Abstract
Introduction
Despite the advances in radiation techniques and chemotherapy, survival with current platinum-based chemotherapy and concomitant thoracic radiation remains dismal. Bortezomib, a proteasome inhibitor, modulates apoptosis and cell cycle through disruption of protein degradation. The combination of bortezomib and carboplatin/paclitaxel and concurrent radiation in unresectable stage III NSCLC was evaluated in this phase I/II study.
Methods
Patients with histologic or cytologic confirmed stage III non-metastatic NSCLC who were candidates for radiation therapy were eligible. In the phase I portion, patients received escalating doses of bortezomib, paclitaxel and carboplatin concomitantly with thoracic radiation(60 Gy/30 daily fractions) using a modified 3 + 3 design. The primary endpoint for the phase II portion was the 12-month survival rate (12MS). A 1-stage design with an interim analysis yielded 81% power to detect a true 12MS of 75%, with a .09 level of significance if the true 12MS was 60% using a sample size of 60 patients. Secondary endpoints consisted of adverse events (AEs), overall survival (OS), progression-free survival (PFS), and the confirmed response rate (RR).
Results
Thirty-one patients enrolled during the phase I portion of the trial, of which 4 cancelled prior to receiving treatment, leaving 27 evaluable patients. Of these 27 patients, 2 dose-limiting toxicities (DLTs) were observed, one (grade 3 pneumonitis) at dose level 1 (bortezomib at 0.5 mg/m2;, paclitaxel at 150 mg/m2 and carboplatin at area under the curve (AUC) of 5) and one (grade 4 neutropenia lasting ≥8 days) at dose level 6 (bortezomib 1.2 mg/m2, paclitaxel 175 mg/m2, and carboplatin at AUC of 6). During the phase I portion, the most common grade 3/4 AEs were leukopenia (44% %), dyspnea (22%), and dysphagia (11%). Dose level 6 was declared to be there commended phase II dose (RP2D) and the phase II portion of the study opened. After the first 26 evaluable patients were enrolled to the RP2D, a per protocol interim analysis occurred. Of these 26 patients, 23 (88%) survived at least 6 months (95% CI: 70 to 98%), which was enough to continue to full accrual per study design. However, due to slow accrual, the study was stopped after 27 evaluable patients were enrolled (6 - phase I RP2D; 21 - phase II). Of these 27 patients, the 12MS was 73% (95% CI: 58 - 92%), the median OS was 25.0 months (95% CI: 15.6 to 35.8), and the median PFS was 8.4 months (95% CI: 4.1 to 10.5). The confirmed RR was 26% (7/27; 95% CI: 11% to 46%), consisting of 4 partial responses and 3 complete responses. Grade 3+ and Grade 4+AEsoccurred in 82%and 56%of patients, respectively. One patient experienced grade 5 pneumonitis that was possibly related to the treatment. Grade 3 and 4 hematological toxicities were observed in 82% and 56% patients, respectively.
Conclusions
The addition of bortezomib to concurrent carboplatin/paclitaxel and radiation appeared to be feasible, although associated with increased hematological toxicities. A favorable median overall survival of 25 months suggests a potential benefit for this regimen.
Introduction
Lung cancer is the most common cause of cancer deaths in the United States with an estimated mortality of 159,480 in 2013 [1]. Non–small-cell lung cancer (NSCLC) accounts for approximately 85-90% of lung cancer diagnoses. Only 25- 30% of patients with NSCLC have early stage and localized disease that is resectable (stage I or II) at the time of diagnosis [2]. For the 30% of patients with regionally advanced, inoperable disease, the recommended therapeutic approach is combined modality therapy with thoracic radiation therapy and chemotherapy [3-6]. Despite the advances in irradiation techniques and improved chemotherapy, local and distant control remain suboptimal, and the majority of patients continue to die from distant metastases [5, 7-10]. In order to improve efficacy of this treatment approach, chemoradiotherapy in corporating novel molecular targeting agents is being actively investigated.
Bortezomib(VELCADE®, PS-341) is a dipeptide boronic acid analogue that reversibly inhibits the 26S proteasome, a large protease complex that degrades ubiquinated proteins. By blocking proteolysis, bortezomib leads to accumulation of proteins involved in multiple signal transduction pathways, resulting in cell cycle arrest, apoptosis and down regulation of angiogenesis. For example, it stabilizes the cyclin-dependent kinase inhibitors p21 and p27, and blocks cell division in the G2-M phase of the cell cycle. It inhibits the degradation of the wild-type tumor suppressor protein p53, and suppresses the activation of oncogene NF-κB. It inhibits overexpression of anti-apoptotic protein B-cell lymphoma–2(BCL-2) and favorably modulates apoptosis [11-14].
Bortezomib has been approved for treatment of multiple myeloma and mantle cell non-Hodgkin's lymphoma (NHL). In NSCLC, bortezomib has been combined with docetaxel, pemetrexed, gemcitabine/carboplatin, carboplatin/bevacizumab, vorinostat and erlotinib in clinical studies with various levels of activity observed [15-21]. In a phase I study combining bortezomib and carboplatin/paclitaxel, the maximum tolerated dose (MTD) and the recommended phase II dose (RP2D) was found to be bortezomib 1.2 mg/m2, paclitaxel 175 mg/m2 and carboplatin at AUC of 6, and a schedule of bortezomib given on days 1, 4 and 8 followed by paclitaxel and carboplatin on day 2 every 21 days was found to be more efficacious than when paclitaxel and carboplatin were given on day 1 of each cycle [22]. Further more, bortezomib was found to be synergistic with radiotherapy in vitro and in animal models [23]. In a twelve-patient phase I study evaluating carboplatin/paclitaxel/bortezomib and concurrent radiotherapy as induction therapy followed by surgical resection in stage III non-small cell lung cancer, five patients achieved complete pathologic response and two other patients were found to have necrosis occurred in 99% of tumor tissue [24]. Here we report a phase I/II study evaluating carboplatin/paclitaxel/bortezomib in combination with concurrent radiation as primary definitive treatment for patients with unresectable local regionally advanced NSCLC.
Patients and Methods
Eligibility Criteria
Patients with histologic or cytologic confirmed inoperable non-metastatic (stage IIIA or IIIB according to American Joint Committee on Cancer (AJCC) staging system 6th edition) non-small cell lung carcinoma (NSCLC) requiring radiation therapy were eligible for the study. Other eligibility criteria included: age ≥18 years; Eastern Cooperative Oncology Group performance status (ECOG PS)≤1; life expectancy of 12 weeks or longer;absolute neutrophil count ≥ 1.5 × 109/L; platelet count ≥ 100 × 109/L; total serum bilirubin ≤ 1.5 mg/dL or direct bilirubin ≤1.5 × upper limits of normal (ULN); and AST ≤3 × ULN and creatinine ≤1.5 × ULN. Patients with weight loss of 10% or greater in past 3 months, forced expiratory volume in 1 second (FEV1) less than 1 L or 35% of predicted FEV1, history of prior radiation therapy to the chest and systemic chemotherapy for NSCLC, major surgery or unhealed wound ≤ 2 weeks prior to registration, New York Heart Association classification III or IV, uncontrolled infection, and peripheral neuropathy ≥ grade 2 were excluded. Written informed consent approved by institutional review boards and the National Cancer Institute were obtained from eligible patients before pre-study assessments.
Study Design
This was a multi-center co-operative group, open-label, single-arm, combined phase I dose-escalation and phase II study to assess the safety, tolerability and efficacy of carboplatin/paclitaxel/bortezomib and concurrent daily thoracic radiation therapy in advanced non-metastatic NSCLC. Patients received escalating doses of carboplatin/paclitaxel/bortezomib, up to the RP2D recommended in a previous phase I study evaluating this combination [22]. After determination of RP2D, additional patients were enrolled for the phase II part of the study.
Eligible patients received treatment in an outpatient setting with chemotherapy and radiotherapy concurrently delivered over a six-week period. Complete blood cell counts were obtained weekly during periods of active study treatment, at four weeks post-radiation, and at each post-treatment follow-up visit. Serum chemistries were collected prior to each cycle of chemotherapy and at each post-treatment follow-up visit. A chest CT was performed at baseline, four weeks post-radiation, three months post-radiation, and then every three months for 1 year, followed by CT every six months for a maximum of 5 years from the time of registration. Full supportive care, including blood-product support, antibiotic treatment, anti-diarrheals, analgesics, antiemetics, and medications used for the prevention and treatment of radiation esophagitis, nutritional evaluation and treatment of other newly diagnosed or concurrent medical conditions was provided. Prophylactic use of colony-stimulating factors during the study was not allowed. Therapeutic use of colony-stimulating factors in patients with serious neutropenic complications such as tissue infection, sepsis syndrome, fungal infection, etc. was allowed at the investigator's discretion. Recombinant erythropoietin to maintain adequate hemoglobin levels and avoid packed red blood cell transfusions was allowed.
Chemotherapy
Bortezomib was administered by intravenous push on days 1,4,8 and 11 of each 21-day cycle. Paclitaxel was administered intravenously over three hours on day 2 of each cycle. Carboplatin was administered as a 30-minute intravenous infusion immediately after paclitaxel infusion on day 2 of each cycle. Thirty minutes prior to the paclitaxel dose, patients were premedicated with 10-20 mg dexamethasone intravenously or orally, 25-50 mg diphenhydramine HCl intravenously, and 50mg ranitidine or 300mg cimetidine or 20mg famotidine intravenously. Bactrim one table twice daily was administered twice per week continuously during treatment.
In the phase I part, groups of three to six patients were entered at different dose levels following the dose escalation scheme given in Table 1. In the phase II part, bortezomib at 1.2 mg/m2, paclitaxel at 175 mg/m2 and carboplatin at AUC of 6 were administered.
Table 1. Dose escalation scheme.
| Dose level | Bortezomib mg/m2 | Paclitaxel mg/m2 | Carboplatin AUC |
|---|---|---|---|
| -1 | 0.5 | 120 | 5 |
| 0 | 0.5 | 135 | 5 |
| **1 | 0.5 | 150 | 5 |
| 2 | 0.8 | 150 | 5 |
| 3 | 1.0 | 150 | 5 |
| 4 | 1.0 | 175 | 5 |
| 5 | 1.0 | 175 | 6 |
| 6 | 1.2 | 175 | 6 |
Starting dose level
Radiation Therapy
Radiation therapy(RT) administering 2 Gy once daily began on day 1 of chemotherapy for all patients. Three-dimensional radiotherapy with no in homogeneity corrections was used. Intensity modulated radiotherapy (IMRT) was not allowed. Elective nodal irradiation was administered for the first 42 to 44 Gy. The primary tumor, mediastinum, and ipsilateral hilum were covered with a two-cm margin to block edge. The minimal mediastinal volume superiorly to inferiorly extended from the top of T1 superiorly to 5 cm below the carina inferiorly. Elective inclusion of the supraclavicular fossa was allowed at the discretion of the treating physician. If supraclavicular disease was present, both fossae were treated. After 42 to 44 Gy was given, off cord oblique fields treated gross disease (primary tumor and enlarged lymph nodes (>1cm in short diameter) with two cm margins between the tumor and block edge. A total dose of 60 Gy was delivered to the isocenterin 30 fractions given 5 days per week at 2 Gy daily. The spinal cord was limited to a maximum dose of 48 Gy. The total lung volume receiving 20 Gy or greater was limited to 40% or less. One third of the heart could not receive greater than 60 Gy, two-thirds of the heart could not receive more than 50 Gy, and the entire heart could not receive more than 40 Gy.
Response and Toxicity Criteria
All toxicities were graded using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (prior to December 31, 2010) or CTCAE v4.0 (after January 1, 2011). Assessment of disease response occurred four weeks post-radiotherapy, 3 months post-radiotherapy, every 3 months for 1 year post-radiotherapy, and every 6 months thereafter for a maximum of 5 years from time of registration. Treatment response was evaluated using RECIST criteria. [25]
End Points and Statistical Analysis
The primary endpoint of the phase I portion was the maximum tolerated dose (MTD) of the treatment combination using a modified cohort of 3 design [26], where the MTD was defined as the highest safely tolerated dose where at most two out of six patients experienced a dose-limiting toxicity (DLT), with the next higher dose level having at least three out of six patients with a DLT. Any of the following were considered DLTs over the 6 weeks of combined chemotherapy/RT, including a 4-week observation, where the DLT needed to be possibly, probably, or definitely related to study treatment: grade 4 radiation dermatitis, grade 4 neutropeniaor thrombocytopenia lasting at least 8 days or grade 4 febrile neutropenia (first 3 weeks of chemotherapy only for hematologic DLTs), grade ≥ 3 esophagitis requiring hospitalization, grade ≥ 3 pneumonitis requiring oxygen, grade 4 dyspnea at rest, and other non-hematologic grade 4 toxicities not manageable with medical interventions (intravenous, narcotic). If the study did not reach the MTD by dose level 6 (see Table 1), dose level 6 would be considered the recommended phase II dose (RP2D).
The primary endpoint of the phase II portion of the study was 12-month survival, where all patients enrolled at the MTD (or RP2D) would be included in the analysis. The 12-month survival rate was calculated using Kaplan-Meier methodology [27]. A one-stage design with an interim analysis was used to test whether there was sufficient evidence to determine that the 12-month survival rate was at least 75% (i.e., clinically promising) versus at most 60% (i.e., clinically inactive). After the first 26 evaluable patients had been on-study for 6 months, an interim analysis was performed. At least 21 of these 26 patients surviving ≥6 months post-registration would be considered sufficient activity to continue to full accrual. Otherwise, the study would be stopped early for lack of efficacy. At least 41 of all 60 (68%) evaluable patients surviving ≥12 months post-registration would be considered adequate evidence of promising activity, and would warrant further testing of this regimen in subsequent studies. This design yielded 81% power to detect a true 12-month survival rate of 75%, with a .09 level of significance if the true 12-month survival rate was 60%.
Secondary end points were adverse event rates, overall survival (defined as the time from registration to death due to any cause), progression-free survival (defined to be the length of time from study registration to the first of either disease progression or death due to any cause), confirmed tumor response (defined to be a complete response (CR) or partial response (PR) noted as the objective status on two consecutive evaluations at least four weeks apart), and treatment tolerability. The maximum grade for each type of adverse event was recorded for each patient, and frequency tables were used to determine adverse event patterns for both phases of the study (regardless of attribution to the study treatment). The commonly occurring grade 3 and greater adverse events(> 5%) were also reported, including all grade 4 or higher adverse events. Kaplan-Meier methodology was used to estimate the distributions of overall survival and progression-free survival [27] for the RP2D patients only. The confirmed response rate was estimated by the number of patients who had documented confirmed responses (PR or CR maintained for a minimum of 4 weeks) divided by the total number of evaluable patients for the RP2D patients only. All patients who were eligible and started treatment were evaluable for all primary and secondary endpoints in this study. All analyses were conducted using SAS Version 9.3 on data available as of 3/18/2014. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Results
Radiation Therapy Quality Assessment
Radiation therapy plans and port films were reviewed for all 48 patients. These were reviewed in detail by 2 NCCTG investigators and one Radiologic Physics Center (RPC) reviewer based on protocol specified criteria. There were 44 (92%) patients with no deviations, 3 (6%)had minor deviations & 1 (2%) had a major deviation.
Patient Characteristics and Reasons off Treatment
Phase I
Thirty-one patients with locally advanced stage IIIA or IIIB NSCLC were enrolled into the phase I portion of the trial from 04/25/2005 to 07/13/2009, of which 27 were deemed evaluable since four patients withdrew prior to receiving treatment. Patients were enrolled to a total of six dose levels (Table 1), where dose level 6 was the dose selected for the phase II portion of the study (see the Table 6 CONSORT diagram for further details). The characteristics of the phase I patients are summarized in Table 2. The median age of the 27 patients was 63 years (range: 45 - 78. Seventeen (63%) patients were men and ten (37%) patients were women. Approximately 33% of patients presented with stage IIIA disease, whereas 67% of patients presented with stage IIIB disease. Fifteen patients (56%) had an ECOG PS of 0, and twelve patients (44%) had an ECOG PS of 1 (Table 2). All patients ended active treatment, with most completing the study per protocol (17/27; 63%). Of the other 10 patients, 5 went off treatment early due to adverse events, 3 went off treatment due to disease progression, and 2 went off treatment for other reasons.
Table 6. Consort Diagram.
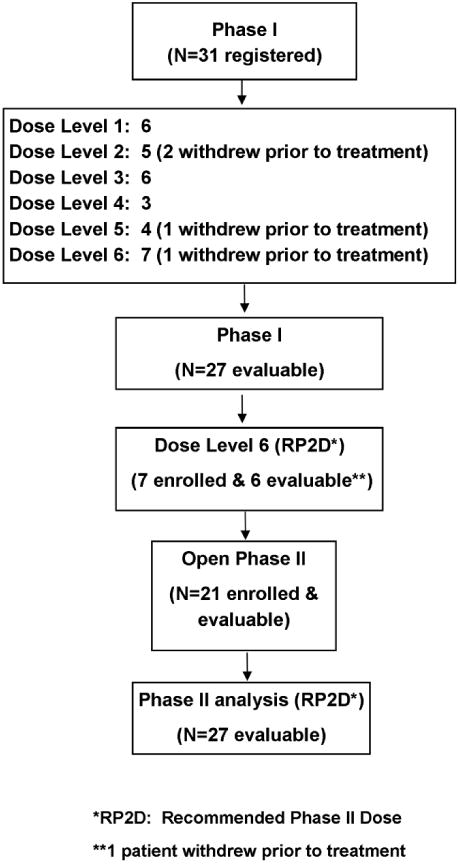
|
Table 2. Baseline Demographics.
| Phase 1 Dose Levels | Combined Phase | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 (N=6) | 2 (N=3) | 3 (N=6) | 4 (N=3) | 5 (N=3) | 61 (N=6) | Phase 1 (N=27) | Phase II RP2D2 (N=27) | |
| Age | ||||||||
| Median | 58.5 | 70.0 | 61.0 | 75.0 | 68.0 | 55.5 | 63.0 | 58.0 |
| Range | (55.0-78.0) | (68.0-76.0) | (55.0-68.0) | (45.0-78.0) | (63.0-70.0) | (47.0-65.0) | (45.0-78.0) | (43.0-79.0) |
|
| ||||||||
| Gender | ||||||||
| Female | 2 (33.3%) | 1 (33.3%) | 1 (16.7%) | 3 (100.0%) | 1 (33.3%) | 2 (33.3%) | 10 (37.0%) | 10 (37.0%) |
| Male | 4 (66.7%) | 2 (66.7%) | 5 (83.3%) | 0 (0.0%) | 2 (66.7%) | 4 (66.7%) | 17 (63.0%) | 17 (63.0%) |
|
| ||||||||
| Tumor Stage | ||||||||
| IIIA | 1 (16.7%) | 1 (33.3%) | 0 (0.0%) | 1 (33.3%) | 2 (66.7%) | 4 (66.7%) | 9 (33.3%) | 13 (48.1%) |
| IIIB | 4 (66.7%) | 1 (33.3%) | 6 (100.0%) | 2 (66.7%) | 1 (33.3%) | 0 (0.0%) | 14 (51.9%) | 11 (40.7%) |
| IIIB w/pleural effusion | 1 (16.7%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (33.3%) | 4 (14.8%) | 3 (11.1%) |
|
| ||||||||
| N-Stage | ||||||||
| N1 | 1 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 2 (7.7%) | 4 (14.8%) |
| N2 | 1 (20.0%) | 2 (66.7%) | 2 (33.3%) | 2 (66.7%) | 2 (66.7%) | 5 (83.3%) | 14 (53.8%) | 16 (59.3%) |
| N3 | 3 (60.0%) | 1 (33.3%) | 4 (66.7%) | 1 (33.3%) | 1 (33.3%) | 0 (0.0%) | 10 (38.5%) | 7 (25.9%) |
|
| ||||||||
| ECOG PS | ||||||||
| 0 | 5 (83.3%) | 1 (33.3%) | 3 (50.0%) | 2 (66.7%) | 2 (66.7%) | 2 (33.3%) | 15 (55.6%) | 9 (33.3%) |
| 1 | 1 (16.7%) | 2 (66.7%) | 3 (50.0%) | 1 (33.3%) | 1 (33.3%) | 4 (66.7%) | 12 (44.4%) | 18 (66.7%) |
|
| ||||||||
| Pre-Treatment Supraclavicular Involvement | ||||||||
| Yes | 3 (50.0%) | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 1 (33.3%) | 1 (16.7%) | 6 (22.2%) | 4 (14.8%) |
| No | 3 (50.0%) | 3 (100.0%) | 5 (83.3%) | 3 (100.0%) | 2 (66.7%) | 5 (83.3%) | 21 (77.8%) | 23 (85.2%) |
|
| ||||||||
| Maximum Pre-Treatment Tumor Size (CM) | ||||||||
| < 3 | 0 (0.0%) | 1 (33.3%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 3 (11.1%) | 4 (14.8%) |
| 3-6 | 4 (66.7%) | 2 (66.7%) | 1 (16.7%) | 3 (100.0%) | 2 (66.7%) | 3 (50.0%) | 15 (55.6%) | 15 (55.6%) |
| > 6 | 2 (33.3%) | 0 (0.0%) | 4 (66.7%) | 0 (0.0%) | 1 (33.3%) | 2 (33.3%) | 9 (33.3%) | 8 (29.6%) |
|
| ||||||||
| Weight Loss Past 3 Months | ||||||||
| <5% | 3 (50.0%) | 3 (100.0%) | 5 (83.3%) | 3 (100.0%) | 3 (100.0%) | 5 (83.3%) | 22 (81.5%) | 22 (81.5%) |
| 5-10% | 3 (50.0%) | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 5 (18.5%) | 5 (18.5%) |
|
| ||||||||
| Diabetes | ||||||||
| No Diabetes | 4 (66.7%) | 3 (100.0%) | 6 (100.0%) | 3 (100.0%) | 3 (100.0%) | 6 (100.0%) | 25 (92.6%) | 26 (96.3%) |
| Type II | 2 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (7.4%) | 1 (3.7%) |
Included in phase II analysis
The recommended phase II dose (RP2D) included 6 phase I patients enrolled at dose level 6 and 21 patients enrolled during the phase II portion only.
Phase II (RP2D)
Only 21 patients were enrolled to the phase II portion because the study was closed early due to slow accrual. Additionally, seven patients were enrolled at the recommended phase II dose (RP2D) during the phase I portion (dose level 6). These 28 patients were enrolled from 07/31/2008 to 01/14/2011. Of the 28 patients enrolled, 27 were deemed evaluable since one patient withdrew prior to receiving treatment (see Table 6 the CONSORT diagram for further details). There were 17 (63%) men and 10 (34%) women, with a median age of 58 years (range: 43 to 79). Approximately 48% of patients presented with stage IIIA disease, whereas 52% of patients had stage IIIB disease. Nine patients (33%) had a baseline ECOG PS of 0, and eighteen patients (67%) had an ECOG PS of 1(Table 2). All patients have ended active treatment. Approximately 67% (18/27) of patients completed the entire protocol treatment. Among the 9 patients who ended treatment early, 4 (44%) discontinued treatment due to adverse events, 2 (22%) discontinued treatment due to disease progression, 1(11%) patient refused further treatment, and the other 2 (22%) went off for other reasons.
Tolerability and Toxicity
Phase I
Twenty-seven patients were evaluable for adverse events across the six phase I dose levels (Table 3). Dose level 1 resulted in one of six patients experiencing a DLT (grade 3 pneumonitis requiring oxygen). Although no patients experienced a DLT per protocol for dose levels 2-5, we did enroll 6 patients at dose level 3 since one of the first 3 patients went off treatment early due to adverse events. Dose level 6 enrolled six evaluable patients with only one patient experiencing a DLT (grade 4 neutropenia lasting ≥8 days). Dose level 6 (bortezomib: 1.2 mg/m2; paclitaxel: 175 mg/m2; carboplatin: AUC of 6 for 2 cycles with concurrent radiation) was chosen as the RP2D since we went through all 6 dose levels without reaching the MTD. Overall, 20/27 patients (74%) experienced grade 3/4 adverse events (regardless of attribution), and 11/27 (41%) experienced grade 4 adverse events (Table 3). No grade 5 adverse events occurred. The most commonly occurring grade 3/4 adverse events (frequency, %) consisted of leukopenia (12, 44%), neutropenia (10, 37%), dyspnea (6, 22%), and dysphagia (3, 11%). All commonly occurring grade 3/4 adverse events (> 5%) are shown in Table 4, including all grade 4 adverse events.
Table 3. Adverse Event1 Summary.
| Phase 1 Dose Levels | Combined Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 (N=6) | 2 (N=3) | 3 (N=6) | 4 (N=3) | 5 (N=3) | 62 (N=6) | Phase 1 (N=27) | Phase II RP2D3,4 (N=27) | |
| DLT (%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 2 (7.4%) | 1 (3.7%) |
| Grade 3+ Overall | 5 (83.3%) | 3 (100.0%) | 4 (66.7%) | 2 (66.7%) | 2 (66.7%) | 4 (66.7%) | 20 (74.1%) | 22 (81.5%) |
| Grade 4+ Overall | 4 (66.7%) | 2 (66.7%) | 1 (16.7%) | 1 (33.3%) | 0 (0.0%) | 3 (50.0%) | 11 (40.7%) | 15 (55.6%) |
| Grade 3+ Heme | 3 (50.0%) | 1 (33.3%) | 2 (33.3%) | 2 (66.7%) | 2 (66.7%) | 4 (66.7%) | 14 (51.9%) | 22 (81.5%) |
| Grade 4 Heme | 2 (33.3%) | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 3 (50.0%) | 6 (22.2%) | 15 (55.6%) |
| Grade 3+ Non-Heme | 4 (66.7%) | 3 (100.0%) | 4 (66.7%) | 1 (33.3%) | 1 (33.3%) | 3 (50.0%) | 16 (59.3%) | 12 (44.4%) |
| Grade 4+ Non-Heme | 2 (33.3%) | 2 (66.7%) | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 5 (18.5%) | 2 (7.4%) |
Adverse events reported regardless of attribution
Included in phase II analysis
The recommended phase II dose (RP2D) included 6 phase I patients enrolled at dose level 6 and 21 patients enrolled during the phase II portion only.
One grade 5 pneumonitis possibly related to treatment occurred.
Table 4. Commonly Occurring (> 5%) Grade 3/4 Adverse Events1, Including All Grade 4 Adverse Events (By Phase of Study).
| Toxicity | Grade 3 N (%) | Grade 4 N (%) | |
|---|---|---|---|
| Phase 1 (N=27) | |||
| Hematologic | Leukocyte count decreased | 9 (33.3%) | 3 (11.1%) |
| Neutrophil count decreased | 4 (14.8%) | 6 (22.2%) | |
| Lymphocyte count decreased | 2 (7.4%) | 0 (0.0%) | |
| Platelet count decreased | 2 (7.4%) | 0 (0.0%) | |
| Non-Hematologic | Dyspnea | 6 (22.2%) | 0 (0.0%) |
| Dysphagia | 3 (11.1%) | 0 (0.0%) | |
| Fatigue | 2 (7.4%) | 0 (0.0%) | |
| Febrile neutropenia | 2 (7.4%) | 0 (0.0%) | |
| Nausea | 2 (7.4%) | 0 (0.0%) | |
| Pneumonitis | 2 (7.4%) | 0 (0.0%) | |
| Dehydration | 1 (3.7%) | 1 (3.7%) | |
| Hypotension | 1 (3.7%) | 1 (3.7%) | |
| Myocardial ischemia | 0 (0.0%) | 2 (7.4%) | |
| Thrombosis | 0 (0.0%) | 2 (7.4%) | |
| Cardiac troponin I increased | 0 (0.0%) | 1 (3.7%) | |
| Phase II RP2D (N=27) | |||
| Hematologic | Leukocyte count decreased | 13 (48.1%) | 4 (14.8%) |
| Neutrophil count decreased | 12 (44.4%) | 6 (22.2%) | |
| Platelet count decreased | 2 (7.4%) | 10 (37.0%) | |
| Hemoglobin decreased | 2 (7.4%) | 0 (0.0%) | |
| Lymphocyte count decreased | 0 (0.0%) | 1 (3.7%) | |
| Non-Hematologic | Fatigue | 6 (22.2%) | 0 (0.0%) |
| Nausea | 3 (11.1%) | 0 (0.0%) | |
| Dyspnea | 2 (7.4%) | 1 (3.7%) | |
| Anorexia | 2 (7.4%) | 0 (0.0%) | |
| Pneumonitis | 2 (7.4%) | 0 (0.0%) | |
| Syncope | 2 (7.4%) | 0 (0.0%) | |
| Depressed level of consciousness | 1 (3.7%) | 1 (3.7%) | |
| Serum sodium decreased | 1 (3.7%) | 1 (3.7%) | |
| Myalgia | 1 (3.7%) | 1 (3.7%) | |
| Serum potassium decreased | 0 (0.0%) | 1 (3.7%) | |
| Hypoxia | 0 (0.0%) | 1 (3.7%) | |
Adverse events reported regardless of attribution
Phase II(RP2D)
Twenty-seven patients were evaluable for adverse events in the phase II portion of the study (Tables 3-4). Although dose reductions were needed at times, most patients received the majority of the doses, as shown in table 5 for the patients enrolled at the recommended phase II dose. Twenty-two of these 27 patients (82%) experienced at least one grade 3 or worse adverse event (regardless of attribution). In addition, 15 (56%) patients experienced at least one grade 4 or worse adverse event, including one patient with a grade 5 adverse event (pneumonitis that was possibly related to treatment) (Table 3). The most commonly occurring grade 3 adverse events (frequency, %) consisted of leukopenia (13, 48%), neutropenia (12, 44%), fatigue (6, 22%), and nausea (3, 11%)(Table 4). The most commonly occurring grade 4 adverse events (frequency, %) were all hematologic and consisted of thrombocytopenia (10, 37%), neutropenia (6, 22%), and leukopenia (4, 15%)(Table4). Non-hematologic grade 4 adverse events (frequency, %) consisted of dyspnea (1, 4%), depressed level of consciousness (1, 4%), myalgia (1, 4%), serum sodium decrease (1, 4%), serum potassium decrease (1, 4%), and hypoxia (1, 4%). All commonly occurring grade 3/4 adverse events (> 5%) are shown in Table 4, including all grade 4 adverse events.
Table 5. Dose-intensity of chemotherapy and the radiotherapy received at the recommended phase II dose.
| Chemotherapy/RT | Cycle | N | % of patients who received full dose* | Median % of per protocol expected dose (range) |
|---|---|---|---|---|
| Bortezomib | 1 | 27 | 85% (23/27) | 99 (50 – 107) |
| 2 | 24 | 67% (16/24) | 98 (13 – 103) | |
| Paclitaxel | 1 | 27 | 100% (27/27) | 100 (99 – 102) |
| 2 | 24 | 79% (19/24) | 100 (50 – 103) | |
| Carboplatin | 1 | 27 | 100% (27/27) | 100 (100 – 100) |
| 2 | 24 | 83% (20/24) | 100 (0 - 100) | |
| RT** | 1-2** | 27 | 85% (23/27) | 100 (3 – 104) |
defined as receiving at least 95% of the expected dose.
The target dose was 6,000 cGy given in 30 daily (except weekends) fractions of 200 cGy each, starting on Day 1 for a total of 6 weeks (2 cycles). Most patients received the full RT dose and most received the 30 total fractions, as expected.
Response and Survival of Phase II portion(RP2D)
A per protocol interim analysis occurred after the first 26 evaluable patients were enrolled. Of these 26 patients, 23 (88%) survived at least 6 months (95% CI: 70 to 98%), which was enough to continue to full accrual per study design. However, due to slow accrual, the study was stopped after 27 evaluable patients were enrolled (6 - phase I RP2D; 21 - phase II). All 27 patients were evaluable for the outcome measures of survival, progression-free survival, and confirmed response. Of all 27 patients, 17(63%) have died, and the median follow-up time was 26.9 months (range: 5.7 to 56.7) for the 10 patients who were still alive. The 12-month survival rate was 73% (95% CI: 58 - 92%). The median survival (Figure 1) was 25.0 months (95% CI: 15.6 to 35.8 months), and the median progression-free survival (Figure 2) was 8.4 months (95% CI: 4.1 to 10.5 months). The confirmed response rate was 26% (7/27; 95% CI: 11% to 46%), which consisted of 4 partial responses (15%) and 3 complete responses (11%). Clinical Benefit Rate (stable disease (10 patients) + confirmed responses (7 patients)) was 63% (95% CI: 42 to 81%).
Figure 1.

Overall Survival (OS) of all patients treated on the RP2D.
Figure 2.

Progression-free survival (PFS) of all patients treated on the RP2D.
Discussion
When administered concurrently with thoracic radiation, chemotherapy can sensitize tumor cells to radiation, and ultimately improve the local control and survival in advanced non-metastatic NSCLC. However, despite the successful application of chemoradiotherapy, the survival rate remains dismal. Novel targeting therapeutic agents, including bevacizumab, erlotinib, gefitinib, and cetuximab, have been tested in combination with thoracic radiation in clinical studies [28-32]. The incorporation of the proteasome inhibitor bortezomib has the presumed advantages of disrupting signal transduction pathways that are responsible for radio-resistance, such as activation of NF-κB [33], loss of p53 [34, 35] and overexpression of BCL-2 [36], and promoting radiation sensitivity by accumulating cells in the radiosensitive G2 and M phases [37, 38], therefore representing a promising strategy. In spite of early termination of the study due to slow accrual, a promising 12-month survival of 73% and an impressive median OS of about 25 months were observed in this small study, which appears to be much higher than the overall survival reported with other chemotherapy regimens [5, 39, 40]. In two phase II studies evaluating weekly carboplatin/paclitaxel and concurrent thoracic radiotherapy (regular fractions or hyperfractionated) followed by two cycles of consolidative carboplatin/paclitaxel chemotherapy, LUN-56 and LUN-63, the median length of overall survival was 17.4 and 14 months, respectively [41], and the 12-month survival rates were 56% and 61%, respectively [42]. When compared with historical data, our results suggested a potential survival benefit associated with the addition of bortezomib and this approach warrants further investigation.
When compared with other concurrent chemotherapy regimens, a much higher rate of grade 3 (82%) and 4 (56%) hematological adverse events were observed in the phase II portion of this study [5, 8, 43]. Since hematological toxicities, especially thrombocytopenia, are common toxicities of bortezomib [44], the significant bone marrow suppression observed with this regimen was not unexpected. However, the overlapping toxicity profile of bortezomib and cytotoxic chemotherapy may render this regimen less tolerable and limit its usage.
Despite the success in the treatment of hematological malignancies, the activity of bortezomib in solid tumors, including NSCLC, overall has been less encouraging. Identification of patients who will likely gain benefit from the addition of bortezomib is essential. Moreover, since increased toxicities were observed with this regimen in comparison with historical data, proper selection of patients will be particularly important in order to avoid unnecessary exposure to toxicities. In mantle cell lymphoma, patients with tumors that had low level proteasome (prosome, macropain) subunit, alpha type 5 (PSMA5) expression or higher NF-κB p65 expression appeared more likely to benefit from the addition of bortezomib as reported in the PINNACLE study, a phase II study comparing rituximab with and without bortezomib [45]. In relapsed/refractory follicular lymphoma, the presence of PSMB1 P11A (G allele) and low CD68 expression (≤50 CD68-positive cells) were found to be associated with improved outcome in patients treated with bortezomib–rituximab versus rituximab [46]. Future studies need to be conducted to identify predictive biomarkers for bortezomib in NSCLC in order to identify the suitable candidate for treatment with bortezomib.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group, Alliance and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-35113, CA-35195, CA-35431, and CA-35119. The study was also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health
Conflicts of Interest and Source of Funding: Jeffrey P. Meyers B.A. received NCI grant for the submitted work, Donald W. Northfelt M.D. received Grant support for the submitted work, James D. Bearden, III, M.D. received Upstate Carolina CCOP grant for the submitted work, Sumithra J. Mandrekar Ph.D received NCCTG NIH grant for the submitted work, Steven E. Schild M.D.received royalties for author of the lung cancer section of UpTo Date®, David B. Johnson M.D.received support for travel from Wichita Community Clinical Oncology Program. For the remaining authors none were declared.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Strauss GM, et al. Multimodality treatment of stage IIIA non-small-cell lung carcinoma: a critical review of the literature and strategies for future research. J Clin Oncol. 1992;10(5):829–38. doi: 10.1200/JCO.1992.10.5.829. [DOI] [PubMed] [Google Scholar]
- 3.Pfister DG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22(2):330–53. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 4.Furuse K, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17(9):2692–9. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 5.Curran WJ, Jr, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–60. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belani CP, et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J Clin Oncol. 2005;23(16):3760–7. doi: 10.1200/JCO.2005.09.108. [DOI] [PubMed] [Google Scholar]
- 7.Choy H, et al. Paclitaxel plus carboplatin and concurrent radiation therapy for patients with locally advanced non-small cell lung cancer. Semin Oncol. 1996;23(6 Suppl 16):117–9. [PubMed] [Google Scholar]
- 8.Vokes EE, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25(13):1698–704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 9.Gandara DR, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21(10):2004–10. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 10.Albain KS, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20(16):3454–60. doi: 10.1200/JCO.2002.03.055. [DOI] [PubMed] [Google Scholar]
- 11.Edelman MJ. The potential role of bortezomib in combination with chemotherapy and radiation in non-small-cell lung cancer. Clin Lung Cancer. 2005;7 Suppl 2:S64–6. doi: 10.3816/clc.2005.s.011. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, et al. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11(3):239–53. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voorhees PM, Orlowski RZ. The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol. 2006;46:189–213. doi: 10.1146/annurev.pharmtox.46.120604.141300. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar SV, et al. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23(3):630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Fanucchi MP, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24(31):5025–33. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 16.Hoang T, et al. Vorinostat and bortezomib as third-line therapy in patients with advanced non-small cell lung cancer: a Wisconsin Oncology Network Phase II study. Invest New Drugs. 2014;32(1):195–9. doi: 10.1007/s10637-013-9980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch TJ, et al. A randomized phase 2 study of erlotinib alone and in combination with bortezomib in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(8):1002–9. doi: 10.1097/JTO.0b013e3181aba89f. [DOI] [PubMed] [Google Scholar]
- 18.Scagliotti GV, et al. A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer. 2010;68(3):420–6. doi: 10.1016/j.lungcan.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Lara PN, Jr, et al. Randomized phase II trial of concurrent versus sequential bortezomib plus docetaxel in advanced non-small-cell lung cancer: a California cancer consortium trial. Clin Lung Cancer. 2011;12(1):33–7. doi: 10.3816/CLC.2011.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piperdi B, et al. Phase-I/II study of bortezomib in combination with carboplatin and bevacizumab as first-line therapy in patients with advanced non-small-cell lung cancer. J Thorac Oncol. 2012;7(6):1032–40. doi: 10.1097/JTO.0b013e31824de2fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies AM, et al. Bortezomib plus gemcitabine/carboplatin as first-line treatment of advanced non-small cell lung cancer: a phase II Southwest Oncology Group Study (S0339) J Thorac Oncol. 2009;4(1):87–92. doi: 10.1097/JTO.0b013e3181915052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma C, et al. A phase I and pharmacologic study of sequences of the proteasome inhibitor, bortezomib (PS-341, Velcade), in combination with paclitaxel and carboplatin in patients with advanced malignancies. Cancer Chemother Pharmacol. 2007;59(2):207–15. doi: 10.1007/s00280-006-0259-9. [DOI] [PubMed] [Google Scholar]
- 23.Russo SM, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys. 2001;50(1):183–93. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 24.Edelman MJ, et al. Phase I trial of carboplatin/paclitaxel/bortezomib and concurrent radiotherapy followed by surgical resection in Stage III non-small cell lung cancer. Lung Cancer. 2010;68(1):84–8. doi: 10.1016/j.lungcan.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45(3):925–37. [PubMed] [Google Scholar]
- 27.Kaplan ELM, Paul Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;25(3):17–24. [Google Scholar]
- 28.van den Heuvel MM, et al. Additional weekly Cetuximab to concurrent chemoradiotherapy in locally advanced non-small cell lung carcinoma: Efficacy and safety outcomes of a randomized, multi-center phase II study investigating. Radiother Oncol. 2013 doi: 10.1016/j.radonc.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Lind JS, Senan S, Smit EF. Pulmonary toxicity after bevacizumab and concurrent thoracic radiotherapy observed in a phase I study for inoperable stage III non-small-cell lung cancer. J Clin Oncol. 2012;30(8):e104–8. doi: 10.1200/JCO.2011.38.4552. [DOI] [PubMed] [Google Scholar]
- 30.Center B, et al. A phase I study of gefitinib with concurrent dose-escalated weekly docetaxel and conformal three-dimensional thoracic radiation followed by consolidative docetaxel and maintenance gefitinib for patients with stage III non-small cell lung cancer. J Thorac Oncol. 2010;5(1):69–74. doi: 10.1097/JTO.0b013e3181c59a0e. [DOI] [PubMed] [Google Scholar]
- 31.Jensen AD, et al. Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity-modulated RT radiotherapy and cetuximab: the NEAR trial. Cancer. 2011;117(13):2986–94. doi: 10.1002/cncr.25888. [DOI] [PubMed] [Google Scholar]
- 32.Hughes S, et al. A brief report on the safety study of induction chemotherapy followed by synchronous radiotherapy and cetuximab in stage III non-small cell lung cancer (NSCLC): SCRATCH study. J Thorac Oncol. 2008;3(6):648–51. doi: 10.1097/JTO.0b013e3181757a60. [DOI] [PubMed] [Google Scholar]
- 33.Wang CY, et al. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5(4):412–7. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 34.Cuddihy AR, Bristow RG. The p53 protein family and radiation sensitivity: Yes or no? Cancer Metastasis Rev. 2004;23(3-4):237–57. doi: 10.1023/B:CANC.0000031764.81141.e4. [DOI] [PubMed] [Google Scholar]
- 35.Clarke AR, et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362(6423):849–52. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 36.Reed JC, et al. BCL -2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996;60(1):23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Terasima T, Tolmach LJ. Changes in x-ray sensitivity of HeLa cells during the division cycle. Nature. 1961;190:1210–11. doi: 10.1038/1901210a0. [DOI] [PubMed] [Google Scholar]
- 38.Sinclair WK, Morton RA. X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. 1966. Radiat Res. 2012;178(2):AV88–101. doi: 10.1667/rrav07.1. [DOI] [PubMed] [Google Scholar]
- 39.Le Chevalier T, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991;83(6):417–23. doi: 10.1093/jnci/83.6.417. [DOI] [PubMed] [Google Scholar]
- 40.Sause WT, et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst. 1995;87(3):198–205. doi: 10.1093/jnci/87.3.198. [DOI] [PubMed] [Google Scholar]
- 41.Kim DW, et al. Long term follow up and analysis of long term survivors in patients treated with paclitaxel-based concurrent chemo/radiation therapy for locally advanced non-small cell lung cancer. Lung Cancer. 2005;50(2):235–45. doi: 10.1016/j.lungcan.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Choy H, Akerley W, DeVore RF., 3rd Concurrent paclitaxel, carboplatin, and radiation therapy for locally advanced non-small cell lung cancer. Semin Oncol. 1999;26(1 Suppl 2):36–43. [PubMed] [Google Scholar]
- 43.Govindan R, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol. 2011;29(23):3120–5. doi: 10.1200/JCO.2010.33.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson PG, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 45.Goy A, et al. Potential biomarkers of bortezomib activity in mantle cell lymphoma from the phase 2 PINNACLE trial. Leuk Lymphoma. 2010;51(7):1269–77. doi: 10.3109/10428194.2010.483302. [DOI] [PubMed] [Google Scholar]
- 46.Coiffier B, et al. Prespecified candidate biomarkers identify follicular lymphoma patients who achieved longer progression-free survival with bortezomib-rituximab versus rituximab. Clin Cancer Res. 2013;19(9):2551–61. doi: 10.1158/1078-0432.CCR-12-3069. [DOI] [PubMed] [Google Scholar]


