Reynolds EK, Potenza MN, Gelernter J, Lejuez CW. The relationship between risk-taking propensity and the COMT Val(158)Met polymorphism among early adolescents as a function of sex. J Psychiatr Res. 2012;46(7):940–945. doi: 10.1016/j.jpsychires.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395(3):235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12 months. J Clin Exp Neuropsychol. 2000;22(2):208–218. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- Becker JB. Oestrogen effects on DArgic function in striatum. In: Chadwick DJ, Goode JA, editors. Neuronal and cognitive effects of oestrogens. West Sussex, England: Wiley; 2000. pp. 134–145. [Google Scholar]
- Bennett BA1, Rusyniak DE, Hollingsworth CK. HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res. 1995;705(1–2):168–176. doi: 10.1016/0006-8993(95)01166-8. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, Nardini M, Weinberger DR, Dallapiccola B. Additive effects of genetic variation in DA regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26(15):3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic DA hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Pace P, Rosenberger P, Nasrallah HA, Para MF, Whitacre C, Fass RJ. Depression and neuropsychological performance in asymptomatic HIV infection. Am J Psychiatry. 1993;150(6):922–927. doi: 10.1176/ajp.150.6.922. [DOI] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Atkinson JH, Heaton RK, Grant I, Everall IP Hnrc Group T. COMT Val158Met Polymorphism, Executive Dysfunction, and Sexual Risk Behavior in the Context of HIV Infection and Methamphetamine Dependence. Interdiscip Perspect Infect Dis. 2010a;2010:678648. doi: 10.1155/2010/678648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Glatt SJ, Atkinson JH, Grant I, Tsuang MT. Impact of COMT Val158Met on executive functioning in the context of HIV and methamphetamine. Neurobehavioral HIV Medicine. 2010b;2:1–11. doi: 10.2147/NBHIV.S8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor D, Cumming N, Scott CEM, Owen AM. Prefrontal cortical involvement in encoding strategies, independent of stimulus modality. Eur J Neurosci. 2004;19:3365–3370. doi: 10.1111/j.1460-9568.2004.03438.x. [DOI] [PubMed] [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361–367. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Sidtis JJ, Petito CK, Price RW. The neurologic complications of AIDS and Human Immunodeficiency Virus Infection. In: Plum F, editor. Advances in Contemporary Neurology. Philadelphia, PA: F.A. Davis Co; 1988. pp. 1–49. [Google Scholar]
- van den Bos R, Homberg J, Gijsbers E, den Heijer E, Cuppen E. The effect of COMT Val158 Met genotype on decision-making and preliminary findings on its interaction with the 5-HTTLPR in healthy females. Neuropharmacology. 2008;56(2):493–498. doi: 10.1016/j.neuropharm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Cai JX, Arnsten AF. Dose-dependent effects of the DA D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1997;283(1):183–189. [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57(6):1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta analytic evidence of a domain-general control network for learning. Cogn Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped DA actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia. 1999;37(4):403–411. doi: 10.1016/s0028-3932(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59(9):1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, Novak RM, Harrow M. Verbal working memory in HIV-seropositive drug users. J Int Neuropsychol Soc. 2000;6(5):548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry. 2012;71(6):538–544. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse. 2009;63(3):181–185. doi: 10.1002/syn.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa Gambling Task. Neuroimage. 2005;24(1):253–259. doi: 10.1016/j.neuroimage.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Garris PA, Collins LB, Jones SR, Wightman RM. Evoked extracellular DA in vivo in the medial prefrontal cortex. J Neurochem. 1993;61(2):637–647. doi: 10.1111/j.1471-4159.1993.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 DArgic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20(3):1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Atkinson JH, Hesselink JR, Kennedy CJ, Richman DD, Spector SA, McCutchan JA. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging. Ann Intern Med. 1989;107:828–836. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Lam MN, Stefaniak M, Zolnikov B. Verbal and spatial working memory performance among HIV-infected adults. J Int Neuropsychol Soc. 2002;8(4):532–538. doi: 10.1017/s1355617702814278. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J Grant I; CHARTER Group; HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci. 2006;17(3):359–367. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O'Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyltransferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol Psychiatry. 2005;10(3):229, 287–298. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol. 2000;99:376–384. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- Jacobs E, D’Esposito M. Estrogen shapes DA-dependent cognitive processes: implications for women's health. J Neurosci. 2011;31(14):5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastak S, Wilkinson GS, Jastak J. Wide Range Achievement Test-Revised. Indianapolis: Jastak Associates Inc; 1984. [Google Scholar]
- Kondo H, Osaka N, Osaka M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage. 2004;23:670–679. doi: 10.1016/j.neuroimage.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, ter Meulen V, Riederer P. Neurotransmission in HIV associated dementia: a short review. J Neural Transm. 2001;108(6):767–775. doi: 10.1007/s007020170051. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17(1):26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Sinsheimer JS, Bilder R, Shapshak P, Singer EJ. Functional polymorphisms in DA-related genes: Effect on neurocognitive functioning in HIV+ adults. J Clin Exp Neuropsychol. 2012;34(1):78–91. doi: 10.1080/13803395.2011.623118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol-O-methyltransferase: a revised mechanism and description of the thermo-labile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Maki PM, Martin E. Women, cognition, and HIV. Neuropsychol Rev. 2009;19(2):204–214. doi: 10.1007/s11065-009-9093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Sullivan TS, Reed RA, Fletcher TA, Pitrak DL, Wedington W, Harrow M. Auditory working memory in HIV-1 infection. J Int Neuropsychol Soc. 2001;7(1):20–26. doi: 10.1017/s1355617701711022. [DOI] [PubMed] [Google Scholar]
- McArthur JC. Neurological and Neuropathological Manifestations of HIV Infection. In: Grant I, Martin A, editors. Neuropsychology of HIV infection. New York: Oxford University Press; 1994. pp. 56–107. [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11(9):797, 867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Xu K, Mitropoulou V, Harvey PD, Finch T, Flory JD, New AS, Goldman D, Siever LJ. Catechol-O-methyltransferase Val158Met genotype variation is associated with prefrontal-dependent task performance in schizotypal personality disorder patients and comparison groups. Psychiatr Genet. 2006;16(3):117–124. doi: 10.1097/01.ypg.0000199448.00163.e6. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petiio CK, Price RW. The AIDS dementia complex: II Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behavior. Curr Opin Neurobiol. 1994;4:207–211. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The attentive brain. Cambridge MA: MIT Press; 1998. pp. 401–423. [Google Scholar]
- Postle BR, Stern CE, Rosen BR, Corkin S. An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. NeuroImage. 2000;11:409–423. doi: 10.1006/nimg.2000.0570. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Cok B, Zani A. Electrophysiological measures of language processing in bilinguals. J Cogn Neurosci. 2002;14(7):994–1017. doi: 10.1162/089892902320474463. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1997;1:385–401. [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1991;82(1):39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory. Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of DA modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sun B, Abadjian L, Rempel H, Calosing C, Rothlind J, Pulliam L. Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodeficiency virus type 1 infection. J Neurovirol. 2010;16(2):115–124. doi: 10.3109/13550280903559789. [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Bishop JR, Rubin LH, Aouizerat B, Wilson TE, Weber KM, Cohen M, Golub E, Anastos K, Liu C, Crystal H, Pearce CL, Maki PM. HIV serostatus differs by catechol-O-methyltransferase Val158Met genotype. AIDS. 2013;27(11):1779–1782. doi: 10.1097/QAD.0b013e328361c6a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Salmon DP, Butters N, Taylor M, Peavy G, Heindel WC, Delis DC, Ryan L, Atkinson JH, Chandler JL, Grant I. Decline in working memory associated with HIV infection. HNRC Group. Psychol Med. 1995;25(6):1221–1232. doi: 10.1017/s0033291700033195. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of DA release in the nucleus accumbens: Genomic-and nongenomic-mediated effects. J Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60(2):141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage. 2003;19(2 Pt 1):271–280. doi: 10.1016/s1053-8119(03)00122-8. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Why am I unsure? Internal and external attributions of uncertainty dissociated by fMRI. Neuroimage. 2004;21(3):848–857. doi: 10.1016/j.neuroimage.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal DA concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- York MK, Franks JJ, Henry RR, Hamilton WJ. Verbal working memory storage and processing deficits in HIV-1 asymptomatic and symptomatic individuals. Psychol Med. 2001;31(7):1279–1291. doi: 10.1017/s0033291701004494. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 DA receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17(21):8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G, Secchiero P, Rodella L, Gibellini D, Mirandola P, Mazzoni M, Milani D, Dowd DR, Capitani S, Vitale M. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem. 2000;275(6):4159–4165. doi: 10.1074/jbc.275.6.4159. [DOI] [PubMed] [Google Scholar]