Abstract
Introduction
Patients with stage III non-small cell lung cancer (NSCLC) and poor performance status (PS) and/or weight loss (WL) do not seem to benefit from standard therapy. Based on the pre-clinical interaction between epidermal growth factor receptor (EGFR) inhibitors and radiation, we designed a trial of induction chemotherapy followed by thoracic radiotherapy (TRT) and concurrent erlotinib.
Methods
Patients with poor risk unresectable stage III NSCLC received 2 cycles of carboplatin at an AUC of 5 and nab-paclitaxel at 100 mg/m2 on days 1 and 8 every 21 days, followed by erlotinib administered concurrently with TRT. Maintenance was not permitted. Molecular analysis was performed in available specimens. Seventy-two eligible patients were required to test whether the 1-year survival rate was <50% or ≥65% with approximately 90% power at a significance level of 0.10.
Results
From March 2008 to October 2011, 78patients were enrolled, 3 of which were ineligible. The median age was 68 (range, 39 to 88) and 32% were ≥75 years of age. Patients were evenly distributed between stage IIIA and IIIB and the majority had PS 2. The overall response rate was 67% and the disease control rate was 93%. Treatment was well tolerated. The median PFS and OS were 11 and 17 months, respectively. The overall 12-month OS was 57%, which narrowly missed the pre-specified target for significance.
Conclusions
Patients with poor risk stage III NSCLC had better than expected outcomes with a regimen of induction carboplatin/nab-paclitaxel followed by TRT and erlotinib. However, as per the statistical design, the 12-month OS was not sufficiently high to warrant further studies.
INTRODUCTION
Patients with locally advanced non-small cell lung cancer (NSCLC) treated with concurrent chemotherapy and thoracic radiotherapy (TRT) have a 20% to 25% probability of long-term disease-free survival (1). However, patients with adverse prognostic features, such as poor performance status (PS) and/or significant weight loss (WL), which represent a sizable percentage of patients, have a worse prognosis and do not seem to benefit from the standard approach (2). No specific treatment guidelines exist for this subset and management options in clinical practice range from palliative radiotherapy to sequential treatment or an attenuated concurrent approach.
Building on the pre-clinical rationale that inhibitors of the epidermal growth factor receptor (EGFR) are strong radiation sensitizers (3), the Cancer and Leukemia Group B (CALGB 30106) published a trial in which a subset of 21 patients with locally advanced disease and poor PS were treated with induction chemotherapy followed by gefitinib, an EGFR tyrosine kinase inhibitor (TKI), administered concomitantly with TRT (4). The median survival was an unprecedented 19 months, which could not be accounted for by the few patients with EGFR mutated tumors.
Based on this experience, we designed a phase II trial of two cycles of induction chemotherapy with carboplatin and nab-paclitaxel followed by TRT and concurrent erlotinib for patients with stage IIIA/B NSCLC and poor risk features. Molecular evaluation for EGFR mutations was performed in available tumor specimens. The trial was conducted by the former CALGB, now Alliance for Clinical Trials in Oncology, and the former Radiation Therapy Oncology Group (RTOG), now NRG Oncology.
PATIENTS AND METHODS
Patients with histologically or cytologically documented NSCLC, with unresectable stage IIIA or IIIB by the AJCC version 6 staging system (T1-3 N2; T4 N0-2; and N3 patients except for contralateral hilar or supraclavicular involvement), were eligible if they had either PS 2 or PS 0-1 and ≥10% weight loss within 3 months prior to enrollment. A formal distinction between a PS 2 on the basis of cancer-related impairment versus pre-existing co-morbidities was not made but the ECOG scale, which was used for eligibility purposes, implies the latter. Prior chemotherapy, radiotherapy, or targeted therapy was not permitted. Measurable disease was required, as was normal renal, liver, and bone marrow function. A PET scan was encouraged but not mandated. Evaluation by a medical and a radiation oncologist prior to study enrollment was required. Participation in the correlative molecular component had to be offered but patients could opt out.
Patients received two cycles of induction chemotherapy with carboplatin and nab-paclitaxel. The first 17 patients were treated, respectively, with an AUC of 6 and 100 mg/m2 on days 1, 8, and 15 every 28 days. A preliminary toxicity analysis showed high rates of grade 3 (29%) and grade 4 (18%) neutropenia, which led to several day 15 omissions, and prompted a protocol modification to carboplatin to an AUC of 5 and the nab-paclitaxel to be administered on days 1 and 8 every 21 days (same doses). Erlotinib at a dose of 150 mg daily was administered from day 1 of TRT until its completion.
Radiation started on week 7, assuming no evidence of progressive disease and recovery from chemotherapy-induced toxicities. Radiation was delivered at 2Gy/day 5 days/week for 33 fractions and a total dose of 66Gy. Radiation planning was based on post-induction scans but originally involved lymph node regions were included in the treatment volume. There was no elective nodal irradiation. All patients were treated with 3-dimensional conformal radiotherapy; IMRT was not allowed. The use of systems to control or compensate for respiratory motion was permitted. Quality assurance was performed by the Quality Assurance Review Center (QARC), and the Chair of the Alliance RT committee. Response was evaluated after induction therapy (8 weeks), after concurrent therapy (16 weeks), and then every 3 months for 1 year and every 6 months until relapse. Patients with progressive disease outside the chest after induction chemotherapy were removed from protocol therapy. Patients with progression of intrathoracic disease within the potential radiation field were considered for protocol therapy after consultation with the study chairs.
The primary objective of this phase II trial was overall survival (OS) at 12 months. Secondary objectives included response rate, progression-free survival (PFS), and correlation of tumor biomarkers with clinical outcomes. Overall survival was defined as the time from registration to death of any cause. Progression-free survival was defined as the time from registration to disease progression or death of any cause, whichever came first. Treatment was deemed a “success” if the patient remained alive for at least 12 months. With 72 eligible patients, the trial was designed to test the null hypothesis that the treatment success rate was less than 50% against the alternative hypothesis that the treatment success rate was greater than 65% at a one-sided Type I error of 0.10 and 90% power. A two-stage phase II design was used to allow early stopping for futility: if less than 19 of the first 40 eligible patients were alive at 12 months, the trial would be stopped. Otherwise, it would proceed to full accrual. If 42 or more “successes” were observed, corresponding to a 12-month survival of 58.3%, further investigation of this regimen would be warranted. For secondary analyses, OS and PFS were estimated with the Kaplan-Meier method. Subgroup analyzes based on PS (0,1 vs 2) and stage (IIIA vs. IIIB) were displayed by Kaplan-Meier curves and tested by log rank tests.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
RESULTS
The study was activated in March 2008 and closed in October 2011. A total of 78 patients were registered, of which 3 patients did not receive protocol treatment and were therefore excluded from the analysis. Median age was 68 years (range, 39 to 88); 32% of the patients were 75 years of age or older and another 41% were between 65 and 74 years of age. Patients were evenly distributed between stage IIIA (51%) and IIIB (49%). The majority of patients had PS 2 (64%). and 55% of patients had a baseline PET scan. The demographic data are shown in Table 1.
Table 1.
Patient Characteristics (N = 75)
| Characteristic | |
|---|---|
| Age (median, range) | 68 (39,88) |
| N (%) | |
| Age | |
| <65 | 20 (27%) |
| 65–74 | 31 (41%) |
| ≥75 | 24 (32%) |
| Gender | |
| Male | 44 (59%) |
| Female | 31 (41%) |
| Stage | |
| IIIA | 38 (51%) |
| IIIB | 37 (49%) |
| Poor risk | |
| PS=0,1 and WL≥10% | 27 (36%) |
| PS= 2 | 48 (64%) |
Protocol therapy was completed by 80% of patients. Progressive disease (5%) and adverse events (5%) were the two most common reasons for lack of completion. Response data included 8% complete response (CR); 59% partial response (PR); 27% stable disease (SD); and 7% progressive disease (PD). The disease control rate (CR+PR+SD) was 93%. Of the 39 patients who relapsed, 16 had local relapse only; 12 had distant relapse only; and 11 had both local and distant relapse.
Overall, the toxicity results demonstrate the feasibility of this approach (Table 2). After the amendment, the frequency of grade 3–4 neutropenia decreased to 7% and 3%, respectively. There was only one episode of documented febrile neutropenia. Grade 3 anemia was seen in 10% of patients. Grade 3 esophagitis was observed in 5% of patients and pneumonitis in 1% of the patients.
Table 2.
Treatment-Related Grade 3–4 Adverse Events
| Grade 3 N (%) | Grade 4 N (%) | |
|---|---|---|
| Anemia | 6 (8) | 1 (1) |
| Neutropenia | 9 (12) | 5 (7) |
| Thrombocytopenia | 3 (4) | 0 |
| Fatigue | 12 (1) | 1 (1) |
| Skin Rash | 3 (4) | 0 |
| Nausea/Vomiting | 3 (4) | 0 |
| Diarrhea | 7 (9) | 0 |
| Esophagitis | 4 (5) | 0 |
| Pulmonary | 1 (1) | 0 |
| Infection | 1 (1) | 0 |
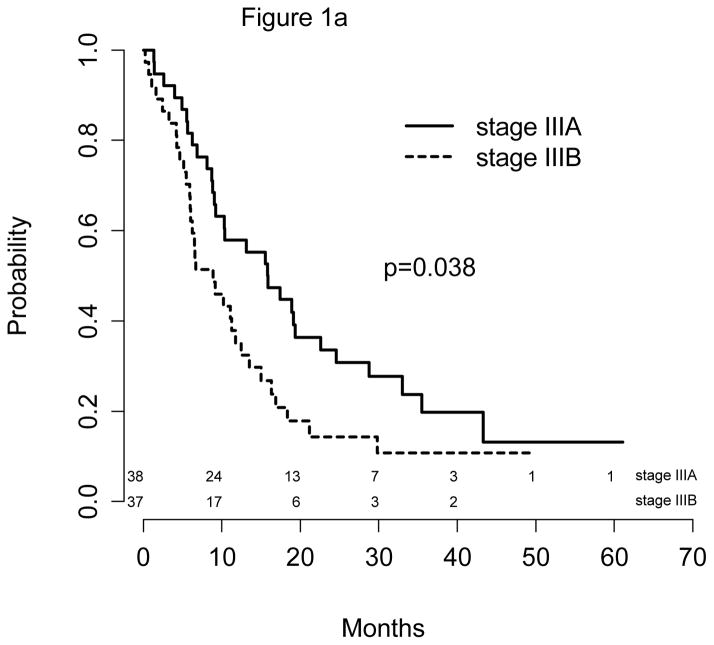
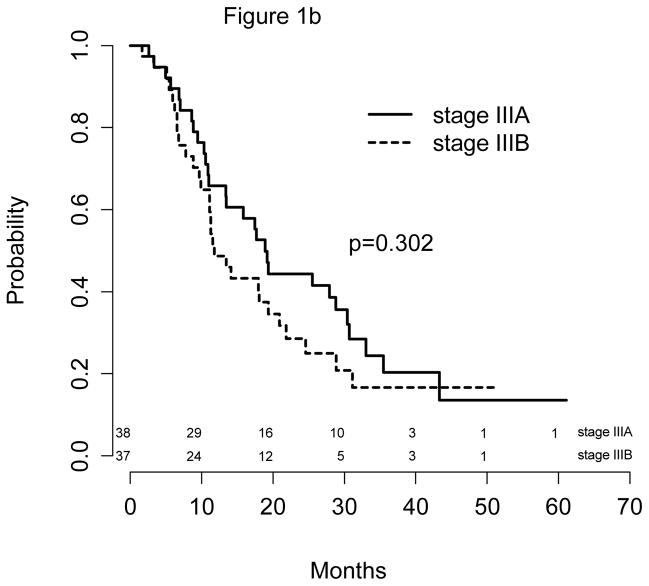
The median follow-up time was 40 months (Table 3). All 75 patients were followed for more than 12 months. The median PFS was 11 months (9, 16 months), and the median survival was 17 months (11, 22 months) for the entire population. Respective 12-month PFS and OS were 47% (95% CI: 37%, 59%) and 57% (95% CI: 47%, 70%). Outcomes by treatment strata by stage (Figure 1a) showed that patients with IIIA disease had a significantly better PFS than stage IIIB patients: 16 vs. 9 months (p=0.038); the respective median survival times were 19 vs. 12 months (p=0.302).
Table 3.
Efficacy Results (N = 75)
| Response | |
| Complete response | 6 (8%) |
| Partial response | 44 (59%) |
| Stable disease | 20 (27%) |
| Progressive disease | 5 (7%) |
| ORR | 67% |
| Progression-free survival (PFS) | |
| Median | 11 months |
| 12-month | 47% |
| Overall survival (OS) | |
| Median | 17 months |
| 12-month | 57% |
| Stage IIIA vs. IIIB | |
| Median PFS | 16 vs. 9 months |
| Median OS | 19 vs.12 months |
| PS 0-1 + WL vs. PS 2 | |
| Median PFS | 16 vs.10 months |
| Median OS | 19 vs.13 months |
Figure 1.
Figure 1a: PFS by Stage
Figure 1b: OS by Stage
Molecular data were available for 31 patients (42% of the eligible patients). Eleven of the samples contained between 1% and 25% of tumor cells, which may have compromised the results. No patients with EGFR mutation were identified; two patients had tumors with KRAS mutations.
DISCUSSION
Our study is the largest cooperative group experience in poor risk patients with stage III NSCLC. Furthermore, our study is the first to incorporate an EGFR TKI in the combined modality therapy (CMT) of locally advanced NSCLC, along with a molecular evaluation of available tumor specimens.
Treatment was well tolerated despite the poor risk features and the advanced age of the population. After the initial dose and schedule adjustment, the combination of carboplatin and nab-paclitaxel proved to be quite tolerable, with no significant hematologic complications. The same applies to the addition of erlotinib to TRT, which did not lead to an increase in esophagitis and/or pneumonitis. Erlotinib was not continued after definitive therapy based on the unfavorable outcomes with gefitinib maintenance after CMT observed in the SWOG 0203 study (5).
While the study results exceed the expected survival for this patient subset, the pre-specified target for significance was narrowly missed. It can be argued that our target was unrealistic based on the available literature. In other words, the survival observed in the 21 poor risk patients in the CALGB 30106 trial (4), which guided our statistical design, may not be reproducible in a larger sample size. Therefore, despite achieving remarkable outcomes for poor risk patients with locally advanced NSCLC, we cannot conclusively reject the null hypothesis, i.e., that the addition of erlotinib to TRT provides no significant benefit over TRT alone after induction chemotherapy in patients not selected by molecular criteria.
Our finding of no EGFR-mutated tumor in the study population is unusual, as the presence of this molecular alteration is expected in approximately 10% to 15% of patients in the US. This underscores the fact that our results reflect a truly unselected study population, Furthermore, the lack of a subset of patients with EGFR mutated tumors in our study prevents any hypothesis about the validity of this approach in selected patients.
Investigators at M.D. Anderson completed a phase II trial of chemotherapy intercalated with erlotinib and concurrent TRT in stage III NSCLC patients with PS 0–1 (6). Forty-six evaluable patients received carboplatin AUC=2 and paclitaxel 45 mg/m² administered every Monday and erlotinib 150 mg orally on Tuesday–Sunday for 7 weeks throughout TRT, followed by two cycles of consolidation carboplatin-paclitaxel. Median time to progression, the primary endpoint, was 13.6 months. Toxicity was acceptable and outcomes did not differ according to EGFR status (4 of the 41 patients tested had EGFR mutated tumors). The investigators concluded that this approach, while effective, did not lead to survival outcomes that justified pursuing it further.
Other trials of EGFR inhibitors given concurrent with TRT in locally advanced disease have been recently reported. RTOG 0617 was a large phase III trial with a 2×2 factorial design, which included an evaluation of cetuximab, an EGFR-directed monoclonal antibody, in combination with chemotherapy. The results showed no advantage for the addition of cetuximab (7).
The National Cancer Institute has recently approved a large phase III cooperative group trial of a molecular-based approach in stage III NSCLC, in which erlotinib or crizotinib is given as a single agent for 3 months in patients with EGFR mutations or ALK rearrangements, respectively, followed by concurrent chemotherapy and TRT (8). This study will determine the value of targeted agents in stage III patients with actionable mutations. In our trial, the results of the molecular component showed no EGFR mutations, which impeded clinical correlation.
Efforts to investigate poor risk patients with locally advanced NSCLC remain meager despite the unmet need. These patients are treated with a variety of approaches in clinical practice and may at times receive substandard therapy, which leads to worse outcomes and, in a circular argument, reinforces the bias that treatment is ineffective. However, at this time, it is not obvious which research venue to pursue to test more appropriate treatments in this patient subset.
In conclusion, a strategy of induction chemotherapy with a well tolerated combination regimen, followed by definitive TRT and concomitant erlotinib, yielded favorable results but failed to reach a pre-specified level of statistical significance. New strategies are required to improve the outcome of poor prognosis patient with locally advanced NSCLC.
Acknowledgments
Source of Funding: Related to the submitted work Dr. Jänne received compensation for consultancy for drug development from Genentech and post marketing royalties from a DCFI owned patent on EGFR mutations. Outside of the submitted work Dr. Jänne received compensation for consultancy with the following entities: Boehringer-Ingelheim, Astra Zeneca, Clovis Oncology, Merrimack Pharmaceuticals, Pfizer, and Chugai Pharmaceuticals. Dr. Jeffrey Bradley has an institutional ViewRay clinical trial grant and a pending NIH RO-1 application. Dr. Bradley was also an invited speaker at the 2014 Brazilian National Radiotherapy Congress. Speaker travel expenses were covered by Varian Medical, Inc. Dr. Vokes received compensation outside of the submitted work for consultancy with the following entities: Amgen, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, Celgene, Clovis, Eisei, Merck, Synta, and VentiRx.
The research for CALGB 30605 (Alliance) was supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair), the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601), the Quality Assurance Review Center (Thomas FitzGerald M.D., CA 29511), and RTOG (CA 21661 and CA 37422). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Christiana Care Health Services, Inc. CCOP, Wilmington, DE, Stephen Grubbs, M.D., supported by CA45418
Cancer Centers of the Carolinas, Greenville, SC, Jeffrey K. Giguere, M.D., supported by CA29165
Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, M.D., Ph.D., supported by CA32291
Duke University Medical Center, Durham, NC, Jeffrey Crawford, M.D., supported by CA47577
Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO, Rakesh Gaur, M.D.
Missouri Valley Consortium-CCOP, Omaha, NE, Gamini S. Soori, M.D.
Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz, M.D., supported by CA45564
Nevada Cancer Research Foundation CCOP, Las Vegas, NV, John A. Ellerton, M.D., supported by CA35421
New Hampshire Oncology-Hematology PA, Concord, NH, Douglas J. Weckstein, M.D.
Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, M.D., supported by CA77658
Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, M.D., supported by CA59518
Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC, James N. Atkins, M.D., supported by CA45808
State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, M.D., supported by CA21060
University of Chicago, Chicago, IL, Hedy L. Kindler, M.D., supported by CA41287
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO, Karl E. Freter, M.D., supported by CA12046
University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, M.D., supported by CA47559
Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, M.D., supported by CA03927
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
References
- 1.Salama JK, Vokes EE. New radiotherapy and chemoradiotherapy approaches for non-small cell lung cancer. J Clin Oncol. 2013;31:1029–1038. doi: 10.1200/JCO.2012.44.5064. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe TE, Hodgson L, Herndon JE, et al. Treatment outcomes of different prognostic groups on Cancer and Leukemia Group B trial 39801: Induction chemotherapy followed by chemoradiotherapy compared to chemoradiotherapy alone for unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2009;4:1117–1125. doi: 10.1097/JTO.0b013e3181b27b33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raben D, Bunn P. Biologically Targeted Therapies Plus Chemotherapy Plus Radiotherapy in Stage III Non–Small-Cell Lung Cancer: A Case of the Icarus Syndrome? J Clin Oncol. 2012;30:3009–3012. doi: 10.1200/JCO.2012.43.1866. [DOI] [PubMed] [Google Scholar]
- 4.Ready NE, Janne PA, Bogart J, et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: cancer and leukemia group B (CALGB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol. 2010;5:1382–1390. doi: 10.1097/JTO.0b013e3181eba657. [DOI] [PubMed] [Google Scholar]
- 5.Kelly K, Chansky K, Gaspar LE, et al. Phase III Trial of Maintenance Gefitinib or Placebo After Concurrent Chemoradiotherapy and Docetaxel Consolidation in Inoperable Stage III Non–Small-Cell Lung Cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 6.Komaki R, Allen P, Wei X, et al. Value of adding erlotinib to thoracic radiation therapy with chemotherapy for stage III non small cell lung cancer: a prospective phase II study. J Thorac Oncol. 2013;8(suppl 2):abstract O02.03. [PubMed] [Google Scholar]
- 7.Bradley J, Masters G, Hu C, et al. An intergroup randomized phase III comparison of standard dose (60Gy) versus high dose (74Gy) chemoradiotherapy +/− cetuximab for stage III non-small cell lung cancer: results on cetuximab from RTOG 0617. J Thorac Oncol. 2013;8(suppl 2):Abstract PL03.05. [Google Scholar]
- 8. [Accessed December 17th, 2013];Erlotinib Hydrochloride or Crizotinib and Chemoradiation Therapy in Treating Patients With Stage III Non-Small Cell Lung Cancer. http://clinicaltrials.gov. NCT01822496.