Abstract
Background
The waiting time for deceased donor renal transplantation in the United States continues to grow. Retransplant candidates make up a small but growing percentage of the overall transplant waiting list and raise questions about the stewardship of scarce resources. The utility of renal transplantation among individuals with two prior renal transplants is not described in the literature and we thus sought to determine the survival benefit associated with a third kidney transplant (3KT).
Methods
Multivariable Cox regression models were created to determine characteristics associated with 3KT outcomes and the survival benefit of 3KT among recipients wait listed and transplanted within the United States between 1995 and 2009.
Results
4,334 patients were waitlisted for a 3KT and 2,492 patients received a 3KT. In a multivariate analysis, 3KT demonstrated an overall patient survival benefit compared to the wait list (HR-0.379, CI=0.302-0.475 p<0.001) for those awaiting their first, second or third kidney transplants, although an inferior graft outcome compared to first kidney transplants. The time to survival benefit did not accrue until 8-months after transplant. Additionally we found that the duration of second graft survival was predictive of third graft survival, such that second graft survival beyond 5 years is associated with superior 3KT graft survival. Second graft loss in 30 days or less was not associated with inferior 3KT graft survival.
Conclusion
3KT achieves a survival benefit over remaining on the wait list, although is associated with inferior graft outcomes compared to first kidney transplants. Graft survival of the second transplant beyond 5 years is associated with superior 3KT graft survival.
Keywords: Kidney Transplant, Graft Survival, Patient Survival, Retransplantation
Introduction
Successful kidney transplantation improves quality of life and increases survival in patients with end-stage renal disease (ESRD), as compared with long-term dialysis treatment (1). Short-term graft survival has significantly improved over the past two decades while long-term graft survival has been more static. Consequently renal allograft failure has become one of the most common causes of ESRD and an increasing number of patients are being relisted and re-transplanted after an initial failed kidney transplant (2-5). Repeat transplantation confers a survival advantage to patients over dialysis and has graft survival approaching primary transplants in selected candidates (6-10). However the modest decrease in graft and patient survival in second transplant recipients has generated unease within the transplant community about overall utility and resource allocation in repeat renal transplantation (2, 6, 11-16). Given the current shortage of renal allografts and the fact that kidney transplant failure is becoming one of the primary indications for kidney transplantation generally, these concerns will likely only increase with time as more repeat transplant recipients are listed. While the utility of a second kidney transplant has been addressed by previous studies, the utility among individuals with two prior kidney transplants is unknown. In this study we report the outcome of 2,492 third kidney transplants (3KT) performed in the US over a 15-year period. The aim of the study was to assess the US experience in 3KT in both decease and living donor kidney transplant, determine the long-term outcome of 3KT, to identify the factors associated with 3KT long-term survival and to determine the survival benefit of a 3KT.
Results
Yearly Number of 3KT Remain Steady Although 3KT Waitlist Additions Continue to Increase
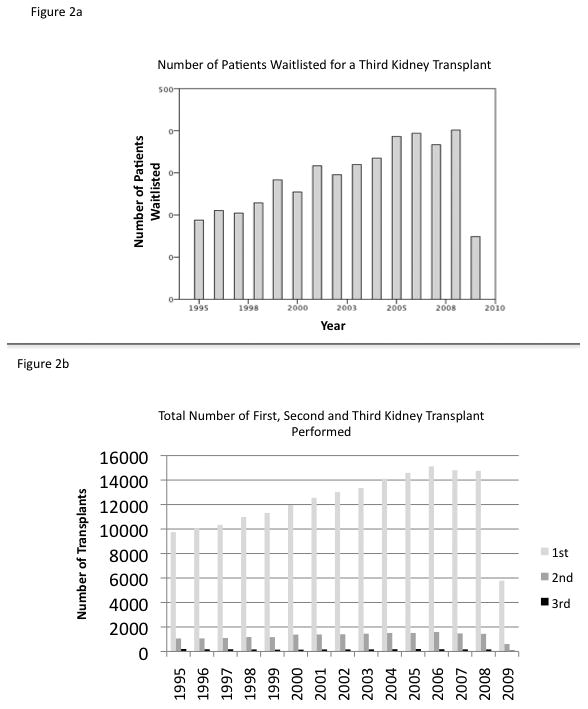
4,334 candidates were listed for a 3KT with a median wait time of 624 days (0-5,234). Figure 1 summarizes the patient selection. Table 1 summarizes the recipient data and table 2 summarizes the donor data. The data for 3KT and their prior (second) transplant are well captured by the UNOS dataset, however the data for their first kidney transplant is very poorly captured by the UNOS dataset, with 69% of the data missing, as a significant number of these transplants occurred before the initiation of UNOS data collection in 1988. As a point of comparison for the study, we included all patients who received their first kidney transplant data during the same study time period in Table 1. 2,492 patients received a 3KT; 26.4% (659) from living donors and 73.6% (1833) from deceased donors. The yearly number of 3KT performed each year has remained relatively steady over the study period (Figure 2a), making up approximately 1% of transplanted patients, despite a significant increase in patients seeking a 3KT over the study period (Figure 2b).
Figure 1.
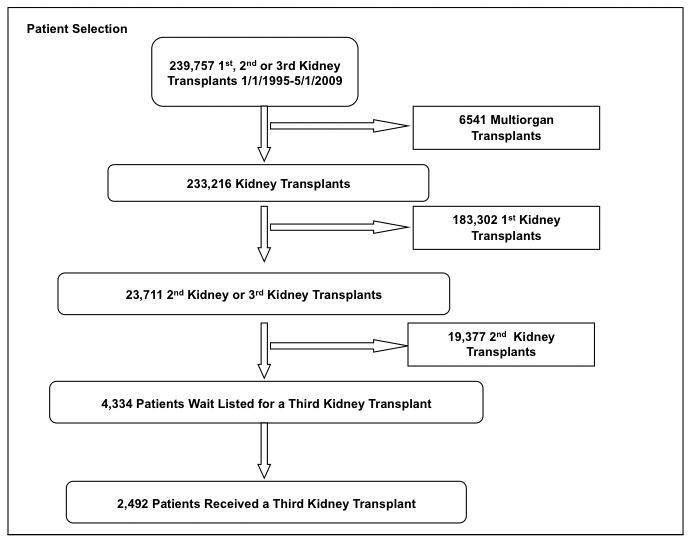
Table 1. Recipient Characteristics.
| 3rd Kidney | 3rd Kidney Deceased Donor | 3rd Kidney Living Donor | Awaiting 3rd Kidney | 2nd Kidney | 1st Kidney (whole list) | p value | |
|---|---|---|---|---|---|---|---|
| Number of Patients | 2492 | 1833 | 659 | 4334 | 4334 | 183302 | |
| Mean Age of Recipient - Years (std) | 39.0 (12.3) | 39.3 (12.3) | 37.6 (11.9) | 39.7 (12.3) | 33.7 (12.5) | 46.2 (15.6) | 0.004 |
| Gender of Recipient | 0.756 | ||||||
| Male (%) | 1462 (58.7) | 1112 (60.7) | 350 (53.1) | 2526 (58.3) | 2526 (58.3) | 110469 (60.3) | |
| Female (%) | 1030 (41.3) | 721 (39.3) | 309 (46.9) | 1808 (41.7) | 1808 (41.7) | 72833(39.7) | |
| Race of Recipient | <0.001 | ||||||
| White | 1736 (69.7) | 1222(66.7) | 521 (79.1) | 2777 (64.1) | 2777 (64.1) | 146829 (58.3) | |
| Black | 444 (17.8) | 378 (20.6) | 65 (9.9) | 955 (22) | 955 (22) | 57844 (23.8) | |
| Other | 312 (12.5) | 233 (12.7) | 73 (11) | 602 (13.9) | 602 (13.9) | 44705 (17.9) | |
| Blood Group | |||||||
| A | 1053 (42.2) | 783 (42.7) | 270 (41.1) | 1790 (41.3) | 1790 (41.3) | 68005 (37.1) | <0.001 |
| B | 256 (10.3) | 180 (9.8) | 76 (11.5) | 462 (10.7) | 462 (10.7) | 23829 (13) | |
| AB | 127 (5.1) | 96 (5.2) | 31 (4.7) | 199 (4.6) | 199 (4.6) | 8798 (4.8) | |
| O | 1056 (42.4) | 774 (42.2) | 282 (42.8) | 1883 (43.4) | 1883 (43.4) | 82669 (45.1) | |
| HLA Match (%) | |||||||
| 0 | 220 (0.8) | 153 (8.4) | 67 (10.5) | 430 (9.9) | 25728 (14.0) | <0.001 | |
| 1 | 457 (18.3) | 329 (20.2) | 88 (13.8) | 858 (19.8) | 41561(22.7) | ||
| 2 | 427 (17.1) | 345 (18.9) | 82 (12.8) | 941 (21.7) | 33748 (18.4) | ||
| 3 | 517 (20.7) | 286 (15.7) | 231 (36.1) | 1036 (23.9) | 43404 (23.7) | ||
| 4 | 312 (12.5) | 238 (13) | 74 (11.6) | 467 (10.8) | 16656 (9.1) | ||
| 5 | 284 (11.4) | 252 (13.8) | 32 (5.0) | 269 (6.2) | 10485 (5.7) | ||
| 6 | 249 (10.0) | 183 (10) | 66 (10.3) | 262 (6.0) | 10077 (5.5) | ||
| missing % | 1.0 | 1.6 | 0.9 | ||||
| Delayed Graft Function (%) | 655 (26.3) | 609 (33.2) | 46 (0.9) | 29.8 | 23.9 | 0.02 | |
| missing % | 1.2 | 0.2 | 1.2 | ||||
| Peak PRA - Mean (std) | 57 (37.2) | 60.15 (36.4) | 42.16 (37.1) | 64.6 (37.6) | 37.9 (37.5) | 11.34 (24.0) | <0.001 |
| missing % | 14.9 | 8.6 | 17.5 | 21.8 | |||
| Mean Wait Time - Days | 624 (0-5936) | 1011 (0-5936) | 517 (0-3792) | 642 (0-5234) | 398 (0-6610) | 532 (0-9536) | <0.001 |
| missing % | 6.9 | 2.1 | 11.9 | 16.5 | |||
| Median Time from last | 2669 | 2577 | 2961 | 2012 | |||
| transplant - Days | (0-7779) | (0-7779) | (0-7393) | (0-7422) | <0.001 | ||
| Preemptive Transplant | <0.001 | ||||||
| No | 2168 (87) | 1661 (90.6) | 507 (76.9) | 3734 (86.2) | 208755 (83.7) | ||
| Yes | 265 (10.6) | 136 (7.4) | 129 (19.6) | 353 (8.1) | 31205 (12.5) | ||
| missing % | 2.4 | 5.7 | 3.8 |
Third Kidney Recipient Characteristics
Table 2. Donor Characteristics.
| 3rd Kidney | 3rd Kidney Deceased Donor | 3rd Kidney Living Donor | 2nd Kidney | p value | |
|---|---|---|---|---|---|
| Mean Age of Donor - Years (std) | 35 (14.7) | 33.95 (15.6) | 37.98 (11.6) | 36 (15.6) | <0.001 |
| Gender of Donor | 0.131 | ||||
| Male (%) | 1408 (56.5) | 1123 (61.3) | 285 (43.2) | 2367 (54.6) | |
| Female (%) | 1084 (43.5) | 710 (38.7) | 374 (56.8) | 1967 (45.4) | |
| Race of Donor | .006 | ||||
| White | 1940 (77.8) | 1411 (77.0) | 529 (80.3) | 3319 (76.6) | |
| Black | 246 (9.9) | 184 (10) | 62 (9.4) | 507 (11.7) | |
| Other | 306 (12.3) | 238 (13) | 68 (10.4) | 507 (11.7) | |
| Cold Ischemic Time - Hours (std) | 16.9 (10.8) | 20.7 (8.3) | n/a | 17.9 (11.9) | 0.01 |
| missing % | 10.0 | 12.9 | |||
| DCD | |||||
| Yes (%) | 65 (2.6) | 65 (3.5) | 0 | 21 (0.8) | <0.001 |
| Pumped | |||||
| Yes (%) | 208 (8.3) | 208 (11.3) | 0 | 397 (9.2) | 0.076 |
| Type of Donor | |||||
| Deceased (%) | 1833 (73.6) | 3261 (75.2) | |||
| Living (%) | 659 (26.4) | 1073 (24.8) | |||
| Extended Criteria Donor (%) | 158 | 158 (8.6) | n/a | 316 | <0.001 |
| missing % | 26.4 | 50.3 | |||
| Blood Group | |||||
| A | 960 (38.5) | 764 (41.7) | 196 (29.8) | 1605 (37.0) | <0.001 |
| B | 223 (8.9) | 164 (8.9) | 59 (9.0) | 374 (8.6) | |
| AB | 52 (2.1) | 44 (2.4) | 8 (1.2) | 95 (3.2) | |
| O | 1257 (50.4) | 861 (47.0) | 396 (60.1) | 2259 (52.1) | |
| History of Hypertension (%) | <0.001 | ||||
| Yes | 304 (12.2) | 299 (16.3) | 5 (0.8) | 473 (10.9) | |
| No | 1805 (72.4) | 1512 (82.5) | 651 (98.7) | 1782 (41.1) | |
| Unkonwn | 22 (0.9) | 19 (1.0) | 3 (0.5) | 23 (0.5) | |
| missing % | 12.2 | 47.4 |
Third Kidney Donor Characteristics
Figure 2.
Recipients Of A 3KT Are Younger and More Sensitized
The mean age of a third transplant recipient was younger (39.0 ± 12.3) than someone obtaining a first transplant over the same 15-year study period (44.4 ± 15.5). It is likely that many of these patients were children or adolescents at the time of their first transplants but these data are too incomplete for thorough analysis. The recipients of a 3KT were more highly sensitized at the time of 3KT than they had been at the time of their second transplant (average peak PRA of 57.0% verses 37.9% respectively, p<0.001), and compared to the pool of patients waiting for their first transplant (57.0% vs. 11.3%). Those waiting for a 3KT were even more sensitized (average peak PRA of 64.6%) than those that were successfully transplanted with a 3KT (57.0%). Additionally, a greater proportion (49.0%) of those on the wait list for a 3KT were highly sensitized (PRA ≥80%), compared to those that were successfully transplanted with a 3KT (36.2%, p<0.001).
African-American Patients Comprise A Reduced Proportion Of 3KT Recipients
33% of the first time kidney waitlist were African American compared to only 22% that were awaiting a 3KT (p<0.001). Furthermore only 17.8% of 3KT recipients were African-American compared to 23.8% of all first time kidney recipients (p<0.001). African-American patients seem to be more highly sensitized, which may be a reason for this disparity. A high degree of sensitization (PRA ≥80%) was more common among African-American patients (66.2%) than white patients (48.7%) on the 3KT waitlist. Whether this causally led to a decrease in the proportion of African-American patients that were successfully transplanted a third time is not determinable. Another possible explanation for this decrease in transplant rate for 3KT for African-Americans seems to be the underutilization of living donors: with only 16.7% of African-American 3KT recipients receiving living donors where as 28.6% of white 3KT recipients received living donors (p<0.001).
3KT Had A Significantly Higher Rate of Early Graft Loss, Higher Rate of DGF, and Reduced 5-Year Graft Survival Than Those Receiving Their First Kidney Transplant
The overall 5-year graft survival was found to be 69.9% for deceased donor and 79.2% for living donor third transplants. This is reduced compared to those that received a first kidney transplant over the same study period: 75.9% for deceased donor and 86.1% for living donor first kidney transplants. There was a significant increase in early (≤30d) graft loss for 3KT over those patients who underwent their first kidney transplant, 9.2% vs. 3.2% (p<0.001). When adjusting for these early graft losses the 3KT graft survival improves but does not equate to the first kidney graft survival – it is 73.6% for deceased donor and 82.5% for living donor 3KT. A multivariate analysis demonstrated a significantly worse 5-year survival over first kidney transplants for both deceased (HR 1.42, CI 1.25 – 1.69, p<0.001) and living donors (1.76, CI 1.18-2.59, P=0.005). Here the degree of HLA matching, donor and recipient age, race, ECD and DCD status, DGF, and peak pra were also associated with graft survival. The incidence of delayed graft function was higher in 3KT deceased donor recipients - 33.2% compared to 23.9% for first kidney deceased donor transplants, p=0.002. Interestingly the rate of DGF was nearly twice that for 3KT living donors compared to first kidney living donor transplants, 7.0% vs. 4.5%. These increased rates could also have contributed to a slightly worse 5-year graft survival.
Deceased Donor 3KT Have an Inferior KDPI/KDRI than First Kidney Transplants
We hypothesized that a rationale for inferior deceased donor 3KT graft survival and increased rates of delayed graft function was due to inferior deceased donor graft quality for 3KT. We therefore calculated the KDPI/KDRI for deceased donor kidneys for 3KT and first kidney recipients. Surprisingly the KDPI/KDRI for 3KT was significantly better than for first kidney transplants. The mean KDPI/KDRI for 3KT was 31%/0.895 (sd 0.276) vs. 48%/0.979 (sd 0.330) for first kidney transplants (p<0.001). The KDPI/KDRI of the second transplant for those patients awaiting a 3KT was not statistically different from those awaiting a first transplant, 48%/0.980 (sd 0.32) vs. 48%/0.979 (sd 0.330), p>0.05).
Second Graft Survival is Predictive of 3KT Graft Survival for Both Living and Deceased Donors
In a multivariable analysis second graft survival was associated with 3KT graft survival for deceased donor kidneys (Figure 3a). Second graft survival of > 5 years was associated with significantly better 3KT graft survival than second graft survival of ≤ 5 year graft survival. Second graft survival ≤ 30 days was not significantly predictive of 3KT graft outcome likely because it was reflective of technical complications, p=0.33. Degree of HLA matching, donor age, ECD status, DGF and peak pra were also associated with 3KT graft survival for deceased donor kidneys. Similarly, graft survival for recipients of a living donor 3KT was also associated with second graft survival (HR 1.63 CI 1.02-2.48 p=0.03 for second graft survival of >30d and < 5 yr compared to second graft survival of >5yr). As found in the deceased donor analysis graft loss in 30 days or less did not indicate a worse graft 3KT graft survival. Here peak PRA, DGF and degree of HLA matching were associated with 3KT living donor graft survival.
Figure 3.
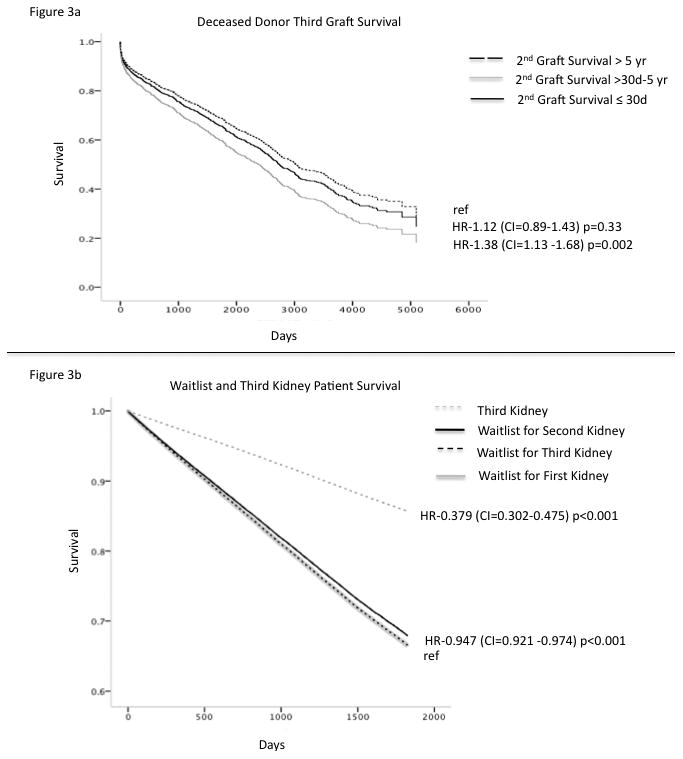
3KT Provides A Survival Benefit Over The Waitlist
To assess overall patient survival, the patient survival for a 3KT was compared to the waitlist survival for those that were waiting for a 3KT. We also compared 3KT survival to those waiting for their first and second kidney transplants to see if any differences in survival could be seen. In a multivariate analysis, 3KT demonstrated an overall patient survival benefit compared to the wait list (HR-0.379, CI=0.302-0.475 p<0.001) for those awaiting their first, second and third kidney transplants (Figure 3b). When comparing the survival benefit of those receiving a 3KT to those on the waitlist a statistically significant survival benefit did not occur until 8 months post-transplant. Importantly a survival benefit for 3KT was observed irrespective of the length of survival of the second transplant.
Discussion
Over time a large proportion of renal transplant recipients will face the prospects of allograft loss. For such patients, repeat transplantation provides better long-term survival and improved quality of life compared to remaining on dialysis, although these studies have only focused on second transplants (17-19). We herein report the analysis of the UNOS experience evaluating outcomes of 3KT and we demonstrate that in selected patients, a third kidney transplant provides significant patient survival advantage over the waitlist while offering a slightly reduced graft outcome compared to first kidney transplants. Early graft loss was significantly higher in 3KT recipients and is one likely cause of inferior graft survival. Transplantation of a 3KT can pose certain surgical challenges. A repeat operation into a previously used or nearby site is inherently more complicated secondary to development of scar tissue and availability or quality of recipient vessels. This may yield higher complication rates, including bleeding, vascular thrombosis, adjacent organ injury, and perioperative infections. These data are poorly captured by UNOS; however, surgical complications may contribute to early graft loss. These patients also by definition have prolonged exposure to immunosuppression medication, complicating their operative and postoperative course. Our data does show that 9.2% of grafts were lost in less than 30d. This is significantly higher than the early graft loss seen in patients undergoing their first kidney transplant during the same study period, 3.2% (p<0.001). These added surgical risks likely contribute to the overall modestly worse graft outcomes. When adjusting for these early graft losses, 3KT graft outcomes are still significantly worse than first kidney transplants. Other reasons for disparate outcomes are potentially due to the increased rates of DGF in 3KT, and the fact that 3KT recipients are more highly sensitized than recipients of first or second renal transplants. We know that there are both recipient and donor factors at play in the development of DGF. The increased rate of DGF seen here in 3KT likely is being driven by recipient factors – like increased immunologic sensitization as indicated by the higher PRA, rather than donor factors like kidney quality since the KDPI/KDRI for 3KT actually suggests higher quality of kidneys being used for 3KT overall.
We acknowledge that a selection bias likely exists in this 3KT graft outcome analysis. First, these patients that received a 3KT were younger than those that received their first kidney transplant (39.0 yo vs. 46 yo to <0.001). This implies that a lot of these patients likely received their first transplant when they were much younger – i.e. in childhood or adolescence - although 69% of the data on 3KT's first transplant is poorly captured by UNOS. It is known that adolescence patients tend to have a poorer post transplant graft survival – typically due to noncompliance. ypically due to noncomplianceWas it worse compliance that led to a 3KT or something else? Did they get poorer quality kidneys at the time of their first and/or second transplants, did they have worse disease processes, or is their immunology more unfavorable. It does not appear to be poorer second kidney quality since the KDRI was comparable to all first kidney transplants, 0.980 (sd 0.32) vs. 0.979 (sd 0.330), p>0.05). To get a 3KT, by definition, you have had two graft failures, which may be selecting for patients with shorter graft outcomes or worse disease processes - thus suggesting a worse graft survival in these selected patients.
Our finding that second graft survival informs 3KT survival provides some context to this. We demonstrate that second kidney graft survival of at least 5 years predicts superior 3KT survival, whereas second graft survival of ≤ 5 year graft survival predicts inferior 3KT survival. Second graft survival ≤ 30 days was not significantly predictive of 3KT graft outcome likely because it was reflective of technical complications - which apparently can be overcome on subsequent transplant attempts. This suggests what many clinicians already know: that multiple recipient factors affect outcomes. Such diverse elements as medication adherence, primary renal disease, immune system function, and access to care make up a risk profile for a particular recipient that may in part be improved with appropriate therapy but which overall connotes a profile of increased risk of graft loss that may span more than one transplant for a given recipient.
Despite these additional risks and inferior graft outcomes, a 3KT provides a survival advantage over the waitlist. This survival advantage does not appear to be statistically significant until 8 months, which is similar to what was reported by Wolf et al for recipients of first kidney transplants (1). Although this analysis was adjusted, a selection bias also likely does exist. Patients that received a 3KT were younger (39 yo vs. 42 yo, p<0.001) and healthier (54% diabetic vs. 67%) than those that remained on the waitlist for a 3KT. Additionally, this analysis can study only those patients at least placed on the waiting list for a 3KT – it is not possible to analyze those patients who were seen and evaluated for a 3KT but deemed not to be a candidate for a 3KT. It stands to reason that these 3KT listed and transplanted patients represent a highly selected group of potential 3KT recipients and these highly selected patients may experience superior outcomes from 3KT than can be attributable to 3KT if applied to all patients without careful patient selection. Those patients with poor second transplant outcomes tended to have less favorable 3KT outcomes and this should raise caution regarding 3KT for this group from a utility perspective, although there was still demonstrable survival advantage of 3KT even in this higher risk group.
Our data also highlights that a lower proportion of African-American patients are listed for and are transplanted with a 3KT relative to first kidney waitlist additions. First transplant outcomes analysis shows that this is not due to lesser rates of first and second transplant graft failure in African-American patients, in fact they suggest higher graft loss if any(11, 20, 21). African-American patients were more highly sensitized and had fewer living donor transplants, which could make it more difficult to get a 3KT. However why a smaller percentage of African-Americans were waitlisted for a 3KT is not clearly identified by this data. We can speculate that since African-American patients tend to be referred later for first transplants and transplanted after more exposure to dialysis than white patients (22) it is thus conceivable that by the time they have lost two kidney transplants they have accumulated enough health problems to be deemed unfavorable 3KT candidates. Improving early access to first transplantation and minimizing health care access disparities may in time diminish the racial disparities in 3KT.
Lastly, when comparing those waiting for a 3KT and those successfully undergoing a 3KT it is clear that those patients waiting are more highly sensitized (average peak PRA is 64.6% versus 57.0%, p<0.001). Obtaining a compatible graft could be a barrier for these patients awaiting a 3KT. These highly sensitized retransplant patients may have improved opportunities to attain a 3KT with increased transplant rates of sensitized patients after adoption of virtual crossmatching, with improved desentization protocols, or via access to national and local kidney exchanges – each of these developments may be too recent to be adequately captured here. New allocation guidelines will give access to regional and national offers for patients with PRA of 98% and above but this should be a minority of even 3KT patients (25%).
This study has some limitations, including the utilization of the UNOS data set, which has well defined data in some areas but some areas where data is less reliable such as rates of rejection particularly after the first year after transplant and attributed factors leading to graft loss. Furthermore we do not know which 3KT recipients had multiple ipsilateral transplants, which could account for increased surgical risk potentially translating into higher early graft loss. Furthermore we have sparse data on perioperative complications, blood loss, vascular problems, length of operation, length of stay, and readmissions. This can be very relevant in the light of 3KT. Also as mentioned above a selection bias likely confounds this analysis and this data supports the careful selection of 3KT recipients and the benefits of 3KT may not accrue to all potential 3KT recipients. Patients with the longest first and second graft survival time and therefore the best outcomes may not be included in this group due to the 25-year maximum time horizon of the UNOS data set.
In conclusion this study supports the continued use of 3KT because it provides a survival advantage over the waitlist with acceptable graft survival, albeit reduced, compared to first kidney recipients. It is clear that there are disparities between 3KT listed and transplanted patients compared to those listed for a first transplant and these may represent discrepancies in access to care, particularly among African-American recipients. Earlier referral and transplantation at each stage in the process may be an opportunity to narrow these differences. The favorable results of 3KT are likely to be in part attributable to careful patient selection and should not be used in isolation to justify broader application of 3KT for patients substantially more complex than those who have received 3KT in the past.
Methods
The study is a retrospective cohort analysis of kidney recipients between 1995 and 2009 using OPTN registry data from UNOS. Patients were identified as having received a 3KT or been waitlisted for a 3KT. Multiple organ transplant recipients were excluded. Time to allograft loss was calculated from transplantation until the date of allograft loss reported to UNOS. Time to waitlist death was calculated from waitlist start date to patient death reported to UNOS. Multivariable Cox regression models were constructed to compare 3KT graft loss between patients who had a second kidney survival time of > 5 years, >30d-5yr and ≤ 30d. Variables deemed to be clinically significant or nominally associated with the outcome (p<0.10) in univariate analysis were entered into the multivariable model. This model included the following covariates: recipient and donor age and race, hypertension, extended criteria donor (ECD) and donation after cardiac death (DCD) status (for deceased donor analysis only), peak panel reactive antibody (PRA), transplant year, delayed graft function (DGF), degree of HLA match, and cold ischemic time. Multivariable Cox regression models were constructed to compare 3KT patient survival after transplant to patients who remain on the waitlist. In our primary analyses, multivariate models were constructed using patients with complete data. In order to demonstrate that eliminating patients with incomplete data did not significantly affect the primary relationships of interest, we conducted sensitivity analyses with multiple imputation of missing covariates for variables missing greater than 5 percent of data. Sensitivity analyses were performed in which missing values for PRA and cold ischemic time were imputed using values corresponding to the 90%, 10%, and mean among patients who did have data for these variables. These secondary analyses demonstrated similar results as the primary analyses and are not shown. KDPI/KDRI was calculated using the formula from OPTN (23). Means of variables were compared between groups using a one-way ANOVA and t-test. All analyses were performed using SPSS version 17.0 (SPSS Inc, Chicago, Il).
Footnotes
Authorships Contribution: R.R.R., P.L.A., M.H.L. Participated in research design
R.R.R., M.G., P.L.A., M.H.L. Participated in the writing of the paper
R.R.R., M.G., E.R., A.W., P.L.A., M.H.L. Participated in the performance of the research
R.R.R., M.G., P.L.A., M.H.L. Participated in data analysis
Disclosure: This work was not supported by any research grants by any of the authors and the authors do not have any potential conflict of interest to disclose.
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Magee JC, Barr ML, Basadonna GP, et al. Repeat organ transplantation in the United States, 1996-2005. Am J Transplant. 2007;7(5 Pt 2):1424. doi: 10.1111/j.1600-6143.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 3.Repeat kidney transplant prevalence. [Last accessed May 23, 2013];2012 http://optn.transplant.hrsa.gov/latestData.
- 4.Rao PS, Ojo A. Organ retransplantation in the United States: trends and implications. Clin Transpl. 2008;57 [PubMed] [Google Scholar]
- 5.Stratta RJ, Oh CS, Sollinger HW, Pirsch JD, Kalayoglu M, Belzer FO. Kidney retransplantation in the cyclosporine era. Transplantation. 1988;45(1):40. [PubMed] [Google Scholar]
- 6.Kienzl-Wagner K, Mark W, Maglione M, et al. Single-center experience with third and fourth kidney transplants. Transpl Int. 2011;24(8):780. doi: 10.1111/j.1432-2277.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- 7.Salvadori M, Bertoni E. Renal transplant allocation criteria, desensitization strategies and immunosuppressive therapy in retransplant renal patients. J Nephrol. 2012;25(6):890. doi: 10.5301/jn.5000207. [DOI] [PubMed] [Google Scholar]
- 8.Ott U, Busch M, Steiner T, Schubert J, Wolf G. Renal retransplantation: a retrospective monocentric study. Transplant Proc. 2008;40(5):1345. doi: 10.1016/j.transproceed.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 9.Rao PS, Schaubel DE, Wei G, Fenton SS. Evaluating the survival benefit of kidney retransplantation. Transplantation. 2006;82(5):669. doi: 10.1097/01.tp.0000235434.13327.11. [DOI] [PubMed] [Google Scholar]
- 10.Hirata M, Terasaki PI. Renal retransplantation. Clin Transpl. 1994;419 [PubMed] [Google Scholar]
- 11.Gruber SA, Brown KL, El-Amm JM, et al. Equivalent outcomes with primary and retransplantation in African-American deceased-donor renal allograft recipients. Surgery. 2009;146(4):646. doi: 10.1016/j.surg.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Pour-Reza-Gholi F, Nafar M, Saeedinia A, et al. Kidney retransplantation in comparison with first kidney transplantation. Transplant Proc. 2005;37(7):2962. doi: 10.1016/j.transproceed.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Sellers MT, Velidedeoglu E, Bloom RD, et al. Expanded-criteria donor kidneys: a single-center clinical and short-term financial analysis--cause for concern in retransplantation. Transplantation. 2004;78(11):1670. doi: 10.1097/01.tp.0000144330.84573.66. [DOI] [PubMed] [Google Scholar]
- 14.Gallichio MH, Hudson S, Young CJ, Diethelm AG, Deierhoi MH. Renal retransplantation at the University of Alabama at Birmingham: incidence and outcome. Clin Transpl. 1998;169 [PubMed] [Google Scholar]
- 15.Troppmann C, Benedetti E, Gruessner RW, et al. Retransplantation after renal allograft loss due to noncompliance. Indications, outcome, and ethical concerns. Transplantation. 1995;59(4):467. [PubMed] [Google Scholar]
- 16.Fasola CG, Kim YS, Morel P, et al. Kidney retransplantation: patients with a failed second kidney transplant should be considered for a third transplant. Transplant Proc. 1991;23(1 Pt 2):1336. [PubMed] [Google Scholar]
- 17.Bryan CF, Baier KA, Nelson PW, et al. Long-term graft survival is improved in cadaveric renal retransplantation by flow cytometric crossmatching. Transplantation. 1998;66(12):1827. doi: 10.1097/00007890-199812270-00043. [DOI] [PubMed] [Google Scholar]
- 18.Coupel S, Giral-Classe M, Karam G, et al. Ten-year survival of second kidney transplants: impact of immunologic factors and renal function at 12 months. Kidney Int. 2003;64(2):674. doi: 10.1046/j.1523-1755.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 19.Delmonico FL, Tolkoff-Rubin N, Auchincloss H, Jr, et al. Second renal transplantations. Ethical issues clarified by outcome; outcome enhanced by a reliable crossmatch. Arch Surg. 1994;129(4):354. doi: 10.1001/archsurg.1994.01420280024003. [DOI] [PubMed] [Google Scholar]
- 20.Chakkera HA, O'Hare AM, Johansen KL, et al. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol. 2005;16(1):269. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]
- 21.Narayanan M, Pankewycz O, Shihab F, Wiland A, McCague K, Chan L. Long-term outcomes in African American kidney transplant recipients under contemporary immunosuppression: a four-yr analysis of the Mycophenolic acid Observational REnal transplant (MORE) study. Clin Transplant. 2013 doi: 10.1111/ctr.12294. [DOI] [PubMed] [Google Scholar]
- 22.Grams ME, Chen BP, Coresh J, Segev DL. Preemptive deceased donor kidney transplantation: considerations of equity and utility. Clin J Am Soc Nephrol. 2013;8(4):575. doi: 10.2215/CJN.05310512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI) [Last accessed December 10, 2013]; http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf.


