Abstract
Background
Extensive stage small cell lung cancer (ES-SCLC) patients who progress following platinum-based chemotherapy are traditionally categorized as platinum-sensitive (progression ≥ 90 days from last platinum dose) or refractory (progression< 90 days), apractice arising from seminal observations of worse survival in refractory patients. Subsequent trials accounted for platinum-sensitivity, resulting in higher sample sizes and increased resource use.
Methods
To assess whether platinum-sensitivity status remains associated with outcomes, patient-level data from recent SWOG trials in 2nd and/or 3rd line ES-SCLC were pooled. Hazard ratios (HRs) for progression-free (PFS) and overall survival (OS) accounting for platinum sensitivity were calculated using unadjusted and adjusted Cox Proportional Hazard models. Recursive partitioning was performed to define prognostic risk groups.
Results
Of 329 patients, 151 were platinum-sensitive, 178 refractory. Hazard ratios from unadjusted Cox PFS and OS models for refractory vs. sensitive disease were 1.0 (95% CI 0.81–1.25, p=0.98) and 1.24 (0.99, 1.57; p=0.06), respectively. Adjusted Cox models showed that only elevated serum LDH (HR 2.04, p<0.001), males (HR 1.36, p=0.04), performance status of 1 (HR 1.25, p=0.02), and weight loss ≥ 5% (1.53, p=0.01) were independently associated with OS. Platinum sensitivity status was not associated with PFS (HR 1.11, p=0.49) or OS (HR 1.25, p=0.14), except in a model that excluded 36 patients who received > 1 prior chemotherapy regimen (HR=1.34, p=0.049). Prognostic groups with differential OS outcomes (high, intermediate, and poor risk) were identified.
Conclusions
Platinum sensitivity status may no longer be strongly associated with PFS or OS in at least one multivariate model. Validation of prognostic risk groups identified here is warranted. These data have critical implications in the design of future SCLC trials.
Background
Disease progression after initial platinum-based chemotherapy is almost universal in patients with extensive stage small cell lung cancer (ES-SCLC). [1] At the time of progression, patients have traditionally been categorized as either platinum-sensitive (defined as progression > 90 days from last platinum dose) or platinum-refractory (progression < 90 days from last platinum dose). Patients who progress while receiving platinum-based therapy are sometimes labeled as “platinum-resistant”; however, many clinicians likewise define these patients as “platinum-refractory” as well.
The practice of categorizing patients according to platinum sensitivity status arose from observations in a phase II trial of the investigational cytotoxic agent teniposide [2]. In this trial that accrued 50 previously-treated SCLC patients, longer time from prior chemotherapy discontinuation (i.e., >2.6 months) and response to prior chemotherapy were found to be associated with response to subsequent teniposide.
Since those seminal observations, subsequent efficacy trials in SCLC began accounting for platinum-sensitivity status. In many cases, this resulted in more complex studies, higher sample sizes and increased resource use. Some studies opted to solely focus on the platinum sensitive group, excluding those with refractory disease [3,4]. In SWOG, trials in previously treated SCLC since 2000 have mandated independent accrual to platinum sensitive and refractory strata in order to account for the possibility of differential outcomes to investigational therapies between these groups. However, recent trials (both SWOG and non-SWOG) have not shown divergent outcomes related to platinum-sensitivity status [5–9].
In the past decade, SWOG has conducted three phase II trials of investigational regimens in platinum-treated SCLC (S0802, S0435, and S0327); these trials are summarized in Table 1 [5–7]. In these studies, progression free survival (PFS) and overall survival (OS) were generally comparable between platinum-sensitive and refractory strata across all trials, except for S0802 where modest advantage for PFS and OS in the aflibercept-containing arm was demonstrated. Similarly, the final results of a recent non-SWOG phase II trial of temozolamide in platinum-treated SCLC also showed no clear differences in outcomes dependent on platinum sensitivity status [10]. We therefore sought to evaluate the association between platinum sensitivity status and SCLC patient outcome in the modern era using the pooled SWOG database.
Table 1.
Recent Phase II SWOG Trials in Extensive Stage Small Cell Lung Cancer
| SWOG Study | Regimen | Number of patients | Performance Status Allowed | Number of prior regimens allowed |
|---|---|---|---|---|
| S0327 | Bortezomib | 57 | 0–1 | ≥1 |
| S0435 | Sorafenib | 83 | 0–1 | 1* |
| S0802 | Topotecan +/− Aflibercept | 189 | 0–1 | 1 |
S0435 was amended to restrict the study population to patients having received only 1 prior regimen after it had enrolled 22 patients.
Methods
Updated patient-level data from S0802, S0435, and S0327 were pooled. S0802 randomized patients to either topotecanalone or topotecan plus the angiogenesis inhibitor aflibercept (VEGF-Trap). S0435 was a single arm trial of the VEGFR-TKI sorafenib. S0327 was a single arm trial of the proteasome inhibitor PS-341 (bortezomib). Each of the trials had consistent eligibility criteria and collected the same baseline demographic variables. The primary endpoint for S0802 was 3-month PFS, while the primary endpoint for S0435 and S0327 was response rate. All staging definitions used by SWOG for these trials preceded the 7th edition of the TNM staging system. All studies were reviewed and approved by Institutional Review Boards and all patients gave written informed consent.
Multivariate Cox regression models were fit to assess the relationship between baseline characteristics and PFS and OS. Progression-free and OS estimates were calculated using the method of Kaplan-Meier. Confidence intervals for the median were constructed using the method of Brookmeyer-Crowley. All p-values were two-sided. To investigate a predictive model for OS, recursive partitioning was performed using the likelihood tree model of LeBlanc and Crowley [11]. The minimum node size was set at 20.
Overall survival was defined as the duration from the date of enrollment to the date of death due to any cause. Patients last known to be alive were censored at the date of last contact. Progression-free survival was defined as the duration from the date of enrollment to the date of first documentation of disease progression, as defined by RECIST, symptomatic deterioration without documented disease progression, or death due to any cause. Patients last known to be alive and without evidence of disease progression or symptomatic deterioration were censored at the date of last contact. Disease assessments were performed every 6 weeks in all 3 protocols.
Results
Three hundred twenty-nine patients constituted the pooled study population. Patient characteristics stratified by platinum sensitivity status are summarized in Table 2. Of 329 patients, 151 were categorized as platinum-sensitive while 178 were platinum-refractory. Median age was 63 years. Males comprised 52% of the group while those with performance status 1 constituted 67%. There were 89 (28%) patients with clinically significant weight loss of >= 5% within the preceding 3 months. Elevated LDH levels were seen in 43%.
Table 2.
Patient Characteristics (Stratified by Platinum Sensitivity Status)
| Variables | Overall (N=329) | |||
|---|---|---|---|---|
| Platinum Refractory (N=178) | Platinum Sensitive (N=151) | |||
| Age, median (range) | 61 | (22–85) | 64 | (33–85) |
| Age ≥ 65 | 70 | 39% | 73 | 48% |
| Male sex | 102 | 57% | 68 | 45% |
| Performance Status | ||||
| 0 | 53 | 30% | 56 | 37% |
| 1 | 124 | 70% | 94 | 63% |
| Smoking Status | ||||
| Never Smoker | 2 | 1% | 1 | 1% |
| Former Smoker | 83 | 47% | 76 | 50% |
| Current Smoker | 64 | 36% | 45 | 30% |
| Smoking status not reported | 29 | 16% | 29 | 19% |
| Weight Loss ≥ 5%* | 50 | 29% | 39 | 27% |
| Elevated LDH* | 70 | 46% | 52 | 39% |
| 2 or more prior chemo regimens* | 20 | 11% | 16 | 11% |
| Prior RT | 122 | 69% | 132 | 87% |
percentages are out of the number of patients with non-missing data
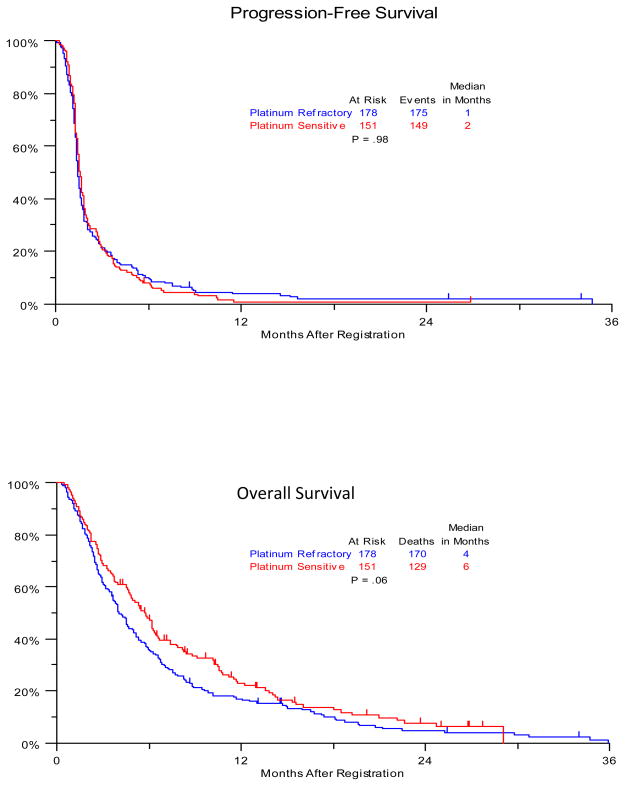
Crude unadjusted analysis of PFS and OS stratified by platinum sensitivity status are summarized in Figure 1. The hazard ratio for PFS (refractory/sensitive) was 1.0 (95% CI 0.81, 1.25), with a p-value of 0.98. The hazard ratio for OS (refractory/sensitive) was 1.24 (95% CI 0.99, 1.57), with a p-value of 0.06.
Figure 1.
Kaplan Meier Curves for Crude Progression-Free and Overall Survival, Stratified by Platinum Sensitivity Status (Unadjusted for Clinical Variables)
Multivariate analysis of baseline clinical variables and PFS (Table 3) showed that only elevated LDH (HR 1.83, p<0.0001) and trial assignment to S0802 (HR 1.82, p=0.001) were independently prognostic for PFS. In contrast, platinum sensitivity status was not associated with PFS. Multivariate analysis for OS (Table 4) showed that platinum sensitivity status was also not associated with OS (HR 1.25, p=0.14). Instead, elevated LDH (HR 2.04, p<0.0001), weight loss (HR 1.53, p=0.01), performance status of 1 (HR 1.43, p=0.02), and male sex (HR 1.36, p=0.04) were found to be the only clinical variables associated with worse OS. In a subsequent analysis, 14 patients (6 from S0435 and 8 from S0802) had their prior chemotherapy status changed from 2 or more to exactly one. An additional 3 patients (2 on S0435 and 1 on S0802) who had missing data were assumed to have received exactly one prior chemo regimen. (The eligibility criteria stated that these patients must have received exactly one prior regimen when all 17 of these patients were enrolled). When these 17 patients who had exactly one prior chemotherapy were reclassified, multivariate analysis subsequently showed that prior chemotherapy was significantly associated with improved OS (HR 0.29, p=0.03). Sex, LDH, weight loss, and performance status remained significantly associated with OS; however, the rest of the variables (including platinum sensitivity status) remained not to be significantly associated with OS. We then analyzed the data excluding the remaining 36 patients who received more than one prior chemotherapy regimen. In this analysis, platinum refractory status becomes associated with OS (HR 1.35, 95% CI 1.00–1.82, p=0.049). There were no substantial changes seen in the PFS multivariate model.
Table 3.
Multivariable Analysis of Progression-Free Survival
| Clinical Variable | Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Platinum Refractory | 1.11 | 0.83 | 1.49 | 0.49 |
| Age ≥ 65 | 1.07 | 0.80 | 1.43 | 0.63 |
| Performance Status = 1 | 1.33 | 0.99 | 1.77 | 0.06 |
| Current Smoker vs. Former or Never | 0.90 | 0.67 | 1.22 | 0.51 |
| Male Sex | 1.14 | 0.87 | 1.51 | 0.35 |
| Elevated LDH | 1.83 | 1.37 | 2.43 | <.0001 |
| 2 or more prior chem regimens vs. only 1 | 0.87 | 0.51 | 1.47 | 0.59 |
| Weight Loss ≥ 5% | 1.09 | 0.80 | 1.48 | 0.59 |
| Prior radiation therapy | 1.22 | 0.87 | 1.70 | 0.26 |
| S0802 vs. (S0435 or S0327) | 1.82 | 1.29 | 2.55 | 0.001 |
Table 4.
Multivariable Analysis for Overall Survival
| Parameter | Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Platinum Refractory | 1.25 | 0.93 | 1.69 | 0.14 |
| Age ≥ 65 | 1.06 | 0.78 | 1.43 | 0.72 |
| Performance Status = 1 | 1.43 | 1.05 | 1.94 | 0.02 |
| Current Smoker vs. (Former or Never) | 1.05 | 0.77 | 1.43 | 0.77 |
| Male Sex | 1.36 | 1.01 | 1.83 | 0.04 |
| Elevated LDH | 2.04 | 1.52 | 2.76 | <.0001 |
| 2 or more prior chemo regimens vs. only 1 | 0.79 | 0.44 | 1.40 | 0.42 |
| Weight Loss ≥ 5% | 1.53 | 1.11 | 2.12 | 0.01 |
| Prior radiation therapy | 0.89 | 0.64 | 1.25 | 0.51 |
| S0802 vs. (S0435 or S0327) | 1.28 | 0.90 | 1.81 | 0.17 |
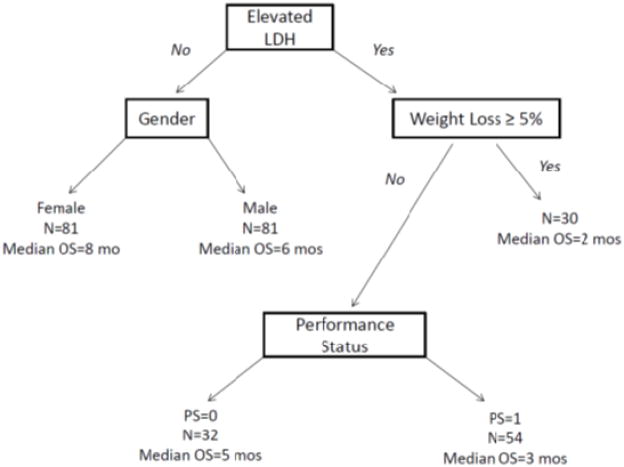
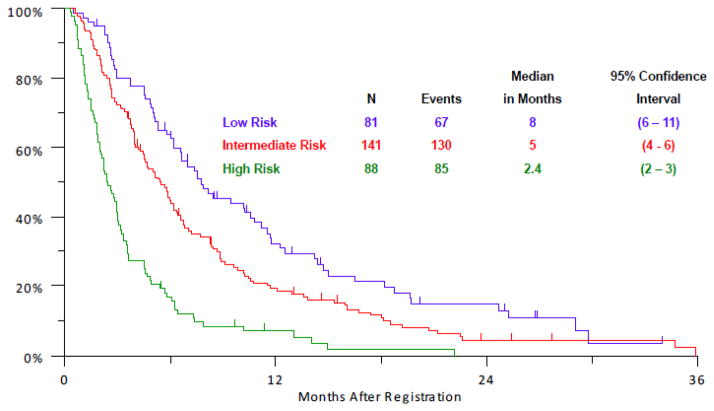
Recursive partitioning analysis showed that specific subsets of patients had differential outcomes dependent on the distribution of baseline clinical variables (Figure 2). Prognostic groups consisting of low, intermediate, and high risk subsets were identified based on differential median OS outcomes of 8, 5, and 2.4 months, respectively (Figure 3), using elevated LDH, >= 5% weight loss, performance status of 1, and male sex as risk factors. Specifically, high risk patients had 2 risk factors (elevated LDH and either >= 5% weight loss or Zubrod performance status > 0), intermediate risk patients had 1 risk factor (either male sex or elevated LDH if not already in high risk group), and low risk patients had no risk factors (i.e., they had normal LDH and were female). A practical model for identifying these subjects is provided in Table 5, representing a modern classifier that no longer includes platinum-sensitivity status as a prognostic indicator.
Figure 2.
Recursive Partitioning Analysis of Platinum-Treated Small Cell Lung Cancer
Figure 3.
Table 5.
Proposed Prognostic Risk Groups for Platinum-Treated Small Cell Lung Cancer Patients
| High Risk (2 Factors) | Elevated LDH | And | ≥ 5% Weight Loss Or Zubrod Performance Status > 0 |
| Intermediate Risk (1 Factor) | Elevated LDH (& not High Risk) | Or | Male |
| Low Risk (0 Factors) | Normal LDH | And | Female |
Note: Lactate dehydrogenase (LDH) and sex were the only variables that define the intermediate and low risk categories
Discussion
In this modern SWOG database analysis, platinum sensitivity status was not significantly associated with survival from the time of post-platinum progression. However, in an unplanned analysis that excluded 36 patients who received >1 prior chemotherapy regimen, platinum sensitivity status was found to be associated with OS. Nevertheless, factors prognostic for survival in this pooled analysis included elevated LDH, weight loss, poor performance status, and male sex.
These results are divergent from prior studies in this context [12] and particularly, from the recent results of a randomized study comparing amrubicin with topotecan in which 637 relapsed SCLC patients were randomized 2:1 to either amrubicin or topotecan [13]. The top-line results of that trial showed that despite a higher response rate in the amrubicin arm (31 vs 17%, p=0.0002), there was no apparent difference in overall survival (HR 0.88, p=0.17). Nonetheless, there was an apparent divergence in OS dependent on platinum refractory status. There were 342 patients in the “sensitive” group compared to 295 patients in the “refractory group”. The former group had better median OS (9.2 months for amrubicin arm; 10 months for the topotecan arm) when compared to the latter group (6.2 months in the amrubicin arm, 5.7 months for the topotecan arm).
The lack of more contemporary consensus information on the value of prospectively stratifying SCLC patients with relapsed disease according platinum-refractory status suggests that this practice ought to be revisited, particularly with regard to phase II drug development. In this context, new agent development often relies on intermediate endpoints such as response rate or PFS to determine whether to proceed to phase III. As reported here, the various phase II agents employed in each of the trials did not yield differential outcomes according to pre-specified strata based on platinum-sensitivity, showing that this differentiation has limited prognostic significance (ie., where survival outcomes are independent of treatment received). A priori, we would have expected to see PFS and OS differences based on historical data on platinum-sensitivity status.
Given the failure of platinum refractory status to provide statistically significant prognostic information in this study, we attempted to develop a new prognostic model based on the best available information from the SWOG database. If validated in an independent pooled dataset containing the same clinical variables described herein, the resulting model will have potential relevance for clinical practice and trial design. We identified clinically relevant prognostic risk groups by recursive partitioning analysis that stratified patients into low, intermediate, and high risk groups based on expected OS outcomes. These risk groupings are potentially useful for individualized patient counseling in clinic and perhaps stratification of patients in prospective phase II trials while remaining agnostic to platinum-sensitivity status. However, it will be necessary to perform a validation study of these risk groupings in an independent SCLC dataset prior to wide use.
This pooled study is limited by its retrospective nature and the heterogeneity of investigational therapies employed in the individual trials. We acknowledge the trials included in this analysis had no or little activity in this disease; therefore, we cannot rule out the possibility that platinum sensitivity status may influence outcome in the context of active therapies. It must be emphasized that this study focused solely on the prognostic value of platinum sensitivity (again, independent of treatment received) and not its predictive value. Nonetheless, the therapeutic effect identified in each of the trials was absent or small, reducing the likelihood that an interaction between platinum refractory status and treatment could have biased our results. Additionally, we lack molecular characterization of the tumors that may be needed to develop a robust prognostic system. The strengths of this analysis include the consistent collection of relevant baseline variables, its relatively robust sample size, and the relative homogeneity of protocol design inherent in SWOG trials.
Conclusion
In this modern SCLC database, platinum sensitivity status may no longer be as strongly associated with PFS and/or OS, calling into question its use in future prospective trials in ES-SCLC. Baseline PS, sex, LDH, and weight loss remain the only independent prognostic indicators of OS outcomes. Here we developed a simple prognostic scoring system from these data that, if validated, would better define prognostic subgroups in the clinic and in future studies.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA46441, and CA58882. Dr. Semrad is supported by the UC Davis Calabresi K12 Clinical Oncology Training Grant (CA138464, PI: Lara).
Footnotes
Presented in part at the 44rd Annual Meeting of the American Society of Clinical Oncology, June 2013, Chicago, IL and the 15th World Conference on Lung Cancer, October 2013, Sydney Australia.
Clinical Trials Registration: ClinicalTrials.gov Identifiers NCT00068289, NCT00182689 and NCT00828139
References
- 1.Schneider BJ. Management of recurrent small cell lung cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2008;6:323–31. doi: 10.6004/jnccn.2008.0027. [DOI] [PubMed] [Google Scholar]
- 2.Giaccone G, Donadio M, Bonardi G, et al. Teniposide in the treatment of small-cell lung cancer: the influence of prior chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1988;6:1264–70. doi: 10.1200/JCO.1988.6.8.1264. [DOI] [PubMed] [Google Scholar]
- 3.Jotte RM, Conkling P, Reynolds C, et al. Second-line amrubicin (AMR) vs topotecan in extensive-disease small cell lung cancer (ED-SCLC) sensitive to first-line platinum based chemotherapy: updated results of a randomized phase 2 trial. Journal of Thoracic Oncology. 2009;4:S399-S. [Google Scholar]
- 4.Morgensztern D, Perry MC, Govindan R. A phase II study of topotecan and docetaxel in patients with sensitive relapse small cell lung cancer. Acta oncologica. 2008;47:152–3. doi: 10.1080/02841860701418853. [DOI] [PubMed] [Google Scholar]
- 5.Lara PN, Jr, Chansky K, Davies AM, et al. Bortezomib (PS-341) in relapsed or refractory extensive stage small cell lung cancer: a Southwest Oncology Group phase II trial (S0327) Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2006;1:996–1001. [PubMed] [Google Scholar]
- 6.Gitlitz BJ, Moon J, Glisson BS, et al. Sorafenib in platinum-treated patients with extensive stage small cell lung cancer: a Southwest Oncology Group (SWOG 0435) phase II trial. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010;5:1835–40. doi: 10.1097/JTO.0b013e3181f0bd78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen JW, Moon J, Gadgeel SM, et al. SWOG 0802: A randomized phase II trial of weekly topotecan with and without AVE0005 (aflibercept) in patients with platinum-treated extensive-stage small cell lung cancer (ESCLC) Journal of Clinical Oncology. 2012:30. doi: 10.1200/JCO.2013.51.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietanza MC, Sima CS, Polley MR, et al. Phase II Study of Temozolomide for Relapsed Sensitive or Refractory Small Cell Lung Cancer (SCLC) Journal of Thoracic Oncology. 2010;5:S507-S. [Google Scholar]
- 9.Krug LM, Crawford J, Ettinger DS, et al. Phase II multicenter trial of voreloxin as second-line therapy in chemotherapy-sensitive or refractory small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6:384–6. doi: 10.1097/JTO.0b013e318200e509. [DOI] [PubMed] [Google Scholar]
- 10.Pietanza MC, Kadota K, Huberman K, et al. Phase II Trial of Temozolomide in Patients with Relapsed Sensitive or Refractory Small Cell Lung Cancer, with Assessment of Methylguanine- DNA Methyltransferase as a Potential Biomarker. Clinical Cancer Research. 2012;18:1138–45. doi: 10.1158/1078-0432.CCR-11-2059. [DOI] [PubMed] [Google Scholar]
- 11.LeBlanc M, Crowley J. Relative risk trees for censored survival data. Biometrics. 1992;48:411–25. [PubMed] [Google Scholar]
- 12.Korkmaz T, Seber S, Kefeli U, et al. Comparison of second-line treatment outcomes between sensitive and refractory small cell lung cancer patients: a retrospective analysis. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2013;15:535–40. doi: 10.1007/s12094-012-0960-6. [DOI] [PubMed] [Google Scholar]
- 13.von Pawel J, Jotte R, Spigel DR, et al. Randomized phase 3 trial of amrubicin versus topotecan as second-line treatment for small cell lung cancer (SCLC) Onkologie. 2011;34:122–4. doi: 10.1200/JCO.2013.54.5392. [DOI] [PubMed] [Google Scholar]