Abstract
Background
Most pediatric kidney transplant recipients eventually require retransplantation, and the most advantageous timing strategy regarding deceased and living donor transplantation in candidates with only one living donor remains unclear.
Methods
A patient-oriented Markov decision process model was designed to compare, for a given patient with one living donor, living-donor-first followed if necessary by deceased donor retransplantation versus deceased-donor-first followed if necessary by living donor (if still able to donate) or deceased donor (if not) retransplantation. Based on Scientific Registry of Transplant Recipients data, the model was designed to account for waitlist, graft, and patient survival, sensitization, increased risk of graft failure seen during late adolescence, and differential deceased donor waiting times based upon pediatric-priority allocation policies. Based on national cohort data, the model was also designed to account for aging or disease development leading to ineligibility of the living donor over time.
Results
Given a set of candidate and living donor characteristics, the Markov model provides the expected patient survival over a time horizon of 20 years. For the most highly sensitized patients (PRA>80%), a deceased-donor-first strategy was advantageous, but for all other patients (PRA<80%), a living-donor-first strategy was recommended.
Conclusions
This Markov model illustrates how patients, families, and providers can be provided information and predictions regarding the most advantageous use of deceased donor versus living donor transplantation for pediatric recipients.
INTRODUCTION
Because of the longevity of children undergoing kidney transplantation (KT), most pediatric recipients will inevitably develop graft failure, requiring a return to dialysis or a second transplant. In fact, approximately 25% of first kidney grafts in pediatric recipients are lost within five years, and among patients whose first transplant fails, 50% undergo retransplantation within five years 1. This frequent need for retransplantation among pediatric candidates complicates decisions about the most advantageous timing strategy for living donor KT: should a patient with only one living donor follow the mantra of “use the best donor first” immediately and select primary living donor KT, or take advantage of expedited deceased donor allocation for children and “save the living donor” for repeat transplantation, hoping the donor remains eligible?
No model currently exists to predict the most beneficial use of living donor KT for pediatric candidates with a single living donor available. Graft survival after pediatric KT is longer with living donor transplants compared to deceased donor transplants 2–4, and therefore use of living donation, when available, has typically been encouraged for primary KT in children. However, since implementation of the Share-35 allocation policy in 2005, there has been a decrease in waiting times for deceased donor grafts, an increase in the use of deceased donor KT 4,5, and a concomitant decrease in the rate of living donor KT among pediatric recipients, suggesting that some pediatric candidates may in fact be selecting deceased donor grafts for primary KT rather than utilizing available living donors 6.
We previously observed that, on average, primary deceased donor KT in pediatric recipients followed by living donor retransplantation does not appear to negatively impact the living donor graft survival advantage and provides similar cumulative graft life compared to living donor KT followed by deceased donor retransplantation 7. However, several critically important factors could not be accounted for in this observational design: sensitization risks, the increased risk of graft loss during adolescence and early adulthood, aging or development of disease in one’s living donor over time, the pediatric advantages of deceased donor allocation that are lost at age 18, and geographic variability in deceased donor waiting times. In addition, the most ideal timing of living donor KT for a given patient is likely dependent at least in part on the specific characteristics of the patient and the potential living donor, so “on average” analyses are inadequate for clinical decision-making.
To address these limitations, and to better inform this important clinical question, the goals of the current study were 1) to develop a Markov decision process model to compare the relative benefit of undergoing primary deceased donor versus living donor KT for a given pediatric patient with a single available living donor, 2) to identify the candidate and living donor characteristics associated with the greatest survival benefit of undergoing either primary deceased donor or living donor KT, and 3) to implement an easily usable tool to help guide patients and providers facing the difficult decision of how best to strategize the benefit of an available living donor versus a deceased donor allocation system that prioritizes pediatric candidates only to a certain age.
MATERIALS AND METHODS
Markov Model Design
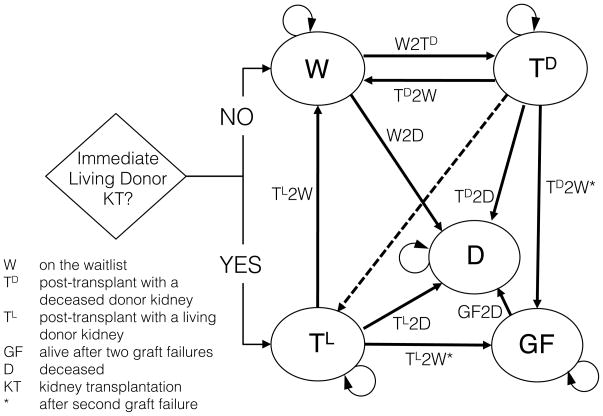
A Markov decision process model 8,9 (Figure 1) was designed to compare the relative outcomes for a given pediatric kidney transplant candidate with only one living donor available who must choose between 1) immediate primary living donor KT, followed if necessary by deceased donor retransplantation, versus 2) waiting to undergo primary deceased donor KT, followed if necessary by living donor retransplantation (if the living donor if still able to donate) or deceased donor retransplantation (if the living donor is no longer able to donate). Time 0 was considered the time at which a living donor was available (for immediate living donor KT, if so chosen). A time horizon of 20 years, a cycle length of one month, and an individual patient perspective were selected. At any given time, a patient could be in one of five states: on the waitlist (W), post-transplant with a deceased donor kidney (TD), post-transplant with a living donor kidney (TL), alive after two graft failures (GF), or deceased (D). The primary outcome of interest was patient survival.
Figure 1. Markov decision process model for order of deceased donor and living donor transplantation among pediatric kidney transplant candidates.
Patients entered into the Markov model must decide whether to utilize their single available living donor for primary living donor transplantation. Declining patients start their first simulated month in the waitlist state (W), and their Markov state over the subsequent 240 months is determined by the probability of deceased donor kidney transplantation (W2TD) or death (W2D). Patients who choose to utilize their living donor undergo immediate living donor transplantation, and their subsequent states are determined by the probability of death (TL2D) and the probability of graft loss (TL2W), necessitating a return to dialysis and immediate relisting for deceased donor retransplantation. Following deceased donor transplantation, a patient’s subsequent states are determined by the probability of death (TD2D) and the probability of graft loss, necessitating either living donor retransplantation (shown with dashed line, if available) or rather a return to dialysis and immediate relisting for deceased donor retransplantation (TD2W) if one’s living donor is no longer eligible for donation. After patients experience two graft failures, subsequent states are determined by the probability of death (GF2D).
Markov Model Input: State Transition Probabilities
With the exception of the waiting times for deceased donor KT, state transition probabilities were estimated based on data from 1987–2012 reported to the Scientific Registry of Transplant Recipients (SRTR), which includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement Transplantation Network (OPTN), and has been described elsewhere 10. The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
All models were based on candidate and donor (where applicable) data, as entered by the user into the Markov model (Table 1). As appropriate, these parameters were updated each cycle (month) as the Markov model progressed; for example, initial age was provided by the user, but the simulated patient aged one month per cycle. Death on the waitlist (W2D) was estimated using a Cox model of time to death among patients on the deceased donor kidney-only waitlist (n=654,832). Death after deceased donor (TD2D) and living donor (TL2D) KT and death after two graft failures (GF2D) were estimated using Cox models of time to death after first and second transplantation and after two graft failures among patients who received their first kidney transplant prior to age 21 (n=21,302). Deceased donor (TD2W) and living donor (TL2W) death-censored graft loss was estimated using a Cox model of time to graft loss for first (n=21,302) and second (n=4777) transplants among patients who received their first kidney transplant prior to age 21. Finally, the expected times to primary deceased donor KT (W2TD) were based (using a gamma distribution) on user input of the expected waiting times in the candidate’s region for pediatric (<18 years) and adult candidates, to reflect the patient’s baseline level of sensitization as well as the pediatric priority through the Share-35 allocation policy 11.
Table 1.
Data sources, models, and variables for the Markov decision process model for order of deceased donor and living donor transplantation among pediatric kidney transplant candidates.
| Data Source | Model | Variables | |
|---|---|---|---|
| Transition Probabilities | |||
| W2D | SRTR | Cox proportional hazards | Candidate age, gender, race, PRA, ABO blood type, and previous transplant |
| TL2D, TD2D TD2W TL2W |
SRTR | Cox proportional hazards | Candidate age, gender, race, etiology of renal disease, previous dialysis, PRA, and previous transplant Donor age, gender, and race HLA mismatch |
| GF2D | SRTR | Cox proportional hazards | - |
| W2TD | User Input | - | - |
| Living Donor Availability | ARIC CARDIA |
Exponential survival | Donor age, gender, race, and smoking history |
| Recipient Sensitization | SRTR | Ordered logistic regression | Donor type HLA mismatch |
SRTR = Scientific Registry of Transplant Recipients, ARIC = Atherosclerosis Risk in Communities, CARDIA = Coronary Artery Risk Development in Young Adults, PRA = panel reactive antibody
Markov Model Input: Potential Living Donor Data
The continued ability of one’s living donor to safely donate over time was estimated using data from the Atherosclerosis Risk in Communities (ARIC) (n=15,792) and Coronary Artery Risk Development in Young Adults (CARDIA) (n=5,111) studies, described in greater detail elsewhere 12,13. Briefly, both cohorts were established to study the natural history of atherosclerosis and utilized patient interviews, physical examinations, and radiographic and laboratory test results. The ARIC study included participants age 45–64 years and consisted of a baseline visit and 3 follow-up visits administered 3 years apart. The CARDIA study included participants age 18–30 years and consisted of a baseline visit and 6 follow-up visits occurring 2, 5, 7, 10, 15 and 20 years thereafter. Healthy ARIC and CARDIA participants (free from hypertension, diabetes, cardiovascular disease, cancer, kidney disease, or morbid obesity at the time of entry into the study) were used to determine the risk a simulated healthy individual (i.e. a potential living kidney donor) would have of developing a contraindication to donation (death, hypertension, diabetes, cardiovascular disease, kidney disease, or morbid obesity) while waiting to donate to a patient in the Markov model.
Markov Model Input: Adolescent Period of Increased Graft Loss
To account for the increased risk of graft failure during late adolescence and early adulthood, the probabilities of graft failure (TD2W and TL2W) were directly increased 1.33-fold while patients were ages 17 to 24, based upon previous work 14,15. In sensitivity analyses, results were compared to having 1) no increased risk of graft failure, or 2) a 2.00-fold increased risk of graft failure during late adolescence and early adulthood. The 1.33-fold increase was used for all reported results unless otherwise stated.
Markov Model Input: Recipient Sensitization
The expected level of sensitization after first graft failure was modeled using pediatric patients in the SRTR who underwent KT between 1995–2012 and had panel reactive antibody (PRA) values both prior to primary KT and after primary graft failure (but before retransplantation) (n=2511). The user-entered peak PRA at time 0 was used for initial state transition probabilities, while a predicted PRA after first graft failure, based upon characteristics of the first transplant, was used for subsequent state transition probabilities. The predicted PRA after first graft failure was also used to inform the waiting time for retransplantation. To account for time to find a kidney exchange and/or undergo desensitization, living donor retransplantation was modeled to occur immediately if predicted PRA was 0–5% and delayed by three months if PRA was 6–80%, six months if 81–95%, and one year if >95%. For deceased donor retransplantation, to account for increased difficulty in finding a compatible deceased donor in the setting of sensitization, the user-entered expected pediatric and adult waiting times for primary deceased donor KT were increased by one year, three years, and five years for those with predicted PRA of 6–80%, 81–95%, and >95%, respectively.
Markov Model Input: State Transition Probability Validation
Multivariable Cox proportional hazards models were developed for the transition probabilities (W2D, TD2D, TL2D, TD2W, and TL2W) between Markov states. State transition models were validated by visually comparing estimated to observed survival curves for a variety of patient phenotypes. An exponential survival model was used to model the probability over time of one’s living donor dying or developing a diagnosis precluding donation. Sensitization by primary transplantation was modeled using a multivariable ordered logistic regression model. These analyses were performed using STATA 12.1/SE (College Station, Texas).
Stochastic Simulation
For a given set of patient and living donor characteristics in the Markov model, patient survival for the subsequent 20 years was simulated 1000 times for the decision to undergo primary living donor KT and 1000 counterfactually seeded times for the decision to forego primary living donor KT and instead wait for a deceased donor kidney. The Markov model and stochastic simulations were implemented in C, and results were graphically exported using R 3.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Classification and Regression Tree Analysis (CART)
The factorial combination of selected patient and donor characteristics (Table 2) generated 336 unique donor phenotypes and 81920 unique candidate phenotypes. The stochastic simulation was used to estimate survival for each of the 27.5 million donor-candidate pairs (using 1000 simulations in each of the two scenarios for each unique pair, for a total of 55 billion simulations). The outputs from the Markov model were then analyzed with CART to identify those patient and donor characteristics that were most predictive of survival benefit from choosing primary living donor KT (versus primary deceased donor KT). The trees were pruned to two levels to minimize over-fitting. CART was performed using the ‘rpart’ package in R 3.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Table 2.
Patient and donor characteristics used in Classification and Regression Tree (CART) analysis.
| Selected levels | |
|---|---|
| Donor Characteristics | |
| Age | 20, 35, 50 |
| Gender | female, male |
| Race | Caucasian, African-American, Hispanic, other |
| HLA mismatches | 0, 1, 2, 3, 4, 5, 6 |
| Smoking history | yes, no |
| Candidate Characteristics | |
| Age | 1, 3, 7, 12, 17 |
| Gender | female, male |
| Race | Caucasian, African-American, Hispanic, other |
| Previous dialysis months | 0, 6, 12, 24 |
| Previous transplant | yes, no |
| Etiology of renal disease | FSGS, other glomerular, CAKUT, other |
| Peak PRA | 0–5%, 6–80%, 81–95%, 96–100% |
| ABO blood type | A, B, AB, O |
| Estimated Deceased Donor Wait | |
| Pediatric (months) | 3, 12 |
| Adult (months) | 12, 48 |
FSGS = focal segmental glomerular sclerosis, CAKUT = congenital anomalies of the kidneys and urinary tract, PRA = panel reactive antibody
RESULTS
Markov Decision Process Model
A functioning web-based implementation of the Markov decision process model can be found at http://www.transplantmodels.com/ped. The user of the model specifies the transplant candidate’s age, sex, race, dialysis history, transplant history, etiology of renal disease, PRA, and ABO blood type. The potential living donor’s age sex, race, human leukocyte antigen (HLA) mismatch with the transplant candidate, and smoking history are also entered, as are the expected region-specific waiting times for a deceased donor KT as a pediatric (<18 years) or adult candidate in the given geographic region. The model then returns estimated 20-year patient survival curves for undergoing primary living donor KT versus waiting for a deceased donor KT and “saving” the living donor.
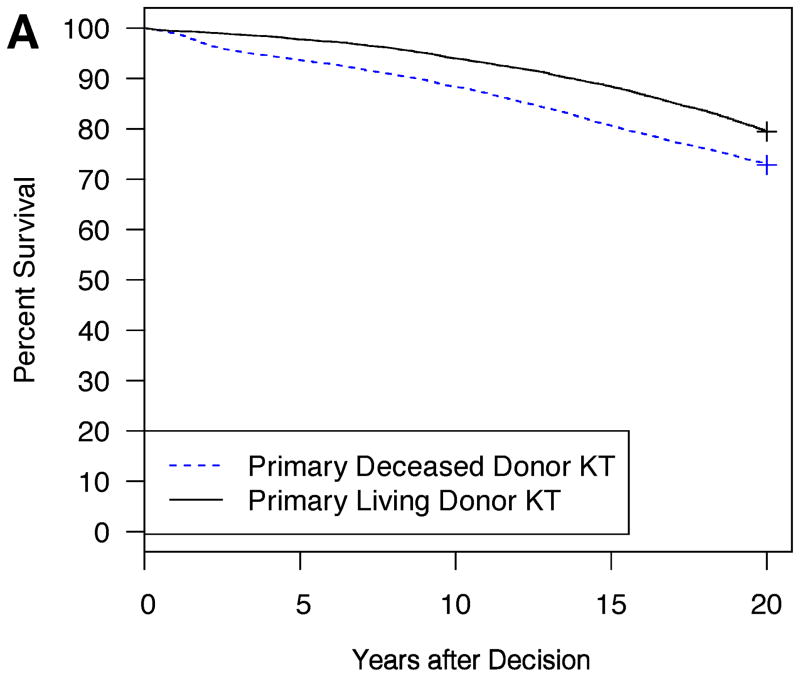
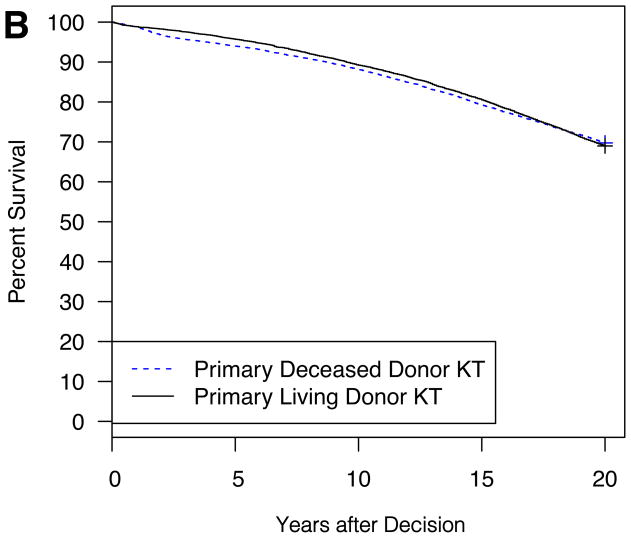
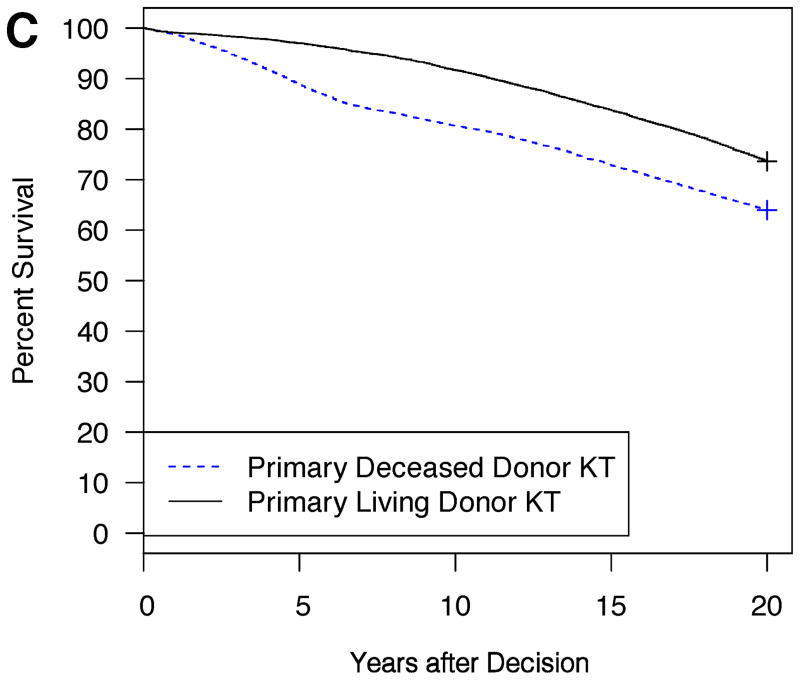
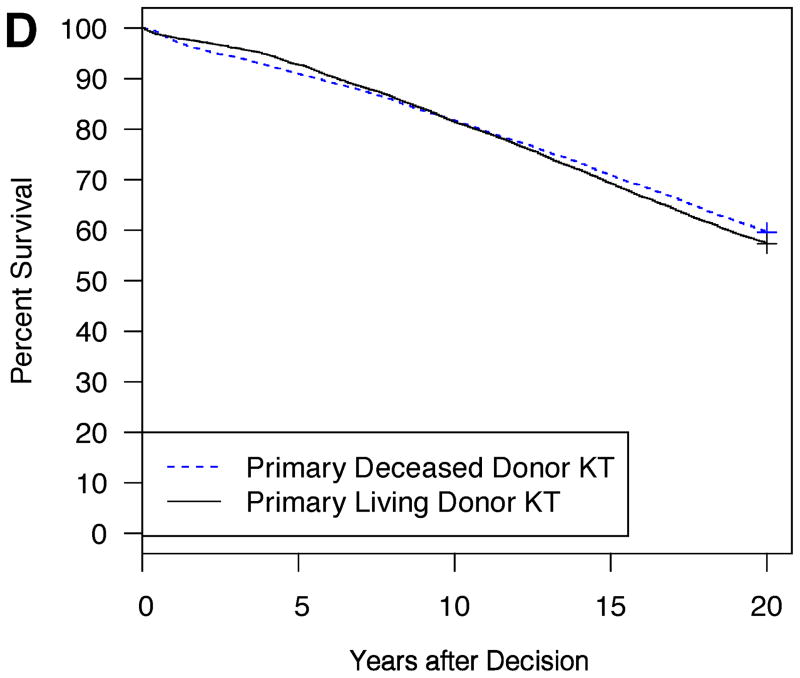
Example model outputs are shown for several illustrative patient and donor phenotypes (Figure 2A–D). For a 5 year-old Caucasian male candidate with congenital anomalies of the kidney and urinary tract (CAKUT), a PRA between 0–5%, blood type O, no prior dialysis or KT, an expected pediatric time to deceased donor KT of 6 months, and an expected adult time to deceased donor KT of 2 years, an estimated survival benefit of 6.6% at 20 years was expected with choosing primary living donor KT if the living donor were a 20 year-old Caucasian female with no smoking history and no HLA mismatches (Figure 2A). On the other hand, the same candidate would have little survival difference (only 0.8% at 20 years) with choosing primary living donor KT if the living donor were instead a 50 year-old Caucasian male with a smoking history and six HLA mismatches (Figure 2B). The same candidate had an estimated survival benefit of 9.7% at 20 years with choosing primary living donor KT if the living donor were a 30 year-old Caucasian male with no smoking history and three HLA mismatches (Figure 2C). On the other hand, for a 15 year-old African-American female candidate with focal segmental glomerulosclerosis (FSGS), a PRA between 96–100%, blood type B, no prior dialysis, and a previous KT with the same available living donor, a survival advantage, albeit only slight (2.3% at 20 years), was expected with choosing primary deceased donor KT (Figure 2D).
Figure 2. Expected patient survival after the choice between primary living donor versus deceased donor KT: illustrative patient and donor phenotypes.
2A: Living donor is a 20 year-old Caucasian female, HLA mismatches = 0, no smoking history
2B: Living donor is a 50 year-old Caucasian male, HLA mismatches = 6, smoking history
2C: Candidate is a 10 year-old Caucasian male, CAKUT, PRA 0–5%, ABO O, no prior dialysis or KT
2D: Candidate is a 15 year-old African American female, FSGS, PRA 96–100%, ABO B, no prior dialysis, previous KT
Candidate for Figure 2A–B: 5 year-old Caucasian male, CAKUT, PRA 0–5%, ABO O, no prior dialysis or KT, expected pediatric time to deceased donor KT = 6 months, expected adult time to deceased donor KT = 2 years. Living donor for Figure 2C–D: 30 year-old Caucasian male, HLA mismatches = 3, no smoking history, expected pediatric time to deceased donor KT = 6 months, expected adult time to deceased donor KT = 2 years.
CART: Characteristics Associated with Survival Difference
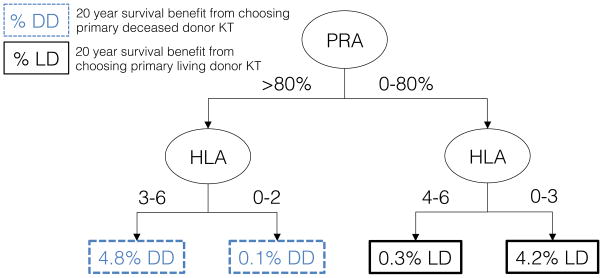
While all parameters entered into the Markov model affected the estimated survival, CART analysis identified peak PRA and HLA mismatch to have the greatest influence on survival differences between choosing primary living donor and deceased donor KT (Figure 3). In general, primary living donor KT was beneficial to candidates with PRA<80%, while primary deceased donor KT was beneficial to highly sensitized (PRA>80%) children (Figure 3).
Figure 3. Classification and regression tree (CART) analysis: patient and donor characteristics that are most predictive of 20-year survival benefit from undergoing primary living donor versus deceased donor KT.
CART data from 336 hypothetical donor phenotypes and 81,920 hypothetical candidate phenotypes. Tree pruned to 2 levels to avoid overfitting. Donor characteristics included age, gender, race, HLA mismatches, and smoking history. Patient characteristics include age, gender, race, previous dialysis, previous KT, etiology of renal disease (FSGS, other glomerular disease, CAKUT, or other), PRA, ABO, and estimated times to deceased donor KT. To navigate the tree, start at the “PRA” node; if the candidate has PRA greater than 80% move left down the tree.
Sensitivity Analyses
When the relative probability of graft loss during late adolescence and early adulthood was varied between 1.00-fold and 2.00-fold, the subgroups predicted to benefit from primary living donor KT had a survival benefit ranging from 4.3% to 4.0%, respectively. Conversely, the subgroups predicted to benefit from primary deceased donor KT had a survival benefit ranging from 5.0% to 4.3%, respectively. In other words, this sensitivity analysis showed that the relative probability of graft loss during late adolescence did not affect overall inferences (whether a patient should choose primary living versus deceased donor KT) and only affected Markov estimates modestly.
DISCUSSION
In this study, we designed a Markov decision process model, using data from a national registry and two large cohort studies, to assist patients, families, and transplant providers with the complex decision to either utilize a pediatric candidate’s available living donor for primary KT or alternatively to “save” this donor for potential retransplantation. Importantly, this tool allows for a personalized approach to this decision and takes into account a number of critical factors that cannot be included when examining only aggregate national data. Our simulations revealed that the estimated optimal strategy of living-donor-first versus deceased-donor-first can vary widely by patient and donor phenotype; however, in general, primary living donor KT is predicted to be most beneficial among patients with lower sensitization, while primary deceased donor KT most benefits patients with a baseline high level of sensitization. While there are clearly limitations to the ability to predict long-term survival on the waiting list and after KT, the robustness of our predictions are supported by (1) the many clinical considerations incorporated into the model; (2) the validation studies of the state transition probabilities; (3) the sensitivity analyses when the impact of various factors was explored; and (4) the fact that these Markov-based comparisons (primary living versus deceased donor KT) are “internally controlled,” i.e. the same assumptions underlying the predictions for primary living donor KT also underlie the predictions for primary deceased donor KT.
Our previous study on this topic using observational data suggested that, on average, primary deceased donor KT followed by living donor retransplantation did not negatively impact the living donor graft survival advantage and provided similar cumulative graft life compared to living donor KT followed by deceased donor retransplantation 7. However, our prior analysis could not evaluate this complex decision with regard to the specific characteristics of a given patient, potential living donor, and geographic region. In addition, we noted several important factors that could not be accounted for in that observational study but that nonetheless would be critical to clinical decision-making, thus motivating the current study in which we developed a Markov model to better address the complexity of the decision.
First, the Markov model accounts for the differential time spent on dialysis after first graft failure (depending on whether living donor KT was performed for primary KT or instead “saved” for repeat transplantation). Living donor retransplantation enables immediate retransplantation (or even preemptive retransplantation) after graft failure, while deceased donor retransplantation may require a significant waiting time for a deceased donor transplant, as the age-based allocation priority given by Share-35 may no longer apply at the time that retransplantation is required (if graft failure occurs when the patient is an adult).
Second, the Markov model accounts for the disproportionately high rate of graft failure seen during ages 17–24 14,15, an important consideration in this decision because this high-risk age window may differentially affect one’s living donor or deceased donor graft based upon the timing of KT with regard to passage through those ages. Interestingly, however, our sensitivity analysis suggests that this variable is not the primary factor determining the benefit of undergoing primary living donor versus deceased donor KT.
Third, the Markov model accounts for the possibility that one’s living donor, healthy and eligible for donation at the time that primary KT is required, may develop health conditions over time or even die prior to graft failure, precluding donation when retransplantation is needed. This probability was modeled through the use of participants in two large cohort studies who at baseline would appear to qualify for kidney donation and represents the inherent risk in “saving” one’s available living donor for retransplantation. In addition to the possibility that one’s living donor may no longer be able donate at the time that retransplantation is required, our model also accounts for the fact that, at the very least, one’s living donor will be older at the time of retransplantation compared to primary KT, which may negatively impact the survival of one’s living donor graft 16.
Finally, the Markov model accounts for sensitization that may occur from primary KT 17 that may negatively impact survival of a subsequent graft should retransplantation be required, allows this sensitization to vary according to the choice between primary living donor or deceased donor KT, and alters the expected waiting time prior to retransplantation based upon one’s expected sensitization after primary KT. Interestingly, CART analysis identified peak PRA (and to a lesser extent HLA mismatch) to have the greatest influence on survival differences between choosing primary living donor and deceased donor KT.
Highly sensitized children may benefit most from primary deceased donor KT because of the high priority given to children for deceased donor kidneys, allowing them to identify compatible donors when they otherwise would be unlikely to do so 18. Poor HLA matching at first transplant has been associated with increased sensitization, longer waiting times for retransplantation, decreased rates of retransplantation, and decreased retransplant graft survival 18–20. Use of the Share-35 priority for deceased donor kidneys among these already sensitized children may thereby prevent further sensitization and better allow for the later use of desensitization protocols to enable living donor retransplantation.
One limitation of this study is the inherent limitation in the data currently available to estimate patient and graft survival after pediatric KT. While 25 years’ worth of data may seem adequate, the decision assessed with this Markov model has even longer-term implications. This limited follow-up is especially true for repeat transplants. Similarly, many of the transplants utilized in building our state transition probability models were performed a number of years ago. Transplant outcomes have changed, and will likely continue to change, over time. We cannot predict how these changes will affect our inferences, nor can we know if these changes will similarly affect living donor and deceased donor KT, highlighting the need to update our models in the future. However, as discussed above, these limitations affect the predictions of both clinical decisions (primary living or deceased donor KT) and as such the comparisons might be thought of as “internally controlled.” Using data on transplants from many years prior could also introduce bias if living donor KT outcomes have improved more so than deceased donor KT outcomes or vice versa; however, this does not appear to be the case 21. This study is also limited by the variables available in the SRTR. Notably, variables such as CMV, EBV, and BK mismatch among donors and recipients, the use of desensitization prior to KT, and the specific maintenance immunosuppression regimens used after KT, all of which may affect transplant outcomes, are poorly captured in the SRTR and thus could not be incorporated into our models.
By design, this Markov model also considers this decision solely from the individual patient perspective. We recognize that other perspectives may be equally valid to consider. For example, while living kidney donation is associated with minimal risk of long-term mortality 22,23, the risk of cardiovascular events 24,25, or the development of end-stage renal disease 26–29 in donors, the long-term risk of other potential consequences such as the development of hypertension is less clear. This Markov model does not incorporate these potential risks of morbidity for the living donor, the possibility that these risks may vary according to the age at which kidney donation is performed, or the individual donor’s feelings regarding the balance between these personal risks and the benefits to the transplant candidate. In addition, our model, by design, does not consider the societal perspective—specifically the impact of these donor type decisions on transplant candidates of other ages given the extreme shortage of deceased donor organs that currently exists in the U.S.
Markov models offer a unique opportunity to utilize observational data from national registries and cohorts to provide estimations of the expected outcomes for each option in a clinical decision. Markov models have also been applied to clinical decision-making elsewhere, including within the field of KT regarding the use of kidneys from infectious risk donors 30. Given the imprecision in modeling clinical outcomes, Markov models should be seen as an adjunct for shared decision-making between healthcare providers and patients but cannot be blindly trusted to provide the correct answer to a clinical dilemma. Nonetheless our study provides another example of how Markov models can be used to simulate the expected outcomes after a clinical decision in order to guide patient and provider decision-making. The implications of this study, given its findings, may include a renewed focus on encouraging primary living donor KT for pediatric recipients instead of deceased donor KT, which has increased in recent years perhaps as a result of Share-35 implementation.
In conclusion, we have developed a patient-perspective Markov decision process model to address a common dilemma facing pediatric KT candidates who have one and only one living donor available. The Markov model estimates the relative benefit of undergoing primary living donor KT versus primary deceased donor KT with potential living donor retransplantation. We have shown that a survival benefit is directly correlated with this choice between primary living donor and deceased donor KT and that this survival benefit varies widely by patient and donor phenotype. In an era of increasing use of deceased donor KT for pediatric candidates, this Markov model provides individualized guidance to assist patients, families, and providers with the complex decision of how best to utilize one’s living donor graft.
Acknowledgments
This work was supported in part by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. The authors wish to thank Mary Grace Bowring and Xun Luo for assistance in developing the web application.
ABBREVIATIONS
- ARIC
Atherosclerosis Risk in Communities
- CAKUT
congenital anomalies of the kidney and urinary tract
- CARDIA
Coronary Artery Risk Development in Young Adults
- CART
Classification and Regression Tree
- D
deceased
- FSGS
focal segmental glomerulosclerosis
- GF
alive after two graft failures
- GF2D
probability of death after two graft failures
- HLA
human leukocyte antigen
- KT
kidney transplantation
- OPTN
Organ Procurement Transplantation Network
- PRA
panel reactive antibody
- SRTR
Scientific Registry of Transplant Recipients
- TD
post-transplant with a deceased donor kidney
- TD2D
probability of death after deceased donor transplant
- TD2W
probability of death-censored graft loss after deceased donor transplant
- TL
post-transplant with a living donor kidney
- TL2D
probability of death after living donor transplant
- TL2W
probability of death-censored graft loss after living donor transplant
- W
on the waitlist
- W2D
probability of death on the waitlist
- W2TD
expected time to primary deceased donor transplantation
Footnotes
Disclosures: The authors declare no conflicts of interest.
KJVA, EKHC, NTJ, and DLS participated in research design, data analysis, interpretation of the results, and writing of the manuscript. BJO, TAE, JMS, and PMC participated in research design, interpretation of the results, and writing of the manuscript.
Contributor Information
Kyle J. Van Arendonk, Email: kvanare1@jhmi.edu.
Eric K.H. Chow, Email: echow8@jhmi.edu.
Nathan T. James, Email: nj1154@gmail.com.
Babak J. Orandi, Email: borandi1@jhmi.edu.
Trevor A. Ellison, Email: telliso1@jhmi.edu.
Jodi M. Smith, Email: jodi.smith@seattlechildrens.org.
Paul M. Colombani, Email: pc@jhmi.edu.
Dorry L. Segev, Email: dorry@jhmi.edu.
References
- 1.Van Arendonk KJ, Garonzik Wang JM, Deshpande NA, et al. Practice patterns and outcomes in retransplantation among pediatric kidney transplant recipients. Transplantation. 2013 Jun 15;95(11):1360–1368. doi: 10.1097/TP.0b013e31828c6d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NAPRTCS. 2010 Annual Transplant Report. 2010 https://web.emmes.com/study/ped/annlrept/2010_Report.pdf.
- 3.Dale-Shall AW, Smith JM, McBride MA, Hingorani SR, McDonald RA. The relationship of donor source and age on short- and long-term allograft survival in pediatric renal transplantation. Pediatr Transplant. 2009 Sep;13(6):711–718. doi: 10.1111/j.1399-3046.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- 4.Magee JC, Krishnan SM, Benfield MR, Hsu DT, Shneider BL. Pediatric transplantation in the United States, 1997–2006. Am J Transplant. 2008 Apr;8(4 Pt 2):935–945. doi: 10.1111/j.1600-6143.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- 5.Abraham EC, Wilson AC, Goebel J. Current kidney allocation rules and their impact on a pediatric transplant center. Am J Transplant. 2009 Feb;9(2):404–408. doi: 10.1111/j.1600-6143.2008.02504.x. [DOI] [PubMed] [Google Scholar]
- 6.Axelrod DA, McCullough KP, Brewer ED, Becker BN, Segev DL, Rao PS. Kidney and pancreas transplantation in the United States, 1999–2008: the changing face of living donation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010 Apr;10(4 Pt 2):987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Arendonk KJ, James NT, Orandi BJ, et al. Order of donor type in pediatric kidney transplant recipients requiring retransplantation. Transplantation. 2013 Sep 15;96(5):487–493. doi: 10.1097/TP.0b013e31829acb10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alagoz O, Hsu H, Schaefer AJ, Roberts MS. Markov decision processes: a tool for sequential decision making under uncertainty. Medical decision making: an international journal of the Society for Medical Decision Making. 2010 Jul-Aug;30(4):474–483. doi: 10.1177/0272989X09353194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Medical decision making: an international journal of the Society for Medical Decision Making. 1993 Oct-Dec;13(4):322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 10.OPTN/SRTR 2010 Annual Data Report. Rockville, MD: 2011. [Google Scholar]
- 11. [Accessed August 2, 2012];Allocation of deceased kidneys. 2011 http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_7.pdf.
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. . Am J Epidemiol. 1989 Apr;129(4):687–702. [PubMed] [Google Scholar]
- 13.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987 Dec;8(4 Suppl):68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 14.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA. Association between age and graft failure rates in young kidney transplant recipients. Transplantation. 2011 Dec 15;92(11):1237–1243. doi: 10.1097/TP.0b013e31823411d7. [DOI] [PubMed] [Google Scholar]
- 15.Van Arendonk KJ, James NT, Boyarsky BJ, et al. Age at graft loss after pediatric kidney transplantation: exploring the high-risk age window. Clinical journal of the American Society of Nephrology: CJASN. 2013 Jun;8(6):1019–1026. doi: 10.2215/CJN.10311012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger JC, Muzaale AD, James N, et al. Living kidney donors ages 70 and older: recipient and donor outcomes. Clin J Am Soc Nephrol. 2011 Dec;6(12):2887–2893. doi: 10.2215/CJN.04160511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akalin E, Pascual M. Sensitization after kidney transplantation. Clinical journal of the American Society of Nephrology: CJASN. 2006 May;1(3):433–440. doi: 10.2215/CJN.01751105. [DOI] [PubMed] [Google Scholar]
- 18.Gebel H, Kasiske B, Gustafson S, Shteyn E, Israni A, Snyder J, Friedewald J, Segev D. Allocating deceased donor kidney to high PRA patients: a simulation. American Journal of Transplantation. 2013 Apr 2013;13(Supplement: 5):178–178. [Google Scholar]
- 19.Foster BJ, Dahhou M, Zhang X, Platt RW, Smith JM, Hanley JA. Impact of HLA mismatch at first kidney transplant on lifetime with graft function in young recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014 Apr;14(4):876–885. doi: 10.1111/ajt.12643. [DOI] [PubMed] [Google Scholar]
- 20.Meier-Kriesche HU, Scornik JC, Susskind B, Rehman S, Schold JD. A lifetime versus a graft life approach redefines the importance of HLA matching in kidney transplant patients. Transplantation. 2009 Jul 15;88(1):23–29. doi: 10.1097/TP.0b013e3181a9ec89. [DOI] [PubMed] [Google Scholar]
- 21.Van Arendonk KJ, Boyarsky BJ, Orandi BJ, et al. National trends over 25 years in pediatric kidney transplant outcomes. Pediatrics. 2014 Apr;133(4):594–601. doi: 10.1542/peds.2013-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axelrod DA, McCullough KP, Brewer ED, Becker BN, Segev DL, Rao PS. Kidney and Pancreas Transplantation in the United States, 1999–2008: The Changing Face of Living Donation. American Journal of Transplantation. 2010;10(4p2):987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim HN, Foley R, Tan L, et al. Long-Term Consequences of Kidney Donation. New England Journal of Medicine. 2009;360(5):459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg AX, Meirambayeva A, Huang A, et al. Cardiovascular disease in kidney donors: matched cohort study. BMJ. 2012 Mar 01;344 doi: 10.1136/bmj.e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavakol MM, Vincenti FG, Assadi H, et al. Long-term renal function and cardiovascular disease risk in obese kidney donors. Clin J Am Soc Nephrol. 2009 Jul;4(7):1230–1238. doi: 10.2215/CJN.01350209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wafa EW, Refaie AF, Abbas TM, et al. End-stage renal disease among living-kidney donors: single-center experience. Exp Clin Transplant. 2011 Feb;9(1):14–19. [PubMed] [Google Scholar]
- 27.Rosenblatt GS, Nakamura N, Barry JM. End-stage renal disease after kidney donation: a single-center experience. Transplant Proc. 2008 Jun;40(5):1315–1318. doi: 10.1016/j.transproceed.2008.03.105. [DOI] [PubMed] [Google Scholar]
- 28.Fehrman-Ekholm I, Norden G, Lennerling A, et al. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006 Dec 27;82(12):1646–1648. doi: 10.1097/01.tp.0000250728.73268.e3. [DOI] [PubMed] [Google Scholar]
- 29.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA: the journal of the American Medical Association. 2014 Feb 12;311(6):579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chow EK, Massie AB, Muzaale AD, et al. Identifying appropriate recipients for CDC infectious risk donor kidneys. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 May;13(5):1227–1234. doi: 10.1111/ajt.12206. [DOI] [PubMed] [Google Scholar]