Abstract
Severe symptoms of cerebral and cardiorenal vascular diseases can be triggered when cerebral, coronary, or glomerular arterioles grow inappropriately as a result of abnormal cell proliferation. The risk factor(s) and molecular mechanisms responsible for microvascular lesion formation are largely unknown. Although controversial, both animal and epidemiological studies have shown that estrogen increases the risk of stroke which may be due to microvascular lesions. Since microvascular diseases are characterized by excessive vessel growth, it is plausible that estrogen-induced neovascularization contributes to the growth of microvascular lesions. We present evidence for how ID3 overexpression in endothelial cells contributes to the development of an estrogen-induced neovascular phenotype with an additional focus on Pyk2 kinase. Our data showed that ID3 overexpression increased neovascularization, cell migration, and spheroid growth of human cerebral microvascular endothelial cells, hCMEC/D3. ID3 overexpressing cells showed significant estrogen-induced G2/M phase transition. Estrogen treatment increased both ID3 phosphorylation and total protein that was inhibited by tamoxifen; and Pyk2 mediated estrogen-induced ID3 mRNA expression. These findings suggest that Pyk2 signals ID3 expression and ID3 is necessary for estrogen-induced neovascularization in hCMEC/D3 cells. A better understanding of how microvascular lesions depend on ID3 may open new avenues for prevention and treatment of neurological diseases.
Introduction
Microvascular disease (small vessel disease or arteriolosclerosis) usually involves proliferation of both endothelial and smooth muscle cells in small arterioles leading to luminal narrowing, and the disease manifests either from the consequence of a restriction in blood supply to tissues, or altered flows and pressures to the distal vascular bed (Kanbay et al. 2011). While arteriolosclerosis can involve multiple organs, there is an increased presence in brain, kidney, and lungs because they are highly vascularized organs. Interestingly, the microvasculature which represents the largest fraction of the total vascular cross-sectional area remains under investigated (Aird 2005). In healthy adults, vessels are quiescent, with <0.5% of endothelial cells (ECs) dividing, however, severe symptoms of neurological, renal, and cardiovascular dysfunction can be triggered when vessels grow inappropriately as vascular lesions (Storkebaum et al. 2011). Thus, microvascular ECs may hold important clues in the study of vascular-driven neurological, renal, and cardiovascular disorders. Risk factors and possible molecular mechanisms responsible for the development of brain microvascular lesions remain incompletely understood. For example, cerebral cavernous malformation (CCM) is a type of microvascular lesion found in the brain which account for 10–15% of all CNS vascular abnormalities (Leblanc et al. 2009; Riant et al. 2010). CCMs are prone to bleeding and lined by multi-layers of hyper-proliferative ECs with a weak blood-brain barrier. Although controversial some researchers have postulated that the increased level of estrogen during pregnancy causes changes to vessel walls of cavernous malformations that make them more susceptible to bleeding. Similarly, the use of estrogen has been implicated with increased risk of stroke, particularly in older women (Heiss et al. 2008). Estrogen has been reported to promote microvascular lesion in stroke-prone spontaneously hypertensive female rats (Stier, Jr. et al. 2003). Chronic 17β-estradiol treatment has been shown to produce ischemic lesions in stroke model of rat (Theodorsson and Theodorsson, E.2005). Furthermore, several epidemiological studies have shown that women taking hormone replacement therapy had increased risk for neurological lesions in stroke (Anderson et al. 2004; Grady et al. 2002; Rossouw et al. 2002; Viscoli et al. 2001). These increased risks may be due to the underlying presence of microvascular disease. It is known that cerebral vessels are directly targeted by estrogen, as they express estrogen receptors (ERs) and the enzyme aromatase that can locally synthesize 17β-estradiol in the vessel wall (Krause et al. 2011). 17β-estradiol is a known mitogen of ECs that promotes new vessel formation. Since microvascular diseases are characterized by excessive vessel growth, it is biologically plausible that estrogen-induced neovascularization may contribute to the development of microvascular lesions in the brain and cardiovascular system.
The Id (inhibitors of DNA binding and differentiation) family of proteins consists of four genes (Id1-Id4). Id protein-protein interactions occur via the helix-loop-helix (HLH) motif in which Id proteins dimerize with basic HLH transcription factors. This type of Id interaction was reported to regulate transcription in a dominant-negative manner (Norton2000); however, besides the negative regulation of cell differentiation, Id proteins are also positive regulators of cell growth and play a major role in neovascularization. We have previously shown that ID3 is an important determinant of cell proliferation of estrogen treated HUVECs (Felty and Porther, N.2008). Since ID3 has been implicated in vascular lesion formation (Forrest et al. 2004a; Matsumura et al. 2001; Nickenig et al. 2002), we hypothesize that ID3 is involved in the development of microvascular lesions in response to estrogen. In this study, we have elucidated the effect of both 17β-estradiol (E2) exposure and ID3 expression on the human cerebral microvascular endothelial cell line, hCMEC/D3.
The focal adhesion kinase Pyk2 has emerged as a “platform kinase” upon which other signaling molecules integrate (Avraham et al. 2000). Overexpression of Pyk2 was reported to increase cell spreading and migration of human brain microvascular ECs (Avraham et al. 2003); thus the dysregulation of these processes could weaken the blood-brain barrier following exposure to estrogen and may provide an explanation for increased risk of CCMs to bleeding during pregnancy. Cell spreading and migration may also be important contributors to excessive vessel growth found in cerebrovascular lesions, so in this study we also determined whether estrogen-induced ID3 effects were associated with Pyk2. A better understanding of how vessel abnormalities cause or contribute to neurological diseases which may depend on Pyk2 - ID3 signaling may create new therapeutic opportunities for prevention and treatment of diseases that harbor microvascular lesions.
Materials & Methods
Cell culture and treatment conditions
The immortalized human cerebral microvascular endothelial cell line hCMEC/D3 was obtained from Dr. B. Weksler, Weill Medical College of Cornell University, NY. The hCMEC/D3 cells have been shown to retain their EC characteristics with a stable normal karyotype, proliferate in response to endothelial growth factors, and form capillary tubes in matrix but no colonies in soft agar (Weksler et al. 2005). The hCMEC/D3 cell line was maintained in DMEM-F12 media with 5% FBS. Cells were cultured at 37°C in a humidified atmosphere with 5% CO2. 17β-estradiol (E2) was purchased from (Sigma) and dissolved in dimethyl sulfoxide (DMSO). Equal volumes of DMSO as in E2 treatment group were added to vehicle control with the final percentage of DMSO in each group to be less than 0.1%.
ID3 overexpression
The hCMEC/D3 cells were stably transfected with either Precision LentiORF for ID3 (Thermo Scientific Open Biosystems) or empty vector lentiviral pLEX-JRED/TurboGFP by the trans-lentiviral packaging kit with Express-in transfection reagent according to the manufacturer’s instructions. We used the MOI (multiplicity of infection) of 25 and selected cells that overexpressed ID3 with blasticidin S (5 µg/ml) as per manufacturer’s instructions. Cells expressing TurboGFP were identified by fluorescence microscopy.
Immunofluorescence
The cells were fixed with methanol and incubated at −20°C for 15 min. Immunofluorescence staining was performed according to standard procedures. Briefly, the cells were blocked with 3% normal goat serum at 4°C for 1h and then incubated with antibodies: anti-CD34, anti-α-smooth muscle actin, or anti-Nanog overnight at 4°C. After washing with 1X PBS, cells were incubated with anti-mouse-IgG Alexa Fluor® 633 for α-SMA (Santa Cruz Biotechnology Inc.), anti-goat -IgG Alexa Fluor® 488 for CD34 (Santa Cruz Biotechnology Inc.), and anti-rabbit-IgG Alexa Fluor® 546 for Nanog (Cell Signaling Technology). Cells were washed with PBS mounted with Fluoromount-G™ reagent. The spheroids with GFP or RFP were photographed and analyzed using a Nikon C1 laser scanning confocal microscope.
Fluorescence activated cell sorting (FACS)
Cell cycle distribution was analyzed with the Guava easyCyte™ using the CytoSoft software program according to the manufacturer's instructions. The percent DNA content quantization was based on propidium iodide (PI) fluorescence. For cell cycle analysis, cells were harvested, rinsed with PBS, and fixed in 73% ethanol for 20 h at −20°C. After incubation at −20°C, the cells were washed with PBS containing 1% BSA, stained with PI (10 µg/ml) in PBS containing RNase A (250 µg/ml), and incubated at 37°C for 30 min in the dark before FACS analysis.
Endothelial spheroid assay
Cells were suspended in serum-free DMEM/F12 (1:1) culture medium supplemented with B27®. For endothelial spheroid formation, approximately 100–150 cells per well were seeded in an ultra low-attachment 96-well plate (Corning Inc, Lowell, MA). Endothelial spheroids were grown for up to 10 days in liquid culture in the absence or presence of E2. A total of 15 endothelial spheroids with a minimum diameter of 50µm were counted in each experimental group. Data were analyzed by ANOVA; Tukey HSD test for multiple comparisons.
Cell migration assay
Cells were cultured in 6-well plates and a sterile plastic 1 mL micropipette tip was used to scratch in the middle area of the well as a line. Then cells were incubated in growth medium for 48 h. The scoring wounds were photographed with a microscope.
Immunoblotting
Whole cell lysates were prepared with lysis buffer containing [25 mM Tris-HCl buffer (pH 8.0), 150mM NaCl, 0.2% NP-40, 10% glycerol, 8 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate, 10 mM NaF, 0.2 mM Na3VO4, 1 mM DTT and 10 µl/ml protease inhibitor cocktail (Sigma Aldrich). Proteins were quantified using the Bradford Assay Reagent (Bio-Rad) according to the manufacturer’s instructions. Proteins (35–75 µg) were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). Membranes were blocked with 5% nonfat milk and incubated with the following antibodies: ID3 (Cal BioReagent), pY402-Pyk2, Pyk2, and p-Ser (Santa Cruz BioTechnology); p-Tyr and β-actin (Cell Signaling). Antibody dilutions used were according to manufacturer’s recommendations for detection by immunoblot. Membranes were then incubated with horseradish peroxidase-conjugated secondary IgG antibodies and visualized with ECL Plus Western blot reagents (GE Healthcare, Amersham). The membranes were re-probed for β-actin as loading control. Electrochemiluminescence (ECL) intensity of detected target proteins was imaged and quantified with a Bio-Rad Versa Doc instrument. All immunoblots were completed a minimum of three times for each experiment.
Immunoprecipitation
For immunoblots of immunoprecipitated (IP) lysate the following method was used. ID3 protein was pulled down using magnetic beads (Dynabeads protein G). In brief, the cell lysates were incubated with 10 µg of antibody and 50 µl (1.5 mg) of Dynabeads (Invitrogen) in microcentrifuge tubes with rotation for 10 minutes at room temperature. Samples were then placed on a magnetic particle concentrator (DynaMag™-2, Invitrogen) and supernatant was discarded. Dynabeads–Ab–Ag complex were washed 3 times with washing buffer and tubes were placed on magnetic particle concentrator to remove the supernatant. The magnetic Dynabeads–Ab–Ag complex was suspended with 20 µl of elution buffer and boiled in SDS sample buffer for 5 min. Samples were placed on the magnetic particle concentrator and supernatant containing protein were separated by SDS-PAGE and immunoblotted as described previously.
Phospho-peptide identification by (MALDI-TOF/TOF) mass spectrometry
The services of Applied Biomics (Hayward, CA, USA) were used for the identification of phosphorylation sites by MALDI-TOF/TOF following a standard protocol. In brief, after the E2 treatment total cell lysate was immunoprecipitated with ID3 and separated on a 1D gel. The gel bands corresponding to ID3 protein were reduced, alkylated, and subjected to trypsin digestion. Supel-Tips (Sigma-Aldrich) were used for phosphopeptide enrichment. Tryptic peptides were desalted by Zip-tip C18 (Millipore), and peptides were eluted from the Zip-tip with 0.5µl of Matrix solution (Agilent Technologies) and spotted on the MALDI plate. MALDI-TOF MS (matrix-assisted laser desorption/ionization–time-of-flight MS) was performed on an AB Sciex Proteomics Analyzer (AB Sciex, Foster City, CA, USA). MS spectra were acquired in positive ion mode and ~4000 laser shots/ per spectrum. A virtual digest was done by submitting protein sequences of interest to University of California–San Francisco Protein Prospector (http://prospector.ucsf.edu/prospector/mshome.htm). The MS precursors matching the virtual digest were submitted for MS fragmentation. The resulting peptide masses were submitted to MASCOT search engine (Matrix Science) to search the database of Swiss-Prot. Candidates with either protein score C.I.% or ion C.I.% > 95 were considered significant. The spectra of all peptides containing potential phosphorylation sites were manually evaluated for the loss of phosphate.
Co-culture tube assay
Primary cells, Human umbilical vein endothelial cells (HUVECs) and normal human dermal fibroblasts (NHDF), were purchased from Cambrex. HUVECs (passages 2–10) were maintained in endothelial basal medium-2 (EBM-2) supplemented with EGM2-MV SingleQuots (Cambrex). NHDF (passages 3–6) were maintained in monoculture in EBM-2 supplemented with FGM-2 SingleQuots (Cambrex). The effect of E2 treatment on HUVECs was studied under the following culture conditions: HUVECs were starved in phenol red-free mammary epithelium basal medium in the absence of serum and growth factors for 3 h. Thereafter, the cells were treated with E2 for the indicated time periods. In a 24-well plate, HUVECs were seeded on top of NHDF in phenol red-free mammary epithelium basal medium. Simultaneously, cells were treated with E2 or the vehicle control (0.1% DMSO) for 3 days. The ELISA was performed as described by Friis et al. (Friis et al. 2006). For RNA interference (RNAi) studies, HUVECs were cultured in 6-well plates to 60% confluency. Transfection was performed using FuGENE 6 (Roche) according to the manufacturer's protocol. Pyk2 siRNA was designed, synthesized, and annealed by Ambion with the following sequence: Scrambled siRNA (Silencer® negative control siRNA, Ambion) was used in all RNAi experiments. RNAi Sequence: 5’-GGUCUGCUUCUAUAGCAACTT-3’, Antisense: 5’-GUUGCUAUAGAAGCAGACCTT-3’. The FuGENE:siRNA mixture (100µl/well) was added to HUVECs in EBM-2 and returned to the incubator for 48 h prior to co-culture experiments.
Determination of Id3 gene expression
Total RNAs were isolated from hCMEC/D3 cells using the TRIZOL Reagent (Invitrogen), according to the manufacturer’s instructions. RNA sample was reverse transcribed into cDNA using the RT² First Strand Kit from SuperArray Bioscience Corporation, Qiagen (Frederick, MD) according to the manufacturer's protocol. The PCR reactions using cDNA were performed in a Applied Biosystems 7300 Real-Time PCR System using RT2 SYBR Green/ROX qPCR Master Mix and the manufacturer's thermal cycler protocol with 2 primers (Catalog no. 330001 PPH00413E, Gene Symbol: ID3, bp: 69, Ref Seq Accession no: NM_002167.3) for ID3 and with 2 primers (Catalog no. 330001 PPH00073E, Gene Symbol : ACTB, bp: 191, Ref Seq Accession no: NM_001101.3) for β-actin (SuperArray Bioscience Corporation, Qiagen). Id3 was quantitated in triplicate for each sample and was determined by a 'delta Ct and delta–delta Ct' calculation with reference to housekeeping gene β-actin control. Id3 expression was normalized and presented as the percentage by using the Id3 level in control as 100%. Results represent the means of 3 independent experiments performed in triplicate.
Statistical analyses
All statistics were performed using VassarStats statistical software (Richard Lowry, Poughkeepsie, NY, USA). One-way analysis of variance (ANOVA) was performed to detect any differences between groups. If the result of the ANOVA is significant (**P < 0.01 or bP < 0.01), pair wise comparisons between the groups were made by a post-hoc test (Tukey’s HSD procedure). The significance level was set at **P < 0.01 or bP < 0.01.
Results
ID3 enhanced E2-induced neovascularization
We previously reported that neovascularization mediated by 17β-estradiol (E2) exposure of HUVECs was dependent on ID3 (Felty and Porther, N.2008). In this study we have extended those findings with the human brain capillary endothelial cell line, hCMEC/D3 (Weksler et al. 2005). Given the biological significance of ID3 as a positive regulator of cell growth, we asked the question of whether overexpression of ID3 increased neovascularization of estrogen exposed brain ECs.
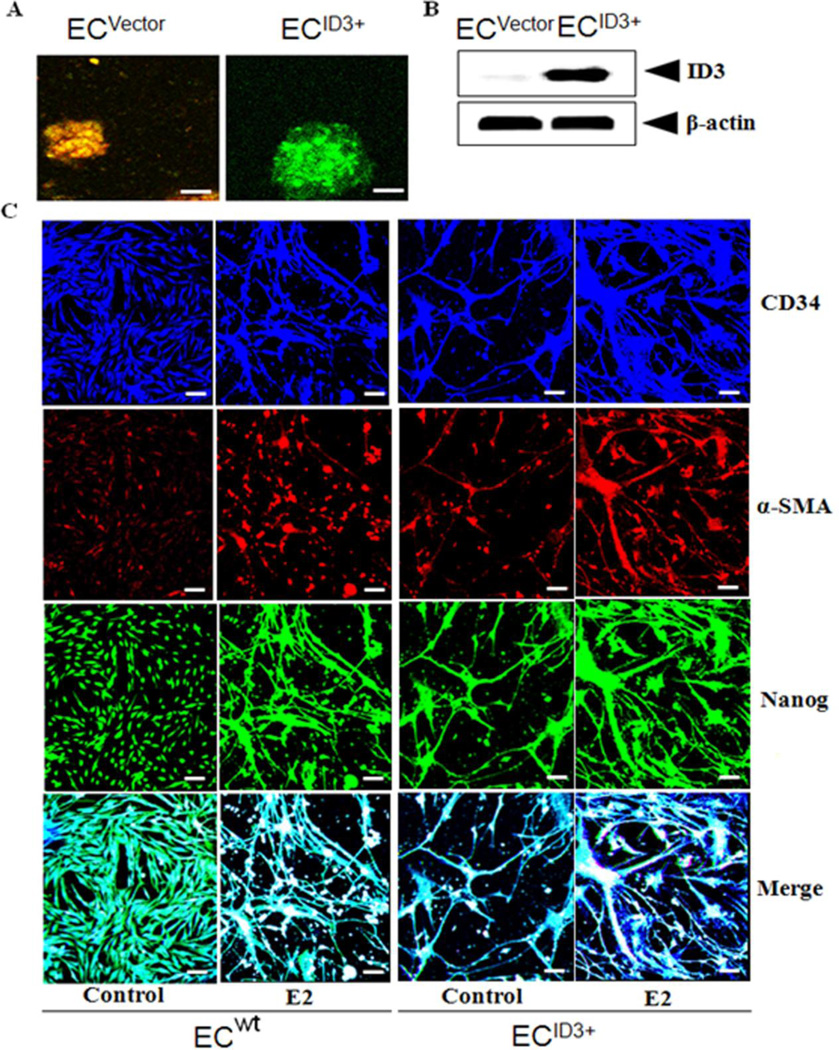
To address this question, hCMEC/D3 cells were stably transfected with the Precision LentiORF for ID3 overexpression or control empty lentiviral vector pLEX-JRED/TurboGFP as described in the Materials & Methods section. As shown in Fig. 1A, fluorescent microscopy confirmed ID3-GFP expression in endothelial spheroids of hCMEC/D3 cells compared to control cells transduced with the empty vector expressing RFP/GFP. Immunoblot analysis of ID3 overexpression indicated a higher level of ID3 protein expression compared to control (Fig. 1B). Next, the following fluorescent microscopy experiments were used to compare the estrogenic response of cells overexpressing ID3 (EC ID3+) to wild-type (EC wt). ECs acquire a proliferative and invasive phenotype in the neovascular process; and there is some evidence that malformatons such as microvascular lesions exploit this phenotype. To our knowledge, the effect of E2 on ID3 in brain ECs has not been previously studied. The complex interplay of ECs with other cell types that compose a vessel makes for a sophisticated multicellular environment that affects the response to estrogen. Therefore, we used a mixed cell co-culture model that included vascular smooth muscle cells (VSMC) and fibroblasts (Fb) with ECs. Cells were seeded in 1:1:1 ratio and allowed to self-organize. Using the endothelial marker CD34, we determined the effect of estrogen treatment on cell morphology. In the vehicle control group, we observed that EC ID3+ formed capillary tube-like structures that stained positive for anti-CD34 antibody while the EC wt displayed a different morphology of a more uniform cell monolayer (Fig. 1C*). As expected we observed tube-like structures stained CD34+ in the E2 treated EC wt cells at 10 days, but to a lesser degree when compared to the EC ID3+. Cells were stained with anti-α-smooth muscle actin (α-SMA) to identify localization of VSMC with hCMEC/D3 cells. Notably, the EC ID3+ group showed thin thread-like cells that stained positive for anti-α-SMA antibody which was absent in EC wt group. In the E2 exposed EC ID3+ group, the thread-like cells showed a higher expression of α-SMA+ stained cells compared to the EC wt group. Based on the merged photo of CD34 and α-SMA antibody staining, these findings show that ID3 overexpression enhanced neovasularization and a morphology that suggests recruitment of VSMCs as the α-SMA+ cells aligned along the hCMEC/D3 (CD34+) tube-like structures. Furthermore, ID3 overexpression showed thicker tube-like structures compared to EC wt (Fig. 1C).
Figure 1. Overexpression of ID3 enhanced neovascularization of brain microvascular endothelial cells.
The functional significance of ID3 on survival of hCMEC/D3 cells was determined by generating a stable ID3 overexpressing cell line. (A) fluorescent microscopy confirmed ID3-GFP expression in endothelial spheroids of hCMEC/D3 cells compared to control cells transduced with the empty vector expressing RFP/GFP. Scale bar=70µm. (B) Immunoblot analysis of ID3 overexpression indicated a higher level of ID3 protein expression compared to control. (C) Fluorescent microscopy experiments were used to compare the estrogenic response (E2 1ng/ml for 10 days) of endothelial cells overexpressing ID3 (EC ID3+) to wild-type (EC wt). A mixed cell co-culture model that included vascular smooth muscle cells (VSMC) and fibroblasts (Fb) was cultured with ECs and allowed to self-organize. Cells were stained with either anti-CD34 or anti-α-smooth muscle actin (α-SMA) antibodies to identify localization of EC and VSMC, respectively. In addition, cells were stained with anti-Nanog antibody to determine the effect of ID3 overexpression had on master transcriptional regulator Nanog. Scale bar=50µm.
ID3 is known to be an inhibitor of differentiation. Although poorly understood, the de-differentiation of ECs has been reported to support the neovascular phenotype (Kohler et al. 2014). Nanog, a master transcription factor associated with the maintenance of cell pluripotency, has been shown to play a central role in regulating EC proliferation, branching point structures, and neovascularization (Kohler et al. 2011). In embryonic stem cells, Nanog was shown to be a downstream target of another Id family member, Id1 (Romero-Lanman et al. 2012); however it is not known whether ID3 can drive the expression of Nanog in brain ECs. Therefore, we determined whether ID3 overexpression correlated with an increase expression of the transcription factor Nanog in hCMEC/D3 cells. In the control EC wt group, we observed a basal level of cells stained positive for the anti-Nanog antibody which appeared localized to nuclei (Fig. 1C). Upon E2 treatment of EC wt, Nanog+ stained cells were aligned along tube-like cells and Nanog+ staining was no longer confined to the nuclei. ID3 overexpression alone showed a similar pattern of Nanog+ cells as seen in estrogen treated EC wt. Taken together these data showed that ID3 increased neovascularization of brain ECs with and without estrogen treatment. Interestingly, ID3 overexpression alone positively correlated with an increase in cells stained positive for Nanog.
ID3 overexpression enhanced E2-induced endothelial spheroid growth
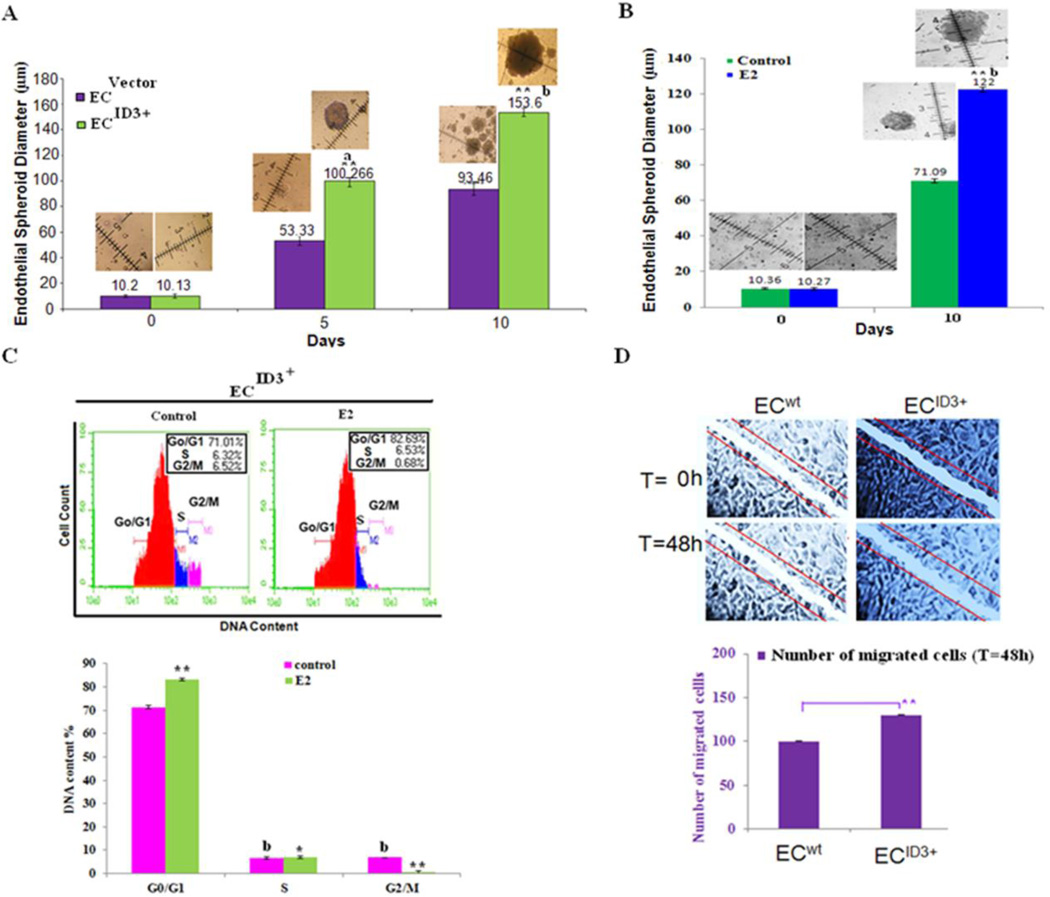
Spheroid formation is known to correlate with cell survival or increased resistance to apoptosis. Since hyper-proliferative, apoptosis-resistant, and monoclonal ECs have been characterized in microvascular lesions (Lee et al. 1998); we used a stem cell sphere forming assay to determine the influence of ID3 overexpression on the formation of endothelial spheroids. Under conditions of the stem cell sphere assay, EC wt and EC ID3+ cells were seeded in an ultra low-attachment 96-well plate and spheroids were grown for up to 10 days in liquid culture. A total of 15 endothelial spheroids with a minimum diameter of 50 µm were counted in each experimental group. We observed that ID3 overexpression resulted in significantly larger endothelial spheroids at both days 5 and 10 compared to wild-type hCMEC/D3 cells when cultured in serum free culture medium supplemented with B27® (Fig 2A). The significant increase in the size of EC ID3+ spheroids at both days 5 and 10 showed that ID3 overexpression contributed to cell proliferation and/or cell survival. Since estrogen exposed EC ID3+ enhanced neovascularization in our previous experiment, we further determined whether E2 treatment would also enhance the growth of endothelial spheroids. As shown in Fig. 2B, E2 treatment resulted in significantly larger endothelial spheroids at 10 days compared to vehicle control (0.1% DMSO).
Figure 2. ID3 overexpression increased E2-induced endothelial spheroid growth.
(A) ID3 overexpression increased the diameter of brain microvascular endothelial spheroids measured at both days 5 and 10. The hCMEC/D3 cell line was cultured with serum free B27 medium in ultra-low attachment 96 well plates. Spheroid diameter was measured at days 5 and 10. The measurement scale was 100 µm at a magnification of 200X. Error bars represent the mean diameter of 15 spheroids ± SD. ap<0.01; bp<0.01 vs control within 5 and 10 days as well as **p<0.01; vs control between 0 and 5 or 10 days. Data were analyzed by ANOVA; Tukey HSD test for multiple comparisons. Representative microphotographs of treatment groups are shown inset. (B) E2 (1 ng/ml) treatment increased the diameter of ID3 overexpressing endothelial spheroids (EC ID3+) measured at 10 days. (C) FACS data shown are representative histograms from three independent experiments showing the effect of ID3 overexpression (EC ID3+) plus E2 (1ng/ml) treatment in hCMEC/D3 cells for 24 h. Cells were subjected to FACS analysis after propidium iodide (PI: X-axis) staining. (D) Representative image of scratch wound assay and graph showing the quantitative effects of ID3 overexpression on cell migration.
Since de-differentiation of ECs has been reported to support the neovascular phenotype, we postulated that ID3 overexpression in combination with estrogen treatment will increase the number of viable cells distributed in the G0/G1 phase of the cell cycle as a biological indicator of proliferative quiescence. We treated EC ID3+ with E2 for 24 h and cells were subjected to FACS analysis after propidium iodide staining. In Fig. 2C, FACS histograms shown are representative of three independent experiments showing the effect of ID3 overexpression plus E2 treatment in hCMEC/D3 cells. As shown in the graph of combined FACS data, our flow cytometry experiments revealed that approximately 71.34% of the DNA content in EC ID3+ resided in the G0/G1 phase and this increased to 83.02% when exposed to estrogen (Fig. 2C). Thus, our results showed a statistically significant 1.2-fold increase in G0/G1 phase in estrogen treated EC ID3+. The percentage of DNA content in S phase from estrogen treatment was 6.86% compared to 6.65% in vehicle control (Fig. 2C) while estrogen treatment showed a decrease in % DNA content in G2/M phase from approximately 6.85% in vehicle control to 0.68% in the estrogen treated cell population (Fig. 2C). Thus, our results showed a statistically significant 10-fold decrease in G2/M phase in estrogen treated EC ID3+. We also evaluated the effect of ID3 overexpression had on cell migration using a wound healing assay. In brief, a brain EC monolayer consisting of hCMEC/D3 wild-type or ID3 overexpressing cells was mechanically scratched to create a wound. After 48 hours, we examined the extent of wound closure. By measuring the wound area compared with the initial wound area, we found that the ability of hCMEC/D3 cells to migrate to the wound area was significantly greater in cells that overexpressed ID3 (Fig. 2D).
17β-estradiol induced ID3 expression depends on ER
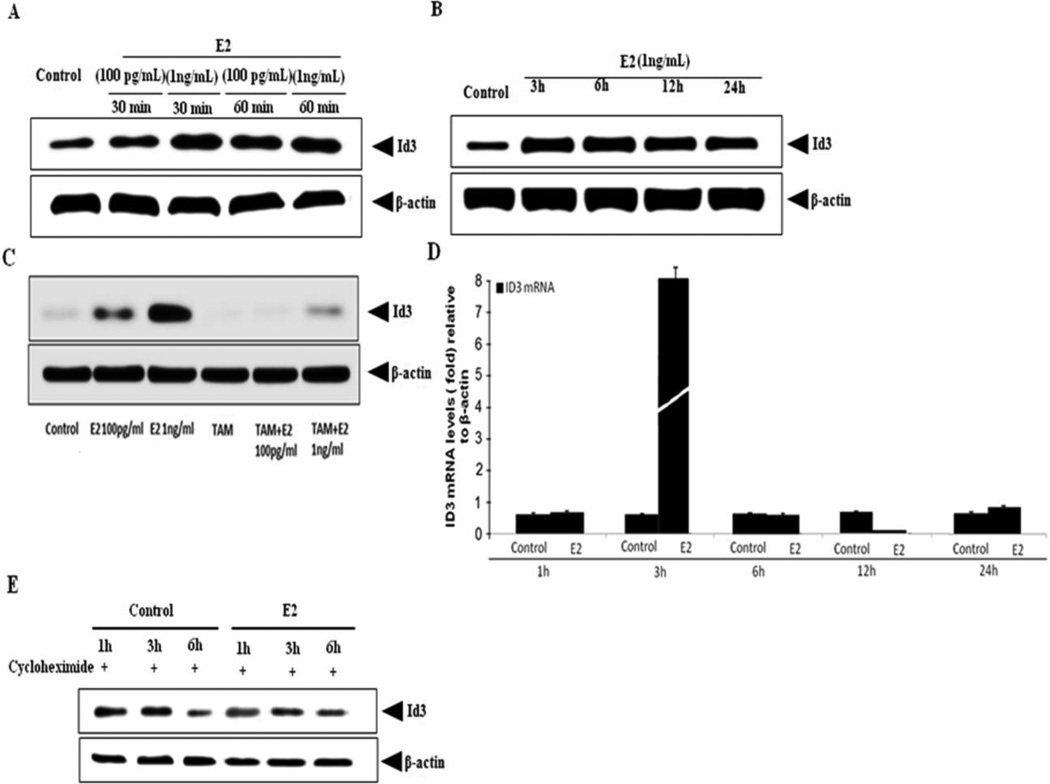
Next, we evaluated the effect of E2 on ID3 expression and phosphorylation in hCMEC/D3 cells. As shown in Fig. 3A, cells exposed to E2 (100 pg/ml - 1 ng/ml) for both 30 min and 60 min time periods showed a significant increase in total ID3 protein compared to vehicle control (0.1% DMSO). We chose the E2 dose of 1 ng/ml to further determine whether the observed increase in ID3 at the early time point was sustained over a longer time course. We demonstrated that 3 h and 6 h exposures to E2 led to higher levels of ID3 protein with the appearance of a slight decrease by 24 h (Fig. 3B). Estrogen receptors (ER-α/β) have been reported to be expressed in cerebral endothelium (Stirone et al. 2003). To investigate whether ER was involved in E2-mediated ID3 protein expression, cells were treated with the known ER antagonist, tamoxifen. As shown in Fig. 3C, tamoxifen inhibited E2-induced ID3 protein levels in hCMEC/D3 cells. Based on the increase we observed in ID3 protein levels by E2, we also determined ID3 mRNA expression in E2 exposed cells by real-time PCR. We observed a 10-fold increase in Id3 mRNA expression from E2 exposure at 3 h (Fig. 3D) which suggested that the early (less than 3 h) increase in ID3 protein was due to protein stabilization. Because the level of ID3 protein is determined by the rates of protein synthesis and protein degradation, we determined whether E2 treatment affected the protein synthesis of ID3. As shown in Fig. 3E, ID3 protein levels in the control group treated with the protein synthesis inhibitor cycloheximide showed a decrease in ID3 levels at 6 h while the ID3 protein levels remained higher in the E2 treatment group. Thus, the observed increase in ID3 by E2 treatment does not depend on protein synthesis in hCMEC/D3, but rather E2 made ID3 more stable from protein degradation during the 1 – 6 hr time period.
Figure 3. E2-induced ID3 expression depends on the estrogen receptor and ID3 protein is stabilized.
(A) E2-induced expression of ID3 protein at 30 & 60 min. (B) Expression of ID3 protein from 3 h – 24 h time points in E2 (1 ng/ml) exposed hCMEC/D3. (C) E2-induced ID3 protein at 3 h was inhibited by pre-treatment with ER antagonist tamoxifen (1 µM) for 6 h prior to 2 h E2 (1 ng/ml) exposure of hCMEC/D3. (D) Id3 mRNA expression showed a 10-fold increase from E2 exposure at 3 h. (E) E2-treated cells in the absence of protein synthesis showed that ID3 protein levels remained higher than control at 3 and 6 h time points. Cells were pretreated with the protein synthesis inhibitor cycloheximide (1 µg/ml) 2 h prior to treatment with E2 (1 ng/ml).
Phosphorylation of ID3 in estrogen exposed brain endothelial cells
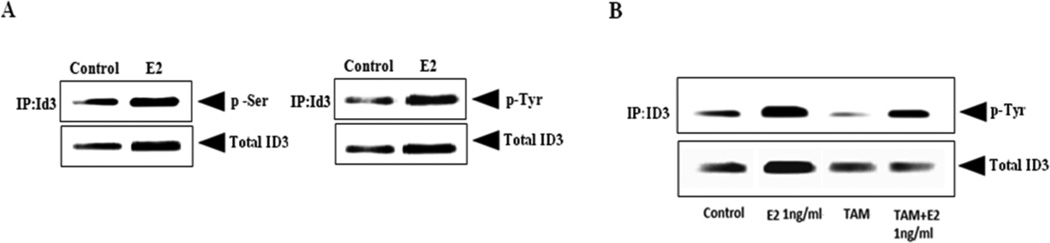
Vascular cell growth has been shown to be regulated by serine phosphorylated ID3 (Forrest et al. 2004b); however, it is not known whether this is also the case for brain microvascular ECs. We previously have reported that E2 exposure to HUVECs increased the serine phosphorylation of ID3 (Felty and Porther, N.2008). So, we determined whether ID3 serine phosphorylation (p-Ser) also occurred in E2 exposed hCMEC/D3 cells. Given that there is no commercially available antibody specific for phospho-ID3, total ID3 was first immunoprecipitated followed by immunoblot with anti-phospho-serine antibody. Immunoprecipitation (IP) was performed to isolate ID3 from cell lysate using a monoclonal anti-ID3 antibody (Cal-Bioreagent). Magnetic bead-based separation was then used to extract the bound ID3 protein via Protein G coupled to superparamagnetic Dynabeads® according to the manufacturer’s instructions. Proteins from immunolysates were separated by SDS-PAGE, transferred to PVDF membrane, and processed as described previously. Western Blots with phosphorylated ID3 were detected by electrochemiluminescence (ECL). The ECL band intensity for p-Ser was imaged with a Bio-Rad Versa Doc instrument. As shown in Fig. 4A, the 3 h treatment with E2 showed a higher relative ratio of p-Ser at molecular weight (MW) of 17 kDa corresponding to ID3. Our data indicated an increase in total serine phosphorylation by E2 treatment, however, it was not possible to determine whether Ser-5 and/or even multiple serine residues were phosphorylated on ID3 by Western Blot.
Figure 4. E2-induced serine and tyrosine phosphorylation of ID3.
(A) E2 treatment increased serine phosphorylation of ID3 protein at 3h. ID3 protein was isolated by immunoprecipitation (IP) technique using magnetic beads and detected by immunoblot. Serine and tyrosine phosphorylation of immuoprecipitated ID3 protein was determined with anti-p-Ser or anti-p-Tyr antibodies. The phosphorylated ID3 bands detected from immunoprecipitated cell lysate corresponded with the molecular weight (MW) of 17kDa for ID3. Electrochemiluminescence (ECL) band intensity for phosphorylated ID3 was imaged with a Bio-Rad Versa Doc instrument. (B) Tamoxifen (1 µM) treatment decreased the level of phospho-Tyr band corresponding to MW~17 kDa for ID3.
We performed a search for the presence of any literature-derived kinase / phosphatase motifs in the human ID3 sequence using the PhosphoMotif Finder program, http://www.hprd.org/ (Keshava Prasad et al. 2009). A search with the PhosphoMotif Finder program revealed that ID3 had 38 serine kinase / phosphatase motifs and 8 tyrosine kinase / phosphatase motifs (data not shown). Although phosphorylated Ser-5 of ID3 has been reported, we have not found published evidence on tyrosine phosphorylated ID3. The search results from PhosphoMotif Finder revealed that the ID3 protein sequences at Tyr-11 and Tyr-48 corresponded to Src, JAK2, and ALK kinase substrate motifs. Using the previous experimental conditions, ID3 was immunoprecipitated from total cell lysate followed by immunoblot with anti-phospho-tyrosine antibody. As we observed with phos-serine there was a basal level of phospho-Tyr that corresponded with the MW ~ 17 kDa for ID3 (Fig. 4A). After 3h of E2 treatment, we observed a higher level of tyrosine phosphorylation than vehicle control. Since our previous data showed that E2-induced ID3 protein levels depended on ER, we also pretreated the cells with tamoxifen as described previously and ID3 was immunoprecipiated from cell lysate using a monoclonal anti-ID3 antibody. In cells that were exposed to E2 for 3 h, tamoxifen treatment decreased the level of phospho-Tyr band corresponding to MW~17 kDa for ID3 (Fig. 4B).
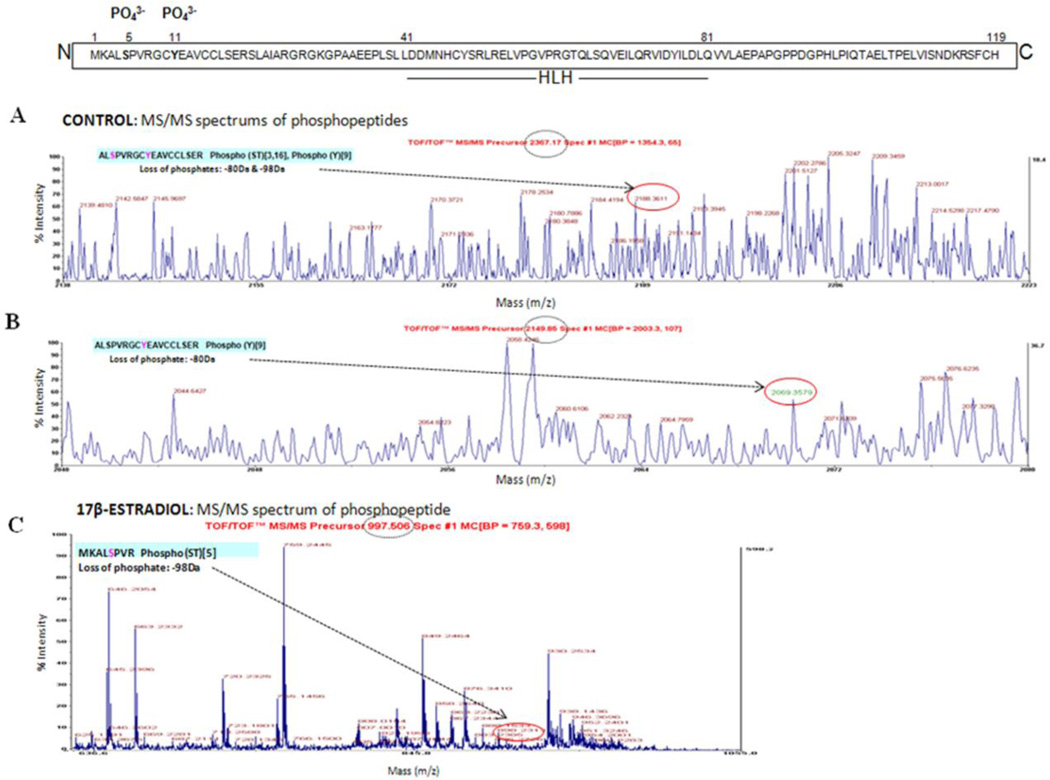
To determine the phospho-ID3 sites induced by E2, total hCMEC/D3 cell lysates were immunoprecipitated with ID3 and prepared for MALDI-TOF analysis as described in Materials & Methods section. MS spectra analysis identified with high confidence the human ID3 sequence with the protein score of 99% from the control sample and 94% from the E2 sample. Presence of phosphorylated residues was identified by the MS/MS peak showing neutral loss of phosphates. In vehicle control, we observed a peptide ALS5PVRGCY11EAVCCLS18ER, m/z 2367.1738, which corresponded to the calculated mass of the loss of phosphate (−98 Da for serine or threonine phosphorylated peptides) plus loss of phosphate (−80 Da for tyrosine phosphorylated peptide) (Fig. 5A). We also observed a peptide ALSPVRGCY11EAVCCLSER, m/z 2149.8459, which corresponded to the calculated loss of phosphate (−80 Da) (Fig. 5B). In the E2 treatment group, the phosphopeptide Tyr-11 was detected at the level of MS, but it could not be resolved at the level of MS/MS. However, phosphorylation at Ser-5 in the E2 treatment group was observed on a peptide MKALS5PVR, m/z 997.5056, which corresponded to the calculated loss of phosphate (−98 Da) for a serine phosphorylated peptide (Fig. 5C). Thus, the peptide spectra data demonstrated that multiple amino acids were phosphorylated on ID3. These phosphorylated amino acids included either Ser-5 or Ser-18 in combination with Tyr-11.
Figure 5. Identification of phosphorylated amino acids in ID3.
(A-C) MALDI-TOF/TOF spectra for peptides extracted from hCMEC/D3 cells. Vehicle control showed that Ser-5 or Ser 18 (A) and Tyr-11 (B) are phosphorylated. Identification of phosphopeptides from E2 treated cells indicated that Ser-5 was phosphorylated (C). Methods: Cell lysate was immunoprecipitated with ID3 antibody from vehicle control and E2 treated hCMEC/D3 cells. The immunoprecipitated lysate was separated by 1D-SDS-PAGE and the protein band corresponding to the MW of ID3 was excised from the gel, subjected to tryptic digestion and phosphopeptide enrichment. The phosphopeptides were spotted on a MALDI plate followed by MS and database search. The spectra of all peptides were manually evaluated for the loss of phosphate which is shown in red circles and the observed mass (dashed circle) and sequence of the peptides are shown. X-axis represents mass and Y-axis represents intensity.
siRNA Pyk2 inhibition of E2-induced neovascularization
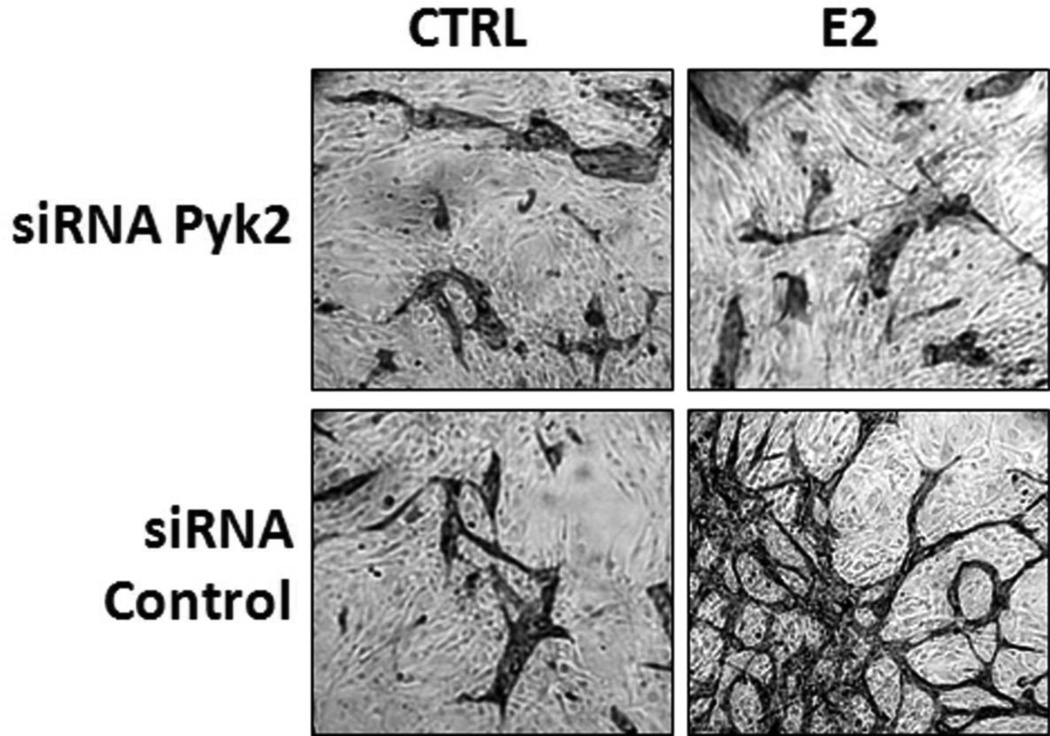
We previously reported that neovascularization mediated by E2 exposure of HUVECs was dependent on ID3 (Felty and Porther, N.2008). Pyk2 plays an important role in cell motility such as spreading and migration necessary for neovascularization (Tang et al. 2002). So, we further determined whether Pyk2 was a positive regulator of E2-induced neovascular phenotype in a well-established HUVECs co-culture model. Although endothelial tube assays performed on matrix gel are commonly used to determine EC vessel formation in vitro, Donovan et al. (Donovan et al. 2001) have demonstrated that endothelial vessel morphology from co-cultured cells better represents EC sprouting that occurs in vivo. In a 24-well plate, HUVECs were seeded on top of normal human dermal fibroblasts in phenol red-free mammary epithelium basal medium. Simultaneously, cells were treated with E2 or vehicle control (0.1% DMSO) for 3 days. Cells were transiently transfected with either Pyk2 siRNA or Silencer® negative control siRNA for 48 h prior to co-culture experiments. As shown in Fig. 6, ECs immunostained with CD31 showed inhibition of E2-induced tube formation by Pyk2 siRNA. These findings indicate that Pyk2 is necessary for the E2-induced neovascular phenotype observed in the HUVEC co-culture model.
Figure 6. siRNA Pyk2 inhibited E2-induced neovascularization of endothelial cells.
E2-induced vessel formation was further characterized in a co-culture model. HUVECs were seeded on top of a confluent layer of fibroblasts. Representative micrographs showing inhibition of endothelial tube formation by siRNA Pyk2. Cells were transiently transfected with either Pyk2 siRNA or Silencer® negative control siRNA for 48 h prior to co-culture experiments. Immunostaining for the endothelial marker CD31 at day 3 showed significant tube formation in the E2 treatment groups compared to vehicle control (0.1% DMSO). Pyk2 siRNA Sequence: 5’-GGUCUGCUUCUAUAGCAACTT-3’, Antisense: 5’-GUUGCUAUAGAAGCAGACCTT-3’(Ambion).
Pyk2 siRNA inhibits 17β-estradiol-induced ID3 expression
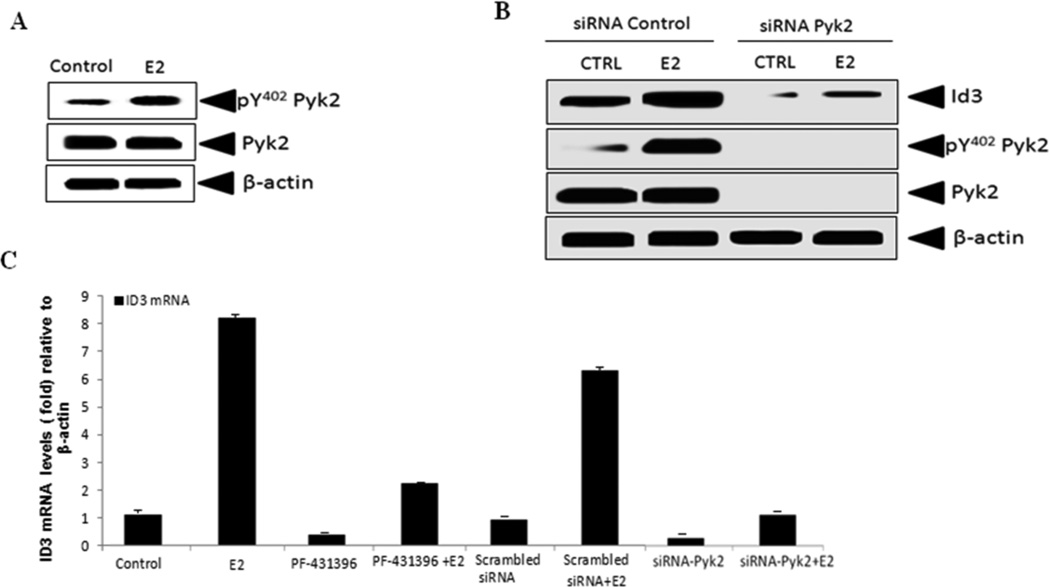
Previously, we reported that ID3 siRNA inhibited E2-induced neovascularization in the HUVEC co-culture model and now based on our data that showed Pyk2 siRNA also inhibited E2-induced neovascularization (Fig. 6), we postulated that the positive effects on tube formation and spheroid growth by ID3 overexpression in hCMEC/D3 cells may be associated with Pyk2. Overexpression of Pyk2 was reported to increase cell spreading and migration of human brain microvascular ECs. Our data from the scratch wound assay showed increased cell migration in hCMEC/D3 cells that overexpressed ID3 (Fig. 2D). Since the tyrosine kinase Src was reported to regulate a different Id family member at both transcript and protein levels (Gautschi et al. 2008), we postulated that ID3 may be a novel target of tyrosine kinase Pyk2. Thus, we determined whether E2 increased phosphorylation of Pyk2 in hCMEC/D3 cells.
The activation of Pyk2 results from its autophosphorylation on Tyr-402 (pY402), which creates a Src homology 2-binding site (SH2). Next, we determined whether E2 increased the level of pY402 Pyk2 in hCMEC/D3 cells. As shown in Fig. 7A, cells treated for 3 h with E2 showed an increase in pY402 Pyk2 while the total level of Pyk2 remained the same compared to vehicle control. Using a loss-of-function approach, cells were treated with siRNA Pyk2 to determine whether ID3 was a downstream target of Pyk2. As shown in Fig. 7B, exposure to the siRNA Pyk2 significantly reduced the total levels of ID3 protein compared to siRNA control. Furthermore, the observed reduction in ID3 by siRNA Pyk2 was not rescued to the level of the siRNA control when treated with E2. We took into account that the observed decrease in ID3 by the siRNA Pyk2 occurred over a 48 h siRNA transfection prior to conducting the E2 treatment. Thus, the observed decrease in ID3 protein may have occurred at the level of mRNA expression.
Figure 7. siRNA Pyk2 inhibited E2-induced ID3 expression.
(A) hCMEC/D3 cells treated for 3 h with E2 showed an increase in pY402 Pyk2 while the total level of Pyk2 remained the same compared to vehicle control. (B) Cells were treated with siRNA Pyk2 to determine whether ID3 was a downstream target of Pyk2. Exposure to the siRNA Pyk2 significantly reduced the total levels of ID3 protein compared to siRNA control. (C) Pyk2 inhibition had on mRNA expression was determined by 1 h pre-treatment of hCMEC/D3 cells with the chemical Pyk2 kinase inhibitor, PF-431396 (1µM), followed by 3 h of E2 exposure. PF-431396 decreased the E2-induced ID3 mRNA expression by 6-fold compared to vehicle control while Pyk2 siRNA decreased the ID3 mRNA level by 7-fold in E2 exposed hCMEC/D3 cells.
The acute effect of Pyk2 inhibition had on mRNA expression was determined by 1 h pre-treatment of hCMEC/D3 cells with the chemical Pyk2 kinase inhibitor, PF-431396, followed by 3 h of E2 exposure. We then measured the effect of PF-431396 treatment had on E2-induced ID3 mRNA expression at 3 h by real-time PCR. As shown in Fig. 7C, we observed that PF-431396 decreased the E2-induced ID3 mRNA expression by 6-fold compared to vehicle control while the basal level of ID3 mRNA expression was decreased by 0.5 fold. Since chemical inhibitors may exert non-specific effects on ID3 mRNA expresson, we compared the observations from the PF-431396 treatment to siRNA Pyk2. ECs were transfected with either Pyk2 siRNA or Silencer® negative control siRNA for 48 h prior to E2 treatments. As shown in Fig. 7C, Pyk2 siRNA decreased the ID3 mRNA level by 7-fold in E2 exposed hCMEC/D3 cells. Pyk2 siRNA treatment also inhibited the basal expression of ID3 in the vehicle control. Taken together these findings demonstrated that Pyk2 mediates E2-induced ID3 mRNA expression.
Discussion
Microvascular diseases are characterized by excessive vessel growth. The effects of estrogen on the endothelium which constitutes the major component of the blood-brain barrier, glomerular, and cardiovascular systems remain under investigated yet may hold promising clues that connect estrogen to abnormal microvessel growth and cerebrovascular and cardiorenal vascular diseases. The fundamental issue of whether estrogens are protective or damaging to the microvasculature of the brain may best be explained by hormone concentration. As mentioned previously several epidemiology studies have reported that estrogens may increase the risk for neurological lesions in stroke. Additionally, epidemiology studies have shown that high levels of endogenous estradiol were associated with increased risk (2-fold) for vascular dementia (Geerlings et al. 2003). More recently, a study reported that diabetic women with high plasma estradiol levels had a 14-fold increased risk of incident dementia over a four-year period compared to those with lower estrogen levels (Carcaillon et al. 2014). With respect to cerebral microvascular endothelial cells, it is important to consider their known expression of the aromatase gene which synthesizes 17β-estradiol in the vessel wall. 17β-estradiol (E2) is a known mitogen of endothelial cells (ECs) that may contribute to the development of brain microvascular lesions and/or increased susceptibility to bleeding. The molecular mechanisms leading to endothelial dysfunction, i.e. excessive vessel growth, are poorly understood and transcription regulators are likely to play a key role in this process. Although there is no single transcription regulator that specifically controls the neovascular activity of ECs, our study has focused on the effects of estrogen on ID3 in the human brain capillary endothelial cell line hCMEC/D3. The major findings from this study are: (i) ID3 overexpression increased neovascularization, cell migration, and endothelial spheroid growth; (ii) ID3 overexpression enhanced estrogen-induced G2/M phase transition in hCMEC/D3 cells; (iii) E2-induced ID3 protein expression was dependent on the ER; (iv) E2 treatment stabilized ID3 protein during the 1 – 6 hr time period; (v) E2 treatment increased serine and tyrosine phosphorylation of ID3; and (vi) Pyk2 kinase mediated estrogen-induced ID3 mRNA expression. The effect of E2 on Id proteins in brain microvascular ECs has not been investigated until now which makes this study novel. In addition, these findings are important because they provide a new paradigm by which estrogens may contribute to the growth of brain microvascular lesions.
Overexpression of ID3 may play an important role in the development of brain microvascular lesions since a previous study reported vascular malformations in the forebrain and an absence of branching and sprouting of blood vessels into the neuroectoderm in Id1-Id3 double knockout mice (Lyden et al. 1999). ID3 is known to be an inhibitor of DNA binding and differentiation. Although poorly understood, the de-differentiation of ECs has been reported to support the neovascular phenotype (Kohler et al. 2014). Our microscopy studies revealed that ID3 overexpression increased cells stained positive for Nanog which is a transcription factor known to maintain cell pluripotency. As pluripotent cells mature, they spend less time in G0/G1 phase due to a more robust proliferative ability as they move toward terminal differentiation. Proliferative quiescence, a state observed in pluripotent cells which reside longer in the G0/G1 phase of the cell cycle, may be a functional consequence of ID3 overexpression that could support de-differentiation of ECs. Our findings suggest that ID3 overexpression promoted G2/M phase transition by estrogen in hCMEC/D3 cells while maintaining a similar percentage of proliferating cells in S-Phase compared to vehicle control. Hence, the increased endothelial spheroid size that we observed may be explained by proliferative quiescence in which a small percentage of the cell population is proliferating in response to E2 while the majority of viable cells reside in G0/G1 ready to proliferate as needed such as in the case of wound healing. With respect to wound healing, we also found that the ability of hCMEC/D3 cells to migrate was significantly greater in cells that overexpressed ID3. The implications of excessive cell migration to endothelial dysfunction could be in supporting the neovascular phenotype that was enhanced when cells were treated with E2.
The connection of ID3 to neovascularization of human brain ECs may be from its direct regulation of vascular endothelial growth factor (VEGF) expression at the protein and promoter level (Cutchins et al. 2012); however, whether this connection between ID3 and VEGF exists in brain microvascular ECs remains to be investigated. Since VEGF plays a significant role in neovascularization after brain injury (Yang et al. 2002); it is plausible that ID3 overexpression is increasing cell migration and endothelial spheroid growth in our model through an ID3 mediated increase in VEGF. As mentioned previously, brain microvascular ECs have been reported to express estrogen receptors which is corroborated by our findings that showed ID3 protein expression was inhibited by treatment with ER antagonist tamoxifen. Although we have not identified the detailed mechanism by which E2 up-regulated ID3 levels, our data demonstrated that E2 treatment increased phosphorylated levels of ID3. An interesting finding from our study is the identification of E2-induced tyrosine phosphorylation of ID3 that was inhibited by tamoxifen treatment. Although tamoxifen is widely used to inhibit ER mediated gene transcription, we suspect that the observed decrease of both phospho-Tyr and total ID3 protein levels by tamoxifen treatment to be a non-genomic effect. This is based on our data that showed E2-induced Id3 mRNA expression does not occur until 3 h while we have observed significant increases in total ID3 protein within 1 h. From our review of the literature, we found that the HLH domain of a different Id family member was essential for the physical interaction between ERβ1 and Id1 (Chen et al. 2009); but whether a similar protein-protein interaction exists between ER and ID3 is not known. Our data suggests that the tamoxifen treatment has either interfered with a protein-protein interaction necessary for ID3 stability such as ID3 binding to ER; or tamoxifen has directly inhibited phosphorylation that is required for ID3 stability.
Pyk2 kinase has been demonstrated to be tyrosine-phosphorylated by VEGF and found to be important for focal adhesion sites assembly in primary human brain microvascular ECs (Avraham et al. 2003). Furthermore, overexpression of Pyk2 increased cell spreading and the migration of brain microvascular ECs. The findings reported from that study form the basis for why we investigated the effects of estrogen on Pyk2 phosphorylation as well as its effect on ID3 expression. Our findings showed that E2 treatment increased Pyk2 autophosphorylation on Tyr-402. This phosphorylation site on Pyk2 creates a SH2 binding domain. Since the SH2 domain recruits other proteins to Pyk2, these protein-protein interactions may be important for signaling to ID3. Cell spreading and migration have been shown to be regulated by Pyk2 in primary brain microvascular ECs. We found that inhibition of Pyk2 kinase, known to control cell migration, correlated to inhibition of ID3 expression at the level of protein and mRNA. We suspect that the dysregulation of Pyk2-ID3 signaling may weaken the blood-brain barrier following exposure to elevated levels of estrogen and may provide an explanation for increased risk of CCMs to bleeding during pregnancy.
Cerebral vessels are directly targeted by estrogen because they express receptors for these hormones. Although our study has implicated estrogen in endothelial dysfunction with respect to excessive vessel growth, the beneficial effect of estrogen on the vascular system has never been without controversy. In relating in vitro studies to in vivo conditions, a critical consideration is the concentration of hormone used. The level of circulating 17β-estradiol in women peaks during ovulation at just over 1 nM (300 to 500 pg/ml), and levels are slightly lower during the luteal phase of the menstrual cycle (Naftolin and Tolis, G. 1978). Thus, our findings are of significant public health importance because they showed that brain EC phenotype changes occurred by estrogen treatments equivalent to physiological and pharmacological levels (100 pg/ml - 1 ng/ml). In summary, the major novel findings which emerged from this study are that Pyk2 signaling leads to the expression of ID3 and that estrogen-induced ID3 is presumably necessary for regulating neovascularization of cerebral microvascular ECs. A better understanding of how microvascular lesions depend on ID3 or its downstream molecular targets can potentially open up new avenues for prevention and treatment of neurological diseases that depend on excessive vessel growth.
Acknowledgments
This research is supported by grants from NIH SC3 Award (1SC3GM084827-01A1).
References
- Aird WC. Spatial and temporal dynamics of the endothelium. J.Thromb.Haemost. 2005;3:1392–1406. doi: 10.1111/j.1538-7836.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van HL, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- Avraham HK, Lee TH, Koh Y, Kim TA, Jiang S, Sussman M, Samarel AM, Avraham S. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J.Biol.Chem. 2003;278:36661–36668. doi: 10.1074/jbc.M301253200. [DOI] [PubMed] [Google Scholar]
- Carcaillon L, Brailly-Tabard S, Ancelin ML, Rouaud O, Dartigues JF, Guiochon-Mantel A, Scarabin PY. High plasma estradiol interacts with diabetes on risk of dementia in older postmenopausal women. Neurology. 2014;82:504–511. doi: 10.1212/WNL.0000000000000107. [DOI] [PubMed] [Google Scholar]
- Chen L, Qiu J, Yang C, Yang X, Chen X, Jiang J, Luo X. Identification of a novel estrogen receptor beta1 binding partner, inhibitor of differentiation-1, and role of ERbeta1 in human breast cancer cells. Cancer Lett. 2009;278:210–219. doi: 10.1016/j.canlet.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Cutchins A, Harmon DB, Kirby JL, Doran AC, Oldham SN, Skaflen M, Klibanov AL, Meller N, Keller SR, Garmey J, McNamara CA. Inhibitor of differentiation-3 mediates high fat diet-induced visceral fat expansion. Arterioscler.Thromb.Vasc.Biol. 2012;32:317–324. doi: 10.1161/ATVBAHA.111.234856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human 'angiogenesis' assays with capillaries formed in vivo. Angiogenesis. 2001;4:113–121. doi: 10.1023/a:1012218401036. [DOI] [PubMed] [Google Scholar]
- Felty Q, Porther N. Estrogen-induced redox sensitive Id3 signaling controls the growth of vascular cells. Atherosclerosis. 2008;198:12–21. doi: 10.1016/j.atherosclerosis.2007.12.048. [DOI] [PubMed] [Google Scholar]
- Forrest ST, Barringhaus KG, Perlegas D, Hammarskjold ML, McNamara CA. Intron retention generates a novel Id3 isoform that inhibits vascular lesion formation. J.Biol.Chem. 2004a;279:32897–32903. doi: 10.1074/jbc.M404882200. [DOI] [PubMed] [Google Scholar]
- Forrest ST, Taylor AM, Sarembock IJ, Perlegas D, McNamara CA. Phosphorylation regulates Id3 function in vascular smooth muscle cells. Circ.Res. 2004b;95:557–559. doi: 10.1161/01.RES.0000142735.67542.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis T, Hansen AB, Houen G, Engel AM. Influence of angiogenesis inhibitors on endothelial cell morphology in vitro. APMIS. 2006;114:211–224. doi: 10.1111/j.1600-0463.2006.apm_189.x. [DOI] [PubMed] [Google Scholar]
- Gautschi O, Tepper CG, Purnell PR, Izumiya Y, Evans CP, Green TP, Desprez PY, Lara PN, Gandara DR, Mack PC, Kung HJ. Regulation of Id1 expression by SRC: implications for targeting of the bone morphogenetic protein pathway in cancer. Cancer Res. 2008;68:2250–2258. doi: 10.1158/0008-5472.CAN-07-6403. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Launer LJ, de Jong FH, Ruitenberg A, Stijnen T, van Swieten JC, Hofman A, Witteman JC, Pols HA, Breteler MM. Endogenous estradiol and risk of dementia in women and men: the Rotterdam Study. Ann.Neurol. 2003;53:607–615. doi: 10.1002/ana.10521. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, Chlebowski RT, Gass M, LaCroix A, Manson JE, Prentice RL, Rossouw J, Stefanick ML. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- Kanbay M, Sanchez-Lozada LG, Franco M, Madero M, Solak Y, Rodriguez-Iturbe B, Covic A, Johnson RJ. Microvascular disease and its role in the brain and cardiovascular system: a potential role for uric acid as a cardiorenal toxin. Nephrol.Dial.Transplant. 2011;26:430–437. doi: 10.1093/ndt/gfq635. [DOI] [PubMed] [Google Scholar]
- Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey A. Human Protein Reference Database--2009 update. Nucleic Acids Res. 2009;37:D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler EE, Baruah J, Urao N, Ushio-Fukai M, Fukai T, Chatterjee I, Wary KK. Low-Dose 6-Bromoindirubin-3'-oxime Induces Partial Dedifferentiation of Endothelial Cells to Promote Increased Neovascularization. Stem Cells. 2014;32:1538–1552. doi: 10.1002/stem.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler EE, Cowan CE, Chatterjee I, Malik AB, Wary KK. NANOG induction of fetal liver kinase-1 (FLK1) transcription regulates endothelial cell proliferation and angiogenesis. Blood. 2011;117:1761–1769. doi: 10.1182/blood-2010-07-295261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DN, Duckles SP, Gonzales RJ. Local oestrogenic/androgenic balance in the cerebral vasculature. Acta Physiol (Oxf) 2011;203:181–186. doi: 10.1111/j.1748-1716.2011.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc GG, Golanov E, Awad IA, Young WL. Biology of vascular malformations of the brain. Stroke. 2009;40:e694–e702. doi: 10.1161/STROKEAHA.109.563692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J.Clin.Invest. 1998;101:927–934. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Matsumura ME, Li F, Berthoux L, Wei B, Lobe DR, Jeon C, Hammarskjold ML, McNamara CA. Vascular injury induces posttranscriptional regulation of the Id3 gene: cloning of a novel Id3 isoform expressed during vascular lesion formation in rat and human atherosclerosis. Arterioscler.Thromb.Vasc.Biol. 2001;21:752–758. doi: 10.1161/01.atv.21.5.752. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Tolis G. Neuroendocrine regulation of the menstrual cycle. Clin.Obstet.Gynecol. 1978;21:17–29. doi: 10.1097/00003081-197803000-00003. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Baudler S, Muller C, Werner C, Werner N, Welzel H, Strehlow K, Bohm M. Redox-sensitive vascular smooth muscle cell proliferation is mediated by GKLF and Id3 in vitro and in vivo. FASEB J. 2002;16:1077–1086. doi: 10.1096/fj.01-0570com. [DOI] [PubMed] [Google Scholar]
- Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J.Cell Sci. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- Riant F, Bergametti F, Ayrignac X, Boulday G, Tournier-Lasserve E. Recent insights into cerebral cavernous malformations: the molecular genetics of CCM. FEBS J. 2010;277:1070–1075. doi: 10.1111/j.1742-4658.2009.07535.x. [DOI] [PubMed] [Google Scholar]
- Romero-Lanman EE, Pavlovic S, Amlani B, Chin Y, Benezra R. Id1 maintains embryonic stem cell self-renewal by up-regulation of Nanog and repression of Brachyury expression. Stem Cells Dev. 2012;21:384–393. doi: 10.1089/scd.2011.0428. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Stier CT, Jr, Chander PN, Rosenfeld L, Powers CA. Estrogen promotes microvascular pathology in female stroke-prone spontaneously hypertensive rats. Am.J.Physiol Endocrinol.Metab. 2003;285:E232–E239. doi: 10.1152/ajpendo.00029.2003. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am.J.Physiol Endocrinol.Metab. 2003;284:E184–E192. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Quaegebeur A, Vikkula M, Carmeliet P. Cerebrovascular disorders: molecular insights and therapeutic opportunities. Nat.Neurosci. 2011;14:1390–1397. doi: 10.1038/nn.2947. [DOI] [PubMed] [Google Scholar]
- Tang H, Hao Q, Fitzgerald T, Sasaki T, Landon EJ, Inagami T. Pyk2/CAKbeta tyrosine kinase activity-mediated angiogenesis of pulmonary vascular endothelial cells. J.Biol.Chem. 2002;277:5441–5447. doi: 10.1074/jbc.M110673200. [DOI] [PubMed] [Google Scholar]
- Theodorsson A, Theodorsson E. Estradiol increases brain lesions in the cortex and lateral striatum after transient occlusion of the middle cerebral artery in rats: no effect of ischemia on galanin in the stroke area but decreased levels in the hippocampus. Peptides. 2005;26:2257–2264. doi: 10.1016/j.peptides.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N.Engl.J.Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Bao WL, Qiu MH, Zhang LM, Lu SD, Huang YL, Sun FY. Role of vascular endothelial growth factor in neuronal DNA damage and repair in rat brain following a transient cerebral ischemia. J.Neurosci.Res. 2002;70:140–149. doi: 10.1002/jnr.10380. [DOI] [PubMed] [Google Scholar]