Abstract
The emergence of insecticide resistance in Anopheles mosquitoes has great implications for malaria control in Nigeria. This study aimed to determine the dynamics of insecticide susceptibility levels and frequency of knockdown resistance (kdr) mutations (L1014F) in wild Anopheles coluzzii Coetzee & Wilkerson sp. n. and An. gambiae Giles ( (Diptera: Culicidae) from Ojoo and Bodija areas of Ibadan, South-West, Nigeria. Insecticide susceptibility to pyrethroids, organophosphates, carbamates and organochlorines was assessed using WHO bioassays. A subset of the mosquitoes exposed to pyrethroids and DDT was used for species and molecular form identification and kdr genotyping was determined using the Taqman real time PCR assay. The mosquitoes were resistant to pyrethroids and DDT but completely susceptible to organophosphates and carbamates. Bodja samples (n=186) comprised of An. gambiae (91.4%) and An. coluzzii (8.1%) while one An. coluzzii / An. gambiae hybrid was recorded. All mosquitoes screened in Ojoo (n=26) were An. gambiae. The 1014F kdr mutation was detected at a frequency of 24.52% and 5.8% in Bodija and Ojoo respectively. No correlation was observed between kdr genotypes and resistance phenotypes. The results indicate that metabolic resistance probably plays an important role in the resistance and highlights the need to implement insecticide resistance management strategies.
INTRODUCTION
Malaria remains a major public health burden in Nigeria which accounts for a quarter of all malaria cases in the 45 malaria-endemic countries in Africa with over 90 million people at risk of malaria every year (NMCP, 2012). Malaria vector control relies heavily on the use of long-lasting insecticide nets (LLINs) and indoor residual spraying (IRS). The gains of the use of these methods have been noted in many countries. However this success is being hampered by the development and spread of insecticide resistance in major malaria vectors in Africa which could compromise the use of these vector control strategies. To date, only a limited number of chemical classes of insecticides are available for mosquito control. Only four insecticide classes (carbamates, organophosphates, organochlorines and pyrethroids) are available for IRS, while LLINs exclusively depend on pyrethroids.
The two major causes of insecticide resistance are through increased metabolic detoxification and decreased target site sensitivity (Hemingway & Ranson, 2000). In metabolic detoxification, the- insecticide is prevented from- reaching its site of action by detoxification enzymes. The three main enzyme families responsible for metabolic resistance are cytochrome P450s, glutathione S-transferases and esterases (Hemingway & Ranson, 2000). Decreased target site sensitivity on the other hand, reduces the rate at which the insecticide binds to its target site. The main target site mutation conferring resistance to pyrethroids and cross resistance to DDT in An. gambiae s.s is the ‘Knock-down resistance’ mutation (kdr). Two amino acid changes in the sodium channel gene at codon 1014 are involved in kdr in An. gambiae s.s: a leucine to phenylalanine substitution (1014F) (Martinez-Torres et al., 1998) and a leucine to serine substitution (1014S) (Ranson et al., 2000). A single amino acid substitution of glycine to serine at position 119 in the ace-1 gene confers resistance to both organophosphates and carbamates in An. gambiae s.s (Weill et al., 2004).
Successful malaria control programmes depend on a good knowledge of the species, abundance, distribution and levels and dynamics of insecticide resistance in the local mosquito population, followed by continuous resistance monitoring in order to detect and monitor resistance to these insecticides. However, in most cases the implementation of vector control measures often precedes careful evaluation of the target mosquito population, putting the success of the control programme at risk. Constant monitoring of the susceptibility status of mosquito vectors is essential to forewarn existence of resistance (Ranson et al., 2009). The Federal Ministry of Health has scaled up vector control interventions for malaria control in Nigeria and knowledge on the susceptibility status of malaria vectors and on the dynamics of resistance to main insecticides in vector populations will provide useful information for malaria control, and future work on the major malaria vectors in these areas. Although resistance to pyrethroid (deltamethrin) and DDT has been previously found in An. gambiae s.l. in South West Nigeria (Kristan et al., 2003; Awolola et al., 2005;Awolola et al., 2007; Djouaka et al., 2008; Oduola et al., 2010; Okorie et al., 2011; Oduola et al., 2012), it is not known whether the strength and scale of resistance have increased over time or even extended beyond locations that previous studies investigated. Therefore, studies were initiated to determine the dynamics of insecticide susceptibility levels and frequency of kdr mutations (L1014F) in wild An. .coluzzii and An. gambiae mosquitoes from Ibadan, South-West, Nigeria. This study also provide the first baseline information on the susceptibility status of An,. coluzzii and An. gambiae s.s (formerly known as An. gambiae M and S molecular forms respectively) to organophosphates and carbamates in the same area. It is hoped that findings from this study will promote and improve effective vector control decision making.
MATERIALS AND METHODS
Study sites and mosquito collections
Mosquito collections were carried out between November and December 2012 in Bodija (7°26’8’’N, 3°54’47’’E) and Ojoo (7°28’4’’N, 3°54’48’’E) areas of Ibadan situated in the forest area of southwestern Nigeria. There are two distinct seasons in the study area: a dry season from November to April and a rainy season which extends from May to October with a short break in August. Bodija field site is urban and situated in a residential area with residents involved mainly in petty trading. Ojoo area is also urban and is a hub for motor mechanics and other petty traders. No extensive spraying or major vector control programmes have been carried out previously in this area until 2013 when LLINs were distributed by the National Malaria and Vector Control Programme. Mosquitoes were sampled as larvae from temporary breeding sites around abandoned construction sites, run-off water from wells and in tire tracks created by vehicles. Sampled mosquitoes were morphologically identified as members of the An. gambiae species complex (Gillies & De Meillon, 1968; Gillies & Coetzee, 1987) and taken to the insectary of the Institute for Advanced Medical Research and Training, University of Ibadan, Nigeria, where they were reared to adult stage for WHO bioassays. Larvae were fed on ground biscuits and adults were provided with 10% sugar solution. Newly emerged adults were separated into females and males. All bioassays were performed on 2 – 3 day old adult females.
WHO insecticide susceptibility tests
For Bodija sample, insecticide susceptibility tests was performed using the WHO protocol (WHO, 1998) for the four classes of insecticides approved for vector control: pyrethroids (0.75% permethrin (n=193), 0.05% lambda-cyhalothrin (n=191), 0.05% Deltamethrin (n=151), 0.15% cifluthrin (n=100), and 0.5% Etofenprox (n=118)); organophosphates (1.0% fenitrothion (n=311) and 5.0% malathion (n=100)); carbamates (Bendiocarb 0.1% (n=100)) and organochlorines (4.0% DDT (n=98)) (Figure 1). Ojoo sample was exposed to pyrethroids (0.75% permethrin (n=96) and 0.05% lambda-cyhalothrin (n=73)); organophosphates (1.0% fenitrothion (n=89)) and organochlorines (4.0% DDT (n=102)) (Figure 2). Cohorts of 20 - 25 mosquitoes were exposed per replicate for 1 hour to the discriminating dose of insecticide-impregnated papers using the WHO cylinder test kit. However, exposure to fenitrothion was done for 2-hours (WHO, 2013). Control mosquitoes were exposed to non treated papers. At least four replicates was done per insecticide per site depending on availability of mosquitoes, the exception was for 0.05% Lambdacyalothrin tested in Ojoo with only three replicates. Following exposure, all knocked down and surviving mosquitoes were transferred to the holding tube of the WHO test kit and offered a 10% sucrose solution. After a 24 h recovery period, individuals were recorded as either dead (susceptible) or alive (resistant), and stored individually over silica gel. All treated papers used were tested in the laboratory using the Kisumu strain to check for efficacy.
Figure 1.
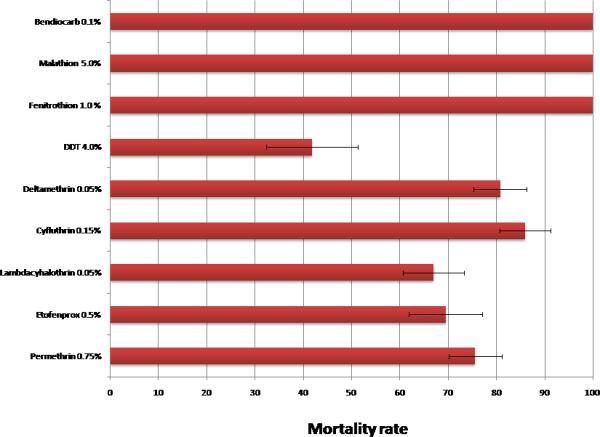
Prevalence of insecticide resistance in Anopheles coluzzii and Anopheles gambiae mosquitoes from Bodija
Figure 2.
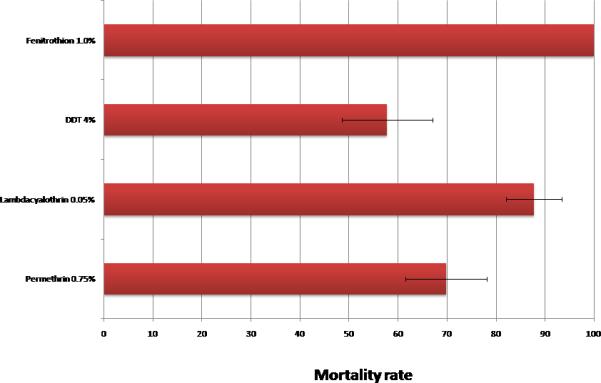
Prevalence of insecticide resistance in Anopheles coluzzii and Anopheles gambiae mosquitoes from Ojoo
Molecular analyses
DNA was extracted from desiccated mosquitoes using the Livak protocol (Livak, 1984). An. gambiae mosquitoes were identified to species and molecular form by PCR (Fanello et al., 2002). For Bodija, DNA was extracted from a subset of the mosquitoes exposed to DDT and pyrethroids (permethrin and deltamethrin) and these were screened for the presence of the kdr alleles (L1014F) using the Taqman real-time PCR assay (Bass et al.,2007) and used to assess the correlation between kdr and resistance phenotypes. For Ojoo, a subset of only the permethrin exposed mosquito sample (n=30) were screened for the presence of the kdr alleles (L1014F) using the Taqman assay.
Statistical analyses
The percentage mortality of each insecticide was calculated as a proportion of mosquitoes that died after twenty four hours and the total number of mosquitoes exposed using 95% confidence intervals. Correction with Abbott's formula (WHO, 1998) was not required as the control mortality was less than 5%.
The WHO criteria for susceptibility used are as follows: mortality in the range 98–100% indicates susceptibility. Mortality of less than 98% is suggestive of the existence of resistance and further investigation is needed (WHO, 2013). The presence of resistant genes in the vector population must be confirmed if the observed mortality is between 90% and 97% but confirmation is not necessary if mortality is less than 90% (WHO, 2013).
The correlation between kdr genotypes and resistance phenotypes was assessed by estimating the odds ratios and the statistical significance based on the Fisher exact probability test.
Ethical Approval
Ethical approval for this study was obtained from University of Ibadan/ University College Hospital (UI/UCH) Ethics Review Committee, Reference number: UI/UCH/EC/12/0246.
RESULTS
Susceptibility profile to insecticides
In total for Bodija, 1362 non blood-fed An coluzzii and An. gambiae females, aged 2-3 days were exposed to the various insecticides. A total of 360 mosquitoes from Ojoo were exposed. The detailed results of the susceptibility tests are given in Figures 1 and 2. In all cases the control mortality was less than 5%, therefore not requiring correction with Abbott's formula (WHO, 1998).
In Bodija, mortality rates with both Type I (permethrin) and Type II (cyfluthrin, lambda-cyhalothrin and deltamethrin) pyrethroids ranged between 67-86%, indicating resistance to these products. Resistance to etofenprox and DDT was also detected and seemed more pronounced toward DDT (42% mortality) than etofenprox (69% mortality) at this site. There was no resistance to bendiocarb, malathion and fenithrothion, mortality rates were constantly 100% (figure 1).
In samples from Ojoo, resistance to permethrin and DDT was detected at a level similar to Bodija whilst full susceptibility to fenithrothion was observed (Figure 2).
Species and molecular form composition
All the mosquitoes tested from both sites were identified as An. coluzzii and An. gambiae. Of the 186 individuals successfully scored to molecular form in Bodija, An. gambiae was predominant representing 91.4% (n=170) of the entire samples while 8.1% (n=15) were An. coluzzii (Table 1). In addition, one An. coluzzii / An. gambiae hybrid (0.5%) was found in the population. Of the 30 mosquitoes screened from Ojoo, 26 samples were successfully identified as An. gambiae (Table 1).
Table 1.
Genotype and mosquito survival after insecticide exposure for An. gambiae from Bodija and Ojoo, Ibadan
| Insecticide | Status | No. | RR | RS | SS | % kdr frequency | Odds Ratio | P value |
|---|---|---|---|---|---|---|---|---|
|
BODIJA
| ||||||||
| DDT | Alive | 17 | 3 | 4 | 10 | 29.4 | ||
| Dead | 24 | 1 | 9 | 14 | 22.9 | |||
| Total | 41 | 4 | 13 | 24 | 25.6 | 1.4 | 0.37 | |
| Deltamethrin | Alive | 20 | 0 | 6 | 14 | 15.0 | ||
| Dead | 22 | 1 | 8 | 13 | 22.7 | |||
| Total | 42 | 1 | 14 | 27 | 19.0 | 0.6 | 0.22 | |
| Permethrin | Alive | 31 | 0 | 10 | 21 | 16.1 | ||
| Dead | 28 | 1 | 14 | 13 | 28.5 | |||
| Total | 59 | 1 | 24 | 34 | 25.0 | 0.47 | 0.04 | |
| Overall Bodija | 142 | 6 (4.2%) | 51 (36%) | 85 (59.8%) | 22.18 | |||
| Control (non exposed) | Alive | 28 | 1 (3.6%) | 13 (46.4%) | 14 (50%) | 26.8 | ||
|
OJOO
| ||||||||
| Permethrin | Alive | 13 | 0 | 1 | 12 | 3. 8 | ||
| Dead | 13 | 0 | 2 | 11 | 7.7 | |||
| Total | 26 | 0 (0%) | 3 (11.5%) | 23 (88.5%) | 5.8 | 0.48 | 0.39 | |
Kdr mutation
The TaqMan assay was successful in detecting kdr genotypes in 186 out of 203 (91.7%) of An. coluzzii and An. gambiae from Bodija. The successfully scored mosquitoes comprised of 157 specimens exposed to the various insecticides and 29 non exposed samples. Of the 157 insecticide exposed mosquitoes 142 were An. gambiae while 14 were An. coluzzii and 1 was an An. coluzzii / An. gambiae hybrid. Of the 29 control samples, 28 were An. gambiae while 1 was An. coluzzii..
For Bodija sample, the 1014F kdr mutation was detected at a frequency of 22.18% and 42.86% in the total An. gambiae and An. coluzzii mosquitoes respectively. Overall, the 1014F kdr mutation was detected at a frequency of 24.52% in the total Bodija sample but only at 5.8% in Ojoo. Analysis of the distribution of the kdr genotypes shows that for Bodija sample, the mutation was mainly present at the heterozygote state in both forms with 36% and 42.9% for the RS genotype respectively in An. gambiae and An. coluzzii mosquitoes in contrast to only 4.2 and 21.4% respectively in An. gambiae and An. coluzzii mosquitoes for the homozygote resistant (RR) genotype (Table 1). The mutation was also mainly present at the heterozygote state in both locations with 36.3 and 11.5% for the RS genotype respectively in Bodija and Ojoo in contrast to only 4.2 and 0% respectively for the homozygote resistant (RR) genotype.
Assessment of the correlation between kdr genotypes and resistance phenotypes did not show a significant association between this mutation and resistance to DDT and deltamethrin in Bodija with low and non significant Odd ratios (Figure 3, Table 1). A marginal significant level was observed for permethrin but with rather a higher frequency of the mutation in susceptible mosquitoes again supporting that the observed resistance is not linked to kdr genotypes. This absence of correlation was also observed in the permethrin sample from Ojoo.
Figure 3.
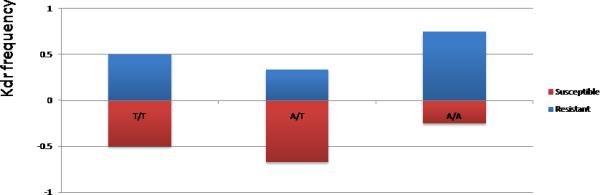
Correlation between kdr genotype and resistance to permethrin for Bodija and Ojoo sample.
DISCUSSION
This study has provided an update on the current level of resistance and frequency of the kdr mutation in An. coluzzii and An. gambiae in Ibadan area of Nigeria..Anopheles coluzzii and An. gambiae were the only Anopheles species observed in this study and the two specieslived in sympatry at varying frequencies.Anopheles gambiaewas the most abundant species found in the two sites. Previous studies in Nigeria (Kristan et al.,2003) and Cameroon (Wondji et al., 2005) have reported that An. coluzzii (M form) was more predominant in urban or peri-urban settings, whereas An. gambiae (S form) was more abundant in rural settings. It has been suggested that competitive exclusion between the two species may be the key factor determining the composition of the species (Wondji et al., 2005). The present study showed that although An. coluzzii and An. gambiae occurred in sympatry, in Bodija area An. gambiae is far more predominant, which corroborates findings from previous reports in Nigeria (Awolola et al., 2005, 2007). However the ratio of An. coluzzii to An. gambiae (8:92) recorded here differs from ratios from previous reports around Ibadan. Previous studies around Ibadan have reported the ratio of An. coluzzii (M form) to An. gambiae (S form) to be 35 : 65 (Awolola et al., 2007); 39 : 61 (Awolola et al., 2005) and 100 : 0 (Djouaka et al., 2007; Djouaka et al., 2008). Infact the most recent study in Ojoo area reported that all mosquitoes collected were identified to be An. coluzzii (M form) in mosquitoes collected in December 2006 and January 2007 (Djouaka et al., 2008). This is in contrast to this study were only An. gambiae (S form) was found in the Ojoo area in mosquitoes collected in November and December 2012. These mosquitoes were collected at about the same time of the year and thus the contrast in the forms may be an indication that the dynamics of the vectors may be changing in this area.
Only one An. coluzzii / An. gambiae hybrid was found in the study area and this was found to be homozygous for the kdr mutation. This is the first time that a hybrid is been reported in Nigeria. The observation of this rare occurrence and with only 1 An. coluzzii / An. gambiae hybrid recorded suggests restricted gene flow between An. coluzzii and An. gambiae providing strong support for reproductive isolation between the two species (Ndiath et al., 2012; Wondji et al., 2005). In West Africa, there are varying levels of hybridization between An. coluzzii and An. gambiae (formerly An. gambiae M and S molecular forms) ranging from none to high frequencies. In some countries, none or very few An. coluzzii and An. gambiae (M/S) hybrids have been observed (Wondji et al., 2005; Yawson et al., 2007) while high frequencies of An. coluzzii and An. gambiae (M/S) hybrid, ranging from 5 to 42% has been reported in The Gambia, Senegal, Guinea-Bissau, and Republic of Guinea (Nwakanma et al., 2013).
This study has provided an insight on the dynamics of insecticide resistance with an update on the current levels of resistance and the underlying resistance mechanisms in An. coluzzii and An. gambiae in Ibadan, Nigeria. It also provides baseline information on the susceptibility status of An. coluzzii and An. gambiae in this location to type II (cyfluthrin, lambda-cyhalothrin and deltamethrin) pyrethroids, etofenprox, the organophosphate (fenitrothion and malathion) and the carbamates (bendiocarb). The WHO bioassay results indicated a high prevalence of resistance to pyrethroids in Ibadan for both Type I pyrethroid (permethrin) and Type II (cyfluthrin, lambda-cyhalothrin and deltamethrin). This pattern is broadly similar between the two locations suggesting that similar selection pressure forces are acting on these populations.
The proportion of An. gambiae surviving permethrin exposure has increased slightly in Ojoo compared to other previous studies_in this same area [80% mortality (Djouaka et al., 2007; Djouaka et al., 2008) vs. 69.8% reported here]. However, the baseline data on An. gambiae from Bodija surviving permethrin exposure reported here (75.6% mortality) is similar to findings from other parts of Ibadan. In Ibadan, the mortality rate of An. gambiae s.l to permethrin was 80.6% for An. coluzzii (M form) and 72.4% for An. gambiae (S form) (Awolola et al., 2005). Resistance to permethrin in populations of An. gambiae s.l has been recorded in different parts of Ibadan with mortality rates of 70% (Bashorun area), 81% (Challenge area) and 100% (UI and Orogun area) (Djouaka et al., 2007; Djouaka et al., 2008). Mortality rates of 72.1% - 78.6% to permethrin has been recorded in field populations of An. gambiae s.s. from Alakia area of Ibadan (Awolola et al., 2007). Thus, our results suggest that resistance to pyrethroids in this area seems to have been stable as the resistance has not gone up significantly after five years. This probably indicates that the selection pressure was not too high, but this could now change with recent introduction of LLINs in the area.
The use of LLINs may be effective for protection against malaria infection under the assumption that the target mosquito populations remain susceptibile to pyrethroids used to treat them and the LLINs are used appropriately and are sufficient in terms of coverage (Okoye et al., 2008). The presence of high resistance to permethrin and deltamethrin in An. gambiae s.s populations from Ibadan is of real concern given the widespread distribution of LLINs impregnated with this insecticide as part of the National Malaria Control Programme efforts to curb malaria. Previous reports have shown that the efficacy of these LLINs could be negatively affected by this resistance as previously observed in Benin (N’Guessan et al., 2007). The results of this study have implications in this region for the current reliance on LLINs and IRS for vector control.
The proportion of An. coluzzii and An gambiae surviving DDT exposure has increased considerably in the two locations compared to other previous studies [71% - 82% mortality recorded in field populations of An. gambiae s.s. from Alakia area of Ibadan (Awolola et al., 2007) vs. 42% and 58% reported here for Bodija and Ojoo areas of Ibadan respectively]. However, DDT resistance in Ibadan remains lower than in the region of Lagos where a mortality rate of (≤ 16%) has been recorded in field populations of An. gambiae s.l in Lagos state, Nigeria (Oduola et al., 2012).
As there was complete susceptibility to fenitrothion, malathion and bendiocarb, no sample was screened for the presence of the ace-1R mutation. The 100% effectiveness of these insecticides on this population indicates that organophosphates and carbamates could be considered as an alternative insecticide for IRS campaign around Ibadan. This result of complete susceptibility to organophosphates and carbamates is in contrast to mortality rates of 25 -77% to propoxur recorded in field populations of An. gambiae s.l from Lagos state, Nigeria (Oduola et al., 2012). The geographical proximity between these two locations and the likelihood of migration implies that the Ibadan population of An. gambiae should continuously be also monitored for carbamate resistance.
The frequency of L1014F kdr mutation in An. gambiae (5.8%) from Ojoo reveals that the kdr frequency remains low in this location. However it also shows that the kdr frequency is increasing in Ojoo as previous studies have reported an absence of this target site mutation in Ojoo area of Ibadan (Djouaka et al., 2007; Djouaka et al., 2008). However, an earlier study in Ibadan reported a kdr frequency of 48.1% for An. gambiae (S form) (Awolola et al., 2005). In the case of Bodija, the frequency of L1014F kdr mutation in An. gambiae (22.18%) reveals that the kdr frequency is also low. Earlier studies in other areas of Ibadan have reported a kdr frequency from 0% (Djouaka et al., 2007; Djouaka et al., 2008) to 48.1% (Awolola et al., 2005) for An. gambiae (S form) and 0% for An. coluzzii (M form) (Awolola et al., 2005) in permethrin resistant populations of An. gambiae. Kdr frequency rates of between 19.1% - 34.2% and 25.9% - 38.8% have been reported in permethrin and DDT resistant populations of An. gambiae (Awolola et al., 2007).
The increase in kdr frequency of 42.86% recorded here for the An. coluzzii from Bodija is slightly higher than what has been reported for An. coluzzii (M form) from other areas of Ibadan (0% (Awolola et al., 2005) and 7.4% (Awolola et al., 2007). The increase in kdr frequency is an indication that this An. coluzzii of Ibadan is still undergoing selection for resistance either through the use of household insecticidal products or the widespread use of LLINs in Ibadan. The low frequency of L1014F homozygotes in this study suggests that there is probably no link between the resistance phenotype and the kdr genotype. This suggests the presence of other mechanisms such as metabolic resistance. Furthermore, the lack of correlation between kdr and resistance suggests that kdr may only play a minor role in the resistance observed. Lack of correlation between the resistance phenotype and the kdr genotype has been previously reported notably in Cameroon (Nwane et al., 2013) while correlation is observed in other populations such as in the Vallee du Kou in Burkina Faso (Kwiatkowska et al., 2013).
The low frequency of this target site mutation even in resistant mosquitoes suggests that metabolic resistance probably plays an important role in the resistance observed to pyrethroids and DDT in these locations notably through the over-expression of cytochrome P450 genes as previously reported (Djouaka et al., 2008; Müller et al., 2008; Koekemoer et al., 2011; Mitchell et al., 2012; Riveron et al., 2012). This is supported by results from a gene expression analysis by microarray pyrethroid resistant An. gambiae mosquitoes from Ojoo area of Ibadan which showed that nine detoxification genes were over expressed in Ojoo compared to a susceptible population from Orogun area of Ibadan (Djouaka et al., 2008). Another gene family potentially involved could be the glutathione S-transferase genes as recently shown in An. funestus where the GSTe2 gene was shown to be the most over-expressed detoxification gene in a permethrin resistant population from Benin and able to metabolise permethrin (Riveron et al., 2014). No extensive spraying or major vector control programme have been carried out previously in this area at the time of the study. The current pyrethroid and DDT resistance observed in this area may have been due to the selection pressure exerted by the extensive use of mosquito coils and aerosols (pyrethroids) which was observed to be used by residents in the area for vector control on the local mosquito populations.
CONCLUSION
The pyrethroid and DDT resistance reported in this study in Anopheles coluzzii andAnopheles gambiae population from Ibadan could have implications in this region for the current reliance on LLINs and IRS for vector control notably for the recent campaign of LLINs distribution in Nigeria. Indeed, pyrethroid resistance could have a huge impact on vector control interventions since these insecticides are the mainstay of various interventions such as IRS and LLINs. It is imperative to implement insecticide resistance management strategies in this region to halt the development of this resistance and maintain the effectiveness of current and future control interventions (Okoye et al., 2007)._The current findings of -an apparent absence- of phenotypic resistance to organophosphates or carbamates means that these insecticides may represent useful alternative chemical classes to DDT- and- pyrethroids -for- vector- control programmes using IRS in Nigeria.
ACKNOWLEDGMENT
This work was supported by a Medical Education Partnership Initiative in Nigeria (R24TW008878) Mentored Research Award to PNO and a Wellcome Trust Research Career Development Fellowship (083515/Z/07/Z) to CSW.
Footnotes
PNO: pnokorie@comui.edu.ng
GOA: ademowo_g@yahoo.com
HI: H.Irving@liverpool.ac.uk
LKH: Louise.Kelly-Hope@lstmed.ac.uk
CW: c.s.wondji@liverpool.ac.uk
AUTHORS' CONTRIBUTIONS
PNO, CSW and LKH conceived and designed the study. PNO and HI performed the experiments. PNO and CSW analyzed the data and wrote the manuscript. GOA and LKH gave conceptual advice and provided editorial feedback on the manuscript. All authors read and approved the final version of the manuscript.
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
- Awolola TS, Oduola AO, Oyewole IO, Obansa JB, Amajoh CN. Dynamics of knockdown pyrethroid insecticide resistance alleles in a field population of Anopheles gambiae s. s. in southwestern Nigeria. Journal of Vector Borne Diseases. 2007;44:181–188. [PubMed] [Google Scholar]
- Awolola TS, Oyewole IO, Amajoh CN, Idowu ET, Ajayi MB, Oduola A, Manafa OU, Ibrahim K, Koekemoer LL, Coetzee M. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Tropica. 2005;95:204–209. doi: 10.1016/j.actatropica.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Bass C, Williamson MS, Wilding CS, Donnelly MJ, Field LM. Identification of the main malaria vectors in the Anopheles gambiae species complex using a TaqMan real-time PCR assay. Malaria Journal. 2007;6:155. doi: 10.1186/1475-2875-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouaka RF, Bakare AA, Bankole HS, Doannio JMC, Coulibaly ON, Kossou H, Tamo M, Basene HI, Popoola OK, Akogbeto MC. Does the spillage of petroleum products in Anopheles breeding sites have an impact on the pyrethroid resistance? Malaria Journal. 2007;6:159. doi: 10.1186/1475-2875-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouaka RF, Bakare AA, Coulibaly ON, Akogbeto MC, Ranson H, Hemingway J, Strode C. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s. s. from Southern Benin and Nigeria. BMC Genomics. 2008;9:538. doi: 10.1186/1471-2164-9-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanello C, Santolamazza F, della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Medical and Veterinary Entomology. 2002;16:461–464. doi: 10.1046/j.1365-2915.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- Gillies M, Coetzee M. A supplement to the Anophellinae of Africa south of the sahara. Publication of the South African Institute for Medical Research; 1987. p. 55.p. 143. [Google Scholar]
- Gillies M, De Meillon B. The Anophelinae of Africa, south of the sahara (Ethiopian zoogeographical region) Publication of the South African Institute for Medical Research; 1968. p. 314. [Google Scholar]
- Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annual Review of Entomology. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- Koekemoer L, Spillings B, Christian R, Lo TC, Kaiser ML, Norton RA, Oliver SV, Choi KS, Brooke BD, Hunt RH, Coetzee M. Multiple insecticide resistance in Anopheles gambiae (Diptera: Culicidae) from Pointe Noire, Republic of the Congo. Vector Borne and Zoonotic Diseases. 2011;11:1193–1200. doi: 10.1089/vbz.2010.0192. [DOI] [PubMed] [Google Scholar]
- Kristan M, Fleischmann H, della Torre A, Stich A, Curtis CF. Pyrethroid resistance/susceptibility and differential urban/rural distribution of Anopheles arabiensis and An. gambiae s.s. malaria vectors in Nigeria and Ghana. Medical and Veterinary Entomology. 2003;17:326–332. doi: 10.1046/j.1365-2915.2003.00449.x. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska RM, Platt N, Poupardin R, Irving H, Dabire RK, Mitchell S, Jones CM, Diabaté A, Ranson H, Wondji CS. Dissecting the mechanisms responsible for the multiple insecticide resistance phenotype in Anopheles gambiae s.s., M form, from Vallée du Kou, Burkina Faso. Gene. 2013;519:98–106. doi: 10.1016/j.gene.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107:611–634. doi: 10.1093/genetics/107.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres D, Chandre F, Williamson M, Darriet F, Bergé J, Devonshire AL, Guillet P, Pasteur N, Pauron D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Molecular Biology. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- Mitchell SN, Stevenson BJ, Müller P, Wilding CS, Egyir-Yawson A, Field SG, Hemingway J, Paine MJ, Ranson H, Donnelly MJ. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6147–52. doi: 10.1073/pnas.1203452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, Warr E, Stevenson BJ, Pignatelli PM, Morgan JC, Steven A, Yawson AE, Mitchell SN, Ranson H, Hemingway J, Paine MJ, Donnelly MJ. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genetics. 2008;4:e1000286. doi: 10.1371/journal.pgen.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Guessan R, Corbel V, Akogbéto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerging Infectious Diseases. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiath MO, Sougoufara S, Gaye A, Mazenot C, Konate L, Faye O, Sokhna C, Trape JF. Resistance to DDT and pyrethroids and increased kdr mutation frequency in An. gambiae after the implementation of permethrin-treated nets in Senegal. PloS One. 2012;7:e31943. doi: 10.1371/journal.pone.0031943. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- NMCP . National Malaria Control Programme 2012 1st & 2nd Quarter report. Federal Ministry of Health; Nigeria: 2012. [Google Scholar]
- Nwakanma DC, Neafsey DE, Jawara M, Adiamoh M, Lund E, Rodrigues A, Loua KM, Konae L, Sy N, Dia I, Awolola TS, Muskavitch MA, Conway DJ. Breakdown in the process of incipient speciation in Anopheles gambiae. Genetics. 2013;193:1221–1231. doi: 10.1534/genetics.112.148718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwane P, Etang J, Chouaїbou M, Toto JC, Koffi A, Mimpfoundi R, Simard F. Multiple insecticide resistance mechanisms in Anopheles gambiae s.l. populations from Cameroon, Central Africa. Parasites & Vectors. 2013;6:41. doi: 10.1186/1756-3305-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduola AO, Idowu ET, Oyebola MK, Adeogun AO, Olojede JB, Otubanjo OA, Awolola TS. Evidence of carbamate resistance in urban populations of Anopheles gambiaes.s. mosquitoes resistant to DDT and deltamethrin insecticides in Lagos, South-Western Nigeria. Parasites & Vectors. 2012;5:116. doi: 10.1186/1756-3305-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduola AO, Olojede JB, Ashiegbu CO, Olufemi A, Otubanjo OA, Awolola TS. High level of DDT resistance in the malaria mosquito: Anopheles gambiae s.l. from rural, semi urban and urban communities in Nigeria. Journal of Rural and Tropical Public Health. 2010;9:114–120. [Google Scholar]
- Okorie PN, McKenzie FE, Ademowo OG, Bockarie M, Kelly-Hope L. Nigeria Anopheles vector database: an overview of 100 years' research. PloS One. 2011;6:e28347. doi: 10.1371/journal.pone.0028347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye PN, Brooke BD, Hunt RH, Coetzee M. Relative developmental and reproductive fitness associated with pyrethroid resistance in the major southern African malaria vector, Anopheles funestus. Bulletin of Entomological Research. 2007;97:599–605. doi: 10.1017/S0007485307005317. [DOI] [PubMed] [Google Scholar]
- Okoye PN, Brooke BD, Koekemoer LL, Hunt RH, Coetzee M. Characterisation of DDT, pyrethroid and carbamate resistance in Anopheles funestus from Obuasi, Ghana. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102:591–598. doi: 10.1016/j.trstmh.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Ranson H, Jensen B, Vulule J, Wang X, Hemingway J, Collins F. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. Insect Molecular Biology. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- Ranson H, Abdallah H, Badolo A, Guelbeogo WM, Kerah-Hinzoumbé C, Yangalbé-Kalnoné E, Sagnon N, Simard F, Coetzee M. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malaria Journal. 2009;8:299. doi: 10.1186/1475-2875-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveron JM, Irving H, Ndula M, Barnes KG, Ibrahim SS, Paine MJI, Wondji CS. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proceedings of the National Academy of Sciences of the United States of America. 2012;110:252–257. doi: 10.1073/pnas.1216705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveron JM, Yunta C, Ibrahim SS, Djouaka R, Irving H, Menze BD, Ismail HM, Hemingway J, Ranson H, Albert A, Wondji CS. A single mutation in the GSTe2 gene allows tracking of metabolically-1 based insecticide resistance in a major malaria vector. Genome Biology. 2014;15:R27. doi: 10.1186/gb-2014-15-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, Raymond M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Molecular Biology. 2004;13:1–7. doi: 10.1111/j.1365-2583.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- WHO . Test procedures for insecticide resistance montoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces. World Health Organization; Geneva, Switzerland: 1998. WHO/CDS/CPC/MAL/98.12. [Google Scholar]
- WHO . Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- Wondji C, Simard F, Petrarca V, Etang J, Santolamazza F, Torre A. Della, Fontenille D. Species and Populations of the Anopheles gambiae Complex in Cameroon with Special Emphasis on Chromosomal and Molecular Forms of Anopheles gambiae s.s. Journal of Medical Entomology. 2005;42:998–1005. doi: 10.1093/jmedent/42.6.998. [DOI] [PubMed] [Google Scholar]
- Yawson AE, Weetman D, Wilson MD, Donnelly MJ. Ecological zones rather than molecular forms predict genetic differentiation in the malaria vector Anopheles gambiae s.s. in Ghana. Genetics. 2007;175:751–761. doi: 10.1534/genetics.106.065888. [DOI] [PMC free article] [PubMed] [Google Scholar]


