Abstract
Over 50% of HIV-infected (HIV+) persons are expected to be over age 50 by 2015. The pathogenic effects of HIV, particularly in cases of long-term infection, may intersect with those of age-related illnesses and prolonged exposure to combined antiretroviral therapy (cART). One potential outcome is an increased prevalence of neurocognitive impairment in older HIV+ individuals, as well as an altered presentation of HIV-associated neurocognitive disorders (HAND).
METHODS
In this study, we employed stepwise regression to examine 24 features sometimes associated with HAND in forty older (55–73 years of age) and thirty younger (32–50 years of age) HIV+, cART-treated participants without significant central nervous system confounds.
RESULTS
The features most effective in generating a true assessment of the likelihood of HAND diagnosis differed between older and younger cohorts, with the younger cohort containing features associated with drug abuse that were correlated to HAND, and the older cohort containing features that were associated with lipid disorders mildly associated with HAND.
CONCLUSION
As the HIV-infected population grows and the demographics of the epidemic change, it is increasingly important to re-evaluate features associated with neurocognitive impairment. Here we have identified features, routinely collected in primary care settings that provide more accurate diagnostic value than a neurocognitive screening measure among younger and older HIV-individuals.
Keywords: HIV, aging, neurocognitive tests, HAND
Introduction
With the success of combined anti-retroviral therapy (cART), the HIV-infected (HIV+) population is living longer. Over 25% of HIV+ adults in the United States are now over the age of 50, and over 50% are expected to be over the age of 50 by 2015 (Heaton et al. 2011). In this aging population, the pathogenic effects of HIV, especially in those with a long duration of infection, may intersect with those of age-related illnesses and the effects of long-term use of cART. Several broad categories of pathologies associated with HIV infection, aging, and long-term cART exist, including lipid disorders, B-cell lymphomas, and neurocognitive disorders (Lamers et al. 2012; Herndier et al. 1994; Bandaru et al. 2013). As such, it is now important that the unique medical challenges posed by the aging HIV+ population be delineated.
HIV-associated neurocognitive disorder (HAND) is one of the most common medical complications of HIV infection (Schouten et al. 2011). It can have a devastating impact on day-to-day functioning (Heaton et al. 2004), and may affect adherence to treatment, resulting in increased morbidity; therefore, it is important to identify HAND early and to begin effective intervention. However, HAND diagnosis remains highly dependent on neuropsychological test batteries that were developed in the pre-cART era, and validated primarily in younger cohorts for whom age-related co-morbidities were not considered. Investigating the pathogenesis of HAND in older HIV+ adults presents unique challenges (Gannon et al. 2011). Long-term exposure to cART and multiple co-morbid conditions that affect the brain may modify the mechanisms that lead to HAND in older HIV+ adults, resulting in a different clinical presentation than that described previously (Everall et al. 2009; Sacktor et al. 2007; Morgello et al. 2001; Heaton et al. 2011; Erlandson et al. 2014). As such, the current diagnostic schema for HAND may not adequately capture the true complexity of neurocognitive impairments that result from both HIV and other age-related factors (such as vascular diseases), and may fail to consider the multifaceted approach required for effective treatment. In addition, the study of HAND has often focused on relatively few potential causative factors at one time (Dwyer et al. 2014; Heaton et al. 2010; Cysique and Brew 2011). As such, a more comprehensive examination of factors affecting neurocognitive functioning in HIV+ adults is needed.
Finally, from a pragmatic perspective in the current age of managed healthcare, it is important to minimize the burdens of assessment for HAND. In research settings, HAND is generally identified via a comprehensive neurological examination and neuropsychological testing, in addition to neuroimaging, virologic, and medical assays. However, such testing is quite expensive and burdensome to patients, making it less feasible to screen a large number of patients in most clinical settings. At present, brief neurocognitive screening measures such as the HIV dementia scale (HDS) (Power et al. 1995), the International HIV Dementia Scale (Sacktor et al. 2005) and others (Zipursky et al. 2013; Milanini et al. 2014) are commonly used to detect HAND in primary or secondary care settings. However, many reviewers opine that these scales have low accuracy (Haddow et al. 2013; Zipursky et al. 2013), especially in milder forms of the disorder. But the availability of data (e.g., virologic, medical, demographic, behavioral, and psychosocial) collected within such clinical environments raises the possibility that consideration of these varied data in clinical screening can improve the detection of HAND without the necessity of comprehensive neurocognitive testing.
In this study, we evaluated extant clinical data from cohorts of both older and younger HIV+ individuals enrolled in a longitudinal study conducted by the National Neurological AIDS Bank at the University of California, Los Angeles (UCLA NNAB) in order to determine those features most predictive of HAND in differently aged groups. We hypothesized that information obtained via standard medical examinations would be more useful in detecting HAND as compared to the HDS, and that those features would differ between older and younger groups.
Methods
Data from participants enrolled in the UCLA NNAB were used for the analyses. The UCLA NNAB is a member of the National NeuroAIDS Tissue Consortium (NNTC) (Morgello et al. 2006). The NNTC was founded in 1998 to respond to researchers’ need for well-characterized human tissues and fluid samples and to study the mechanisms of HIV neurological disease. The NNAB uses standardized NNTC protocols to assess and classify neurocognitive impairment and to assign diagnoses according to established criteria (Morgello et al. 2006; Woods et al. 2004; Antinori et al. 2007). NNAB recruits adult volunteers with advanced HIV infection to enroll in the study. Eligibility is as follows: willing and able to give an informed consent or had a legal guardian to do so; agreed to donate brain and other tissues in the event of death; had a CD4+ count <50 cells/mm3 at enrollment and/or one or more of the following conditions: systemic lymphoma or other malignancy, mycobacterium avium complex, wasting with loss of ≥30% body weight, primary central nervous system (CNS) lymphoma, progressive multifocal leukoencephalopathy, congestive heart failure, renal failure, end-stage liver disease, or serum albumin <3.2 g/dL. Once enrolled, participants continue to be followed in the event that their health improves. All participants or their legal guardians are consented in accordance with UCLA IRB-approved protocols. The subjects are both male and female, from a variety of ethnicities, racial backgrounds and socioeconomic groups. All study material is coded in accordance with NNAB protocols.
Participants
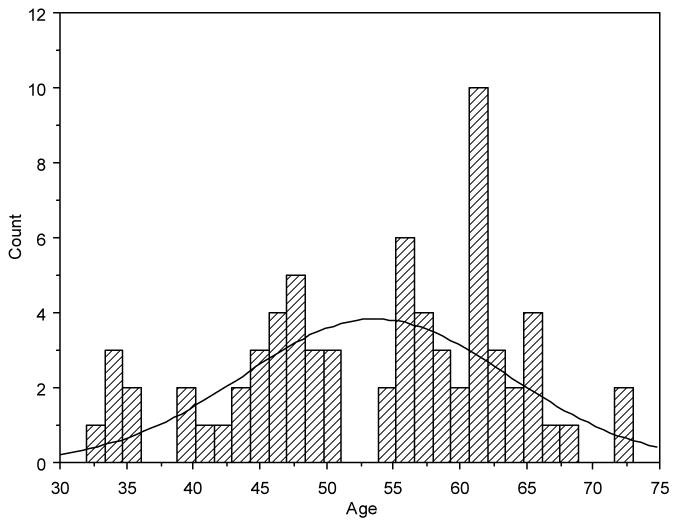
The primary NNAB dataset contained over 200 living HIV+ persons aged 21–80 with a variety of CNS conditions. From this primary dataset we identified 70 cART-treated HIV+ participants that met our inclusion criteria, ranging in age from 32–73 years (Figure I). For inclusion in the study, we required that each patient have associated data for all parameters of interest for at least one time point. Longitudinal data was not was considered in the study. In the case that a single patient received all tests on a single day more then once, only the last time point, where all variables were collected was considered for our analysis. The histogram of patient age provides a natural breakpoint of five years to divide the participants into two categories of younger and older patients. Thirty of these participants were 32–50 years of age with a mean age of 43.8±5.62 and a mean known duration of their HIV infection of 17.7±6.79 years. Forty were aged 55–73 years with a mean age of 61.1±4.56 years and a mean known duration of their HIV infection of 19.3±6.14 years. Duration of known HIV infection (years infected) was calculated as the number of years from the first positive HIV serology to the time of participant assessment for this study; however, the precise time of infection was rarely available and was likely to be of longer duration. Interestingly, the mean length of infection for the both cohorts was quite long (17.7 – 19.3 years), thus, each cohort represents individuals with moderately successful cART treatment over time. These two age-stratified datasets were used in our analysis to identify features related to HAND and global deficit score (GDS).
Fig. I. Histogram of Patient Age.
The bar graph shows the distribution of age in the two categories (younger vs. older) given a threshold at 53 years of age.
Procedures
As part of the standard NNAB protocol, participants were asked to complete a standardized medical and neurological examination including administration of the HDS performed by a board-certified neurologist who specializes in HIV neuro-disease; a standardized set of neuropsychological tests; a psychiatric/substance abuse inventory based on the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition DSM-IV (DSM-IV 2000), or Composite International Diagnostic Interview (CIDI) (Kessler and Ustun 2004), or the Psychiatric Research Interview for Substance and Mental Disorders (PRISM) (Hasin et al. 1996); the Beck Depression Inventory-Second Edition (BDI) (Beck et al. 1996); a urine screen for drugs of abuse; a blood draw for complete blood count (CBC) including white blood count, hemoglobin, hematocrit, and platelet count; CD4+ cell subsets, plasma HIV viral load, HCV serology (at entry) and RPR (syphilis serology); an optional lumbar puncture for cerebrospinal fluid (CSF) cell count, glucose, total protein, VDRL, and CSF HIV viral load; and in a subset of subjects, brain magnetic resonance imaging (MRI). In all cases, aggressive efforts were made to review and abstract the participants’ current medical records and verify their cART regimens and adherence. In addition, all participants were asked to sign a release of medical information so that their healthcare providers could be contacted and medical records obtained on a regular basis.
Criterion Variables
HAND was established based on the results of the procedures described above. HAND was determined via consensus conference by the study neurologist and neuropsychologist, with the consideration of the participants’ neuropsychological test scores and other pertinent data, including medical and substance use history and psychosocial background. Participants found to have neurocognitive impairment (NCI) attributable to other disease processes including head trauma, severe psychiatric disorders, learning disorder, metabolic encephalopathy, cerebral opportunistic infections, tumors, etc. were excluded from this study. The remaining participants were given a HAND classification based on either the previous AAN criteria (Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force 1991) if their evaluation occurred prior to the publication of the newer HAND research criteria in 2007, or according to the “Frascati” criteria if after 2007 (Antinori et al. 2007). Because the NNTC included a “subsyndromic” impairment classification since its inception, and because the criteria for MCMD and HAD (AAN) remained essentially identical to MND and HAD (Antinori et al 2007), we were able to group participants from both diagnostic eras. For simplicity, we use the Frascati designations in this paper, as follows: neurocognitively normal (0), asymptomatic neurocognitive impairment (1), possible minor neurocognitive disorder (2), probably MND (3), possible HIV–associated dementia (4), probable HAD (5). The Global Deficit Score (GDS) was determined by averaging individual neuropsychological (NP) domain deficit scores (Carey et al. 2004). The test battery is shown in Table I.
Table I.
Neurological domains and measures for the calculation of GDS.
| Neurocognative Domain | Test | Reference |
|---|---|---|
| I. Working Memory | Letter–Number Sequencing | WAIS–III; Wechsler, 1997 (Wechsler 1997) |
| PASAT Trial 1 | Wiens, Fuller, & Crossen, 1997 (Wiens et al. 1997) | |
| II. Motor | Grooved Pegboard, dominant and nondominant hand | Klove, 1963 (Klove 1963) |
| III. Information Processing Speed | Digit Symbol | WAIS–III; Wechsler, 1997 (Wechsler 1997) |
| Symbol Search | WAIS–III; Wechsler, 1997 (Wechsler 1997) | |
| Trail Making Test–Form A | Battery A., 1944 (Battery 1944) | |
| IV. Learning | HVLT–Revised Learning Trials total | Shapir et al., 1999 (Shapiro et al. 1999) |
| BVMT–Revised Learning Trials total | Benedict et al., 1996 (Benedict et al. 1996) | |
| V. Memory | HVLT–Revised Free Recall | Shapiro et al., 1999 (Shapiro et al. 1999) |
| BVMT–Revised Free Recall | Benedict et al., 1996 (Benedict et al. 1996) |
Modeling Variables
For each patient, 24 features routinely collected as part of the NNAB protocol were used as input for our statistical analysis to determine those features that best predicted, or explained variance in, HAND and GDS. Table II provides a description of the mean and standard deviation or the frequency for these features for both the entire participant set in addition to the younger and older cohorts.
Table II.
Sample Characteristics.
| Variable (units) | All Patients Mean (SD) (n=70) |
Younger Cohort Mean (SD) (n=30) |
Older Cohort Mean (SD) (n=40) |
|---|---|---|---|
| HAND | 1.24 (1.40) | 1.20 (1.32) | 1.28 (1.47) |
| GDS | 0.74 (0.62) | 0.85 (0.64) | 0.65 (0.59) |
| HDS | 12.2 (3.1) | 12.7 (2.7) | 11.8 (3.3) |
| Years infected (years) | 18.61 (6.43) | 17.70 (6.79) | 19.30 (6.14) |
| Age (years) | 53.7 (10.0) | 43.9 (5.6) | 61.1 (4.6) |
| Absolute CD4+ count (cells/mm3) | 411.46 (303.55) | 299.87 (241.11) | 495.15 (320.98) |
| Plasma viral load (IU/L) | 14,036.14 (50,163.34) | 23,994.63 (62,103.08) | 6,567.05 (38,087.31) |
| Nadir CD4+ count (cells/mm3) | 202.01 (211.69) | 134.47 (143.26) | 252.68 (240.59) |
| Bld Hemoglobin (g/L) | 13.8 (1.8) | 13.4 (2.2) | 14.1 (1.5) |
| BDI | 11.76 (9.47) | 13.53 (11.03) | 10.43 (8.00) |
| # Comorbidities | 2.41 (1.65) | 2.07 (1.28) | 2.68 (1.85) |
| Variable | All Patients (%) | Younger Cohort (%) | Older Cohort (%) |
|---|---|---|---|
| HCV | 25.7 | 20.0 | 30.0 |
| Chronic renal disease | 13.2 | 10.0 | 15.8 |
| Lipodystrophy | 27.5 | 20.1 | 32.5 |
| Tobacco smoking | 48.6 | 46.7 | 55.0 |
| Hypertension | 50.7 | 40.0 | 59.0 |
| Diabetes | 21.7 | 23.3 | 20.5 |
| Cardiovascular | 24.6 | 24.1 | 25.0 |
| Liver disease | 7.1 | 3.3 | 10.0 |
| Cerebrovascular | 10.4 | 13.8 | 7.8 |
| Hyperlipidemia | 39.1 | 26.7 | 48.7 |
| Cocaine/Meth | 8.5 | 3.3 | 12.5 |
| Race | |||
| Black | 34.3 | 40.0 | 30.0 |
| Hispanie (Caucasian) | 17.1 | 30.0 | 7.5 |
| White | 38.6 | 23.3 | 50.0 |
| Other (Native American) | 10.0 | 6.7 | 12.5 |
| Gender | |||
| Male | 84.3 | 90.0 | 80.0 |
| Female | 15.7 | 10.0 | 20.0 |
Nadir absolute CD4+ cell count was determined by chart review and/or participant recollection. Tobacco smoking was determined by self-report, participant examination and/or chart review. Depression was determined by the BDI. Chronic renal disease was assessed by self-report and/or review of medical records including recent measurements of creatinine (Cr) and glomerular filtration rate (GFR). Hypertension was assessed by self-report, review of medications, chart review, and review of patient’s serial blood pressures taken on multiple NNAB visits. Hyperlipidemia was assessed by self-report, review of current medications, and chart review of serum lipid panels. Diabetes was assessed by self-report, use of medications specific for diabetes, chart review for elevated hemoglobin A1C or elevated fasting blood sugars (≥100mg/dL). Lipodystrophy was determined by NNAB physical examination looking for evidence of abnormalities such as facial lipoatrophy, centripetal fat accumulation, and/or wasting of fat in the extremities, and confirmed by anthropomorphic measurements at each study visit. Cardiac disease was determined by participant history, medication review (prescription of one or more medications specific for cardiac disease) and/or medical record review demonstrating abnormal cardiac function. Liver disease was determined by self-report, physical examination, and review of medical records for elevated liver enzymes, elevated alpha fetoprotein, or abnormal liver biopsy. Cerebrovascular disease was assessed by the NNAB neurological exam and history as well as medication review and review of medical records, including neuroimaging scans when available. Cocaine or methamphetamine use was assessed via urine toxicology; we focused on these two drugs due to their consistent association with additive neurocognitive problems in HIV+ individuals (Rippeth et al. 2004; Carey et al. 2006; Levine et al. 2006; Meade et al. 2011; Gaskill et al. 2009; Silverstein et al. 2012) and because they are recognized as an intersecting presenting condition of HIV infection (High et al. 2012). The study neurologist administered the HDS test. The HDS test generates a score between from 0–16, which was used in a continuous manner for these analyses. An HDS score of ≤10 was considered to be an indication of impairment (Power et al. 1995).
Statistical models
For each age group and also with all participants pooled together as a single dataset, stepwise regression using StatView (SAS International) was used to identify multiple linear regressions relating patient features to the dependent variable of HAND or GDS. For each of these dependent variables we first considered HDS score on its own to evaluate the utility of this single metric in clinical evaluation. For each of the dependent variables above we then used a standard process of stepwise regression to evaluate multiple linear regressions using all available subject features. Stepwise regression resulted in multiple linear regressions that used as few terms as possible with maximal correlation (R2) to the dependent variable. Terms in the regressions were reviewed to ensure that an appropriate p-value had been attained and that coefficients associated with the features were reasonable.
Results
HAND
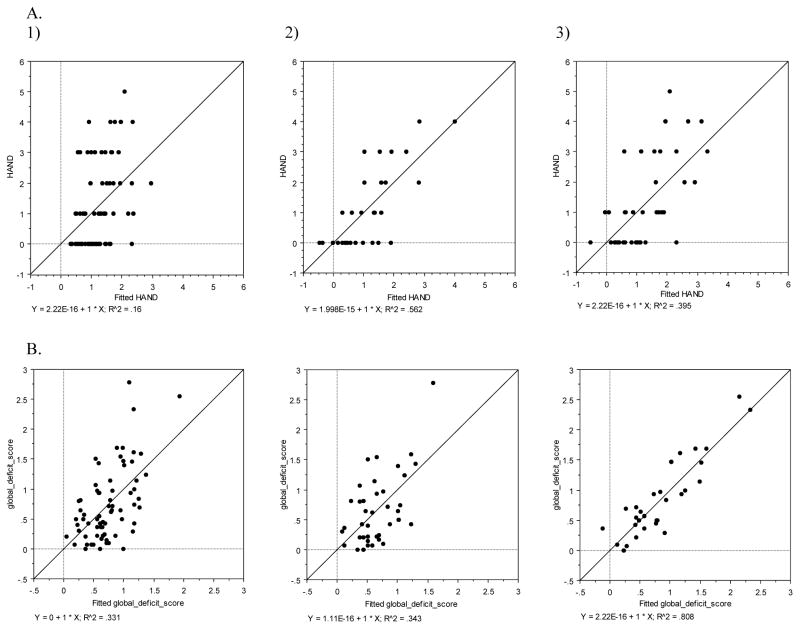
When combining all 70 participants and identifying variables of greatest concern using stepwise regression, a two-term linear regression was identified with correlation to HAND (y = −0.120 (HDS) + 0.041 (BDI) + 2.234; adj R2 = 0.135). All terms in this model are significant to the p<0.05 level. However, examining HDS on its own relative to HAND provided a weaker correlation of adj R2 = 0.071, thus indicating the limitations of this test on its own in a cART-exposed, longer-lived patient cohort.
When examining only participants in the younger cohort (n=30), stepwise regression identified a 4-term model (y = −0.110 (years infected) + 0.091 (age) − 0.356 (Bld hemoglobin) + 3.416 (cocaine/methamphetamine) + 3.788; adj R2=0.492). All terms in this model were significant to the p<0.05 level. Interestingly, cocaine/methamphetamine use on its own provided a stronger correlation to HAND (adj R2=0.130) than HDS on its own (adj R2=0.005).
When examining only the older cohort (n=40), stepwise regression identified a model with three terms that generated a useful regression (y = 0.077 (years infected) + 0.108 (BDI) − 0.766 (hyperlipidemia) − 0.236; adj R2=0.345). Only BDI was significant to p<0.0001 in this model, all other terms were significant to p<0.05. HDS on its own provided a weaker correlation of adj R2=0.094 to HAND in the older cohort. A summary of these regressions is provided in Figure IIA.
Fig. II. Multiple Regression Plots.
A) HAND relative to the output of three stepwise regressions (fitted HAND) for 1) all patients, 2) the young cohort, and 3) the old cohort being examined. B). Global deficit score relative to the output of three stepwise regressions (fitted global deficit score) for 1) all patients, 2) the young cohort, and 3) the old cohort being examined.
Global deficit score
When combining all 70 participants and using GDS as the dependent variable, stepwise regression resulted in a four term model (y = −0.093 (HDS) − 0.018 (age) + 0.015 (BDI) − 0.292 (diabetes) + 3.004; adj R2=0.290). Of these terms, only HDS, age, and BDI were significant to the p<0.05 level. Diabetes (p=0.0567) was retained in the model in light of its large coefficient. HDS on its own generated a weak correlation with GDS (adj R2=0.145) over the entire cohort.
When examining the younger cohort, a 6-term model was realized (y = − 0.111 (HDS) − 0.028 (years infected) − 0.134 (Bld hemoglobin) − 0.721 (liver disease) − 0.096 (# comorbid) + 1.664 (cocaine/methamphetamine) + 5.432; adj R2=0.758). This model included all the terms that correlated to HAND for this cohort. Of these terms, only the number of comorbid conditions was not statistically significant at the p=0.05 level. (p=0.0598). HDS, Bld hemoglobin, and cocaine/methamphetamine were all significant to the p<0.0001 level. HDS on its own for the younger cohort had a much weaker correlation of adj R2=0.271 to GDS.
When examining the older cohort, a 3-term model was realized (y = −0.071 (HDS) + 0.647 (liver disease) + 0.391 (gender) + 0.079; adj R2=0.289). All of these terms were significant (p<0.02) with the exception of gender (p=0.0570). HDS on its own resulted in a weaker correlation of adj R2=0.106 to GDS for the older cohort. A summary of these regressions is given in Figure IIB. Table III provides a summary of all results by dependent variable and by cohort age.
Table III.
Features Identified Through Stepwise Regression. Terms marked by asterisk have the highest coefficient in each of the realized stepwise regressions.
| Dependent Variable | All Patients | Younger Cohort | Older Cohort |
|---|---|---|---|
| HAND | HDS* BDI |
Years infected Age Bld hemoglobin Cocaine/meth* |
Years infected BDI* Hyperlipidemia |
| GDS | HDS Age BDI Diabetes* |
HDS Years Infected Bld hemoglobin Liver disease # Comorbid Cocaine/meth* |
HDS Liver disease* Gender |
Discussion
In this study we sought to determine which variables, routinely collected in primary care settings, predicted both HAND diagnosis and neurocognitive impairment. In addition, we determined if these variables differed between older and younger HIV+ individuals. Not surprisingly (Zipursky et al. 2013), in the overall sample the HDS was weakly correlated with GDS but not HAND, suggesting that it is a poor indicator of neurocognitive dysfunction compared to approaches that consider multiple features. This is consistent with recent studies that have found the HDS to be of limited utility in identifying the majority of cART-exposed individuals with HAND (i.e., those with mild impairment) (Bottiggi et al. 2007; Smith et al. 2003; Cross et al. 2013). The model was strengthened when HDS was combined with BDI. This finding is also not unexpected, as there is reason to believe that depression is related to HAND both via molecular and behavioral avenues (Atkinson et al. 2008; Leserman 2008; Dantzer and Kelley 2007; Raison et al. 2006; Fialho et al. 2013); however, the relationship is far from unequivocal.
When examining the young vs. old cohorts, positive urine toxicology for cocaine/methamphetamine, years of known infection, age, and blood hemoglobin in the younger population become useful for predicting HAND, whereas in the older population a small and different set of features were significant (depression, hyperlipidemia, and known years of infection). The finding that hyperlipidemia may be associated with neurocognitive disorders in older HIV+ individuals agrees with other studies that suggest there is a cerebrovascular component to the development of NCI (Foley et al. 2010; Becker et al. 2009; Cysique et al. 2010; McCutchan et al. 2012). This finding could also indicate increased inflammation in the older cohort, as both HIV dementia and hyperlipidemia have been linked to inflammatory cytokines (Gongvatana et al. 2012; Shiramizu et al. 2011; Bandaru et al. 2013; Bernstein et al. 2006; Lo et al. 2007; Correia et al. 2013). The number of known years infected is considered as an important feature for both the younger and older cohorts, but was not statistically significant in the best multiple linear regression associated with HAND over all participants, suggesting that this might be the result of a limited participant sampling. In all cases when predicting HAND, these multiple linear regressions outperformed HDS on their own, indicating the need for additional features to be used in combination with that screening measure. Further, the difference between HDS on its own and the use of a multiple linear regression for the prediction of HAND was pronounced in the younger cohort. Such a result confirms the need for additional participant features for detecting HAND in both young and older cART-treated cohorts so that improved models can be developed.
With GDS as the dependent variable, HDS remained important for the sample as a whole and in both age groups. However, beyond this one feature, each division of the data by age resulted in different features that enhanced the regression to GDS, demonstrating that NCI in the two groups may have different etiological factors contributing to HAND. As was the case with HAND, prediction of GDS in the younger cohort benefited the most from the addition of other parameters in addition to HDS, presenting a drastic improvement over HDS alone. The younger cohort model made use of more terms than those that were indicative of HAND: years known infected, blood hemoglobin, cocaine/methamphetamine use, liver disease, and number of co-morbidities. This set of features may have its own internal correlations, for example cocaine/methamphetamine use could result in low hemoglobin due to poor nutrition, is often associated with alcohol abuse, which could result in liver disease and increase the number of co-morbidities due to overall bad health combined HIV infection. This finding further underscores the importance of these features when examining younger HIV+ patients for neurocognitive issues. The features associated with GDS in the older cohort were different from those associated with HAND, thus highlighting the need to consider a wider range of variables when predicting HAND in older individuals. In the older cohort, it is interesting that hyperlipidemia was correlated to HAND and liver disease was correlated to GDS because uncontrolled hyperlipidemia can lead to liver disease (Yang 2004). Table IV compares the difference between using the HDS on its own relative to HAND or GDS versus the multiple linear regression with additional terms.
Table IV.
Differences in HIV dementia scale (HDS) vs. multiple linear regression (MLR) correlation (adjusted R2) to HAND and GDS given different age cohorts.
| Dependent Variable | All Patients Combined | Younger Cohort | Older Cohort | |
|---|---|---|---|---|
| HAND | HDS MLR |
0.071 0.135 |
0.005 0.492 |
0.094 0.345 |
| GDS | HDS MLR |
0.145 0.290 |
0.271 0.758 |
0.106 0.289 |
Using stepwise regression we have identified combinations of commonly collected clinical features relevant for these HIV- and age-related impairments. Clearly this approach would benefit from the examination of a larger number of participants. As the HIV+ population ages, this contemporary and quantitative method could be used to understand the contributions of multiple viral, age, and cART-related factors in the pathogenesis of NCI. Here we have elucidated the relative importance of the different clinical factors that are often measured as HAND or GDS.
For appropriate diagnosis, statistical methods that can handle the complex and multi-factorial features that may lead to neurocognitive impairment are necessary to identify older HIV+ individuals at the highest risk. Moreover, the development of a method that can incorporate a wide range of newer biological tests in the pipeline, including MRI (Li et al. 2011), novel biomarkers (Burdo et al. 2011), and information about the CNS brain penetration effectiveness (CPE) of cART (Shapshak et al. 2011) would be a highly valuable asset to the medical community. Such models may require nonlinear multivariate models such as neural networks or support vector machines that are able to merge the information from significantly different types of data (images, demographics, etc.) and determine a classification/probability of neuropsychological condition based on age. Cysique et al. (Cysique et al. 2010) developed a screening algorithm for neuropsychological impairment on a similarly sized cohort of individuals using support vector machines that used age, CD4+ cell count, past CNS HIV-related diseases, and current treatment duration to classify neurocognitively impaired vs. normal individuals. Our study has made use of many more features than those used by Cysique et al. (Cysique et al. 2010) and has also focused on the identification of age-specific features that can be used to diagnose HAND in older individuals. A recent study by the same group used age and neurodiagnosis in various statistical analyses and found no significant interaction of the two variables (Cysique et al. 2011). Noted in that analysis is that the other cohort consisted of a homogenous group of primarily educated men, whereas our data sets contained patients from diverse backgrounds, including 15.7% women. In the Cysique study, the age separation between the means of the two age groups was a seven years, whereas in our study the difference between the means was 17.2 years. Moreover, we considered varied types of pathological features occurring over a long period of time. In the younger cohort we found features that are likely synergistic, and as such may have more total impact on CNS injury. For example, low blood hemoglobin is a correlate of comorbidities and drug use, both of which were identified as meaningful correlates of HAND. In the older cohort it is likely that new biomarkers for NCI are required for adequate diagnosis. Therefore, our findings differ from the study by Cysique et al. primarily because of the greater diversity of our cohort, an increased age spread (a 14 year difference between the means of the two age groups in our cohort) and the inclusion of multiple risk factors in the analysis. Further, our data supports Becker et al.’s recent determination that non-HIV factors are significant in older neurocognitively impaired individuals (Becker et al. 2011).
Continuous re-evaluation of the diagnostic methods and scales used to assess HAND are necessary because both therapies and HIV disease pathogenesis change with time (Gandhi et al. 2010; Becker et al. 2011). This is especially the case for the growing number of older, cART-treated HIV+ individuals, where there is concern that other age related features that influence neurological decline should be considered, such as duration of infection, cardiovascular disease or lipodystrophy. The features found in this analysis that were associated with HAND or GDS in older vs. younger HIV+ individuals would be greatly enhanced with more data as these individuals continue to age. Our initial evaluation in this direction suggests that additional focus should be applied toward a revised method for NP evaluation in the older HIV+ population.
Acknowledgments
The authors thank the patients and staff associated with the NNAB. The project was supported by National Institutes of Health grants U24MH100929, U01MH083500 and R24 NS-38841 (EJS, MVS), U01MH083500 (PS), U01MH083500, R24 NS-38841 and R01DA030913 (AJL), UM1CA181255 (MSM) and R01MH100984 (SLL, GBF EJS, MSM).
Footnotes
Conflict of Interest: The authors, Gary B. Fogel, Susanna L. Lamers, Andrew J. Levine, Miguel Valdes-Sueiras, Michael S. McGrath, Paul Shapshak, Elyse J. Singer declare that they have no conflict of interest.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. 01.WNL.0000287431.88658.8b [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Heaton RK, Patterson TL, Wolfson T, Deutsch R, Brown SJ, Summers J, Sciolla A, Gutierrez R, Ellis RJ, Abramson I, Hesselink JR, McCutchan JA, Grant I. Two-year prospective study of major depressive disorder in HIV-infected men. J Affect Disord. 2008;108 (3):225–234. doi: 10.1016/j.jad.2007.10.017. S0165-0327(07)00381-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Mielke MM, Sacktor N, McArthur JC, Grant I, Letendre S, Chang L, Wojna V, Pardo C, Calabresi P, Munsaka S, Haughey NJ. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013;81 (17):1492–1499. doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battery AIT. Manual of directions and scoring. Adjunctant General’s Office; Washington, D.C: 1944. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II 1996 [Google Scholar]
- Becker JT, Dew MA, Aizenstein HJ, Lopez OL, Morrow L, Saxton J. Concurrent validity of a computer-based cognitive screening tool for use in adults with HIV disease. AIDS Patient Care STDS. 2011;25 (6):351–357. doi: 10.1089/apc.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, Ragin A, Sacktor N, Selnes OA, Visscher BR. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73 (16):1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. 73/16/1292 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH, Schretlen D, Groningger L, Dobraski M. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment. 1996;8 (2):145–153. [Google Scholar]
- Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Archives of internal medicine. 2006;166 (8):902–908. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiggi KA, Chang JJ, Schmitt FA, Avison MJ, Mootoor Y, Nath A, Berger JR. The HIV Dementia Scale: predictive power in mild dementia and HAART. J Neurol Sci. 2007;260 (1–2):11–15. doi: 10.1016/j.jns.2006.03.023. S0022-510X(07)00217-1 [pii] [DOI] [PubMed] [Google Scholar]
- Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. The Journal of infectious diseases. 2011;204 (8):1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26 (3):307–319. doi: 10.1080/13803390490510031. 2898QRA0RWTH996U [pii] [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10 (2):185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Correia S, Cohen R, Gongvatana A, Ross S, Olchowski J, Devlin K, Tashima K, Navia B, Delamonte S. Relationship of plasma cytokines and clinical biomarkers to memory performance in HIV. Journal of neuroimmunology. 2013;265 (1–2):117–123. doi: 10.1016/j.jneuroim.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S, Onen N, Gase A, Overton ET, Ances BM. Identifying risk factors for HIV-associated neurocognitive disorders using the international HIV dementia scale. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2013;8 (5):1114–1122. doi: 10.1007/s11481-013-9505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. Journal of neurovirology. 2011;17 (2):176–183. doi: 10.1007/s13365-011-0021-x. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Bain MP, Wright E, Brew BJ. HIV and age do not substantially interact in HIV-associated neurocognitive impairment. J Neuropsychiatry Clin Neurosci. 2011;23 (1):83–89. doi: 10.1176/appi.neuropsych.23.1.83. 23/1/83 [pii] [DOI] [PubMed] [Google Scholar]
- Cysique LA, Murray JM, Dunbar M, Jeyakumar V, Brew BJ. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11 (10):642–649. doi: 10.1111/j.1468-1293.2010.00834.x. HIV834 [pii] [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21 (2):153–160. doi: 10.1016/j.bbi.2006.09.006. S0889-1591(06)00300-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-IV. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Dwyer R, Wenhui L, Cysique L, Brew BJ, Lal L, Bain P, Wesselingh S, Wright EJ. Symptoms of depression and rates of neurocognitive impairment in HIV positive patients in Beijing, China. Journal of Affective Disorders. 2014;162:89–95. doi: 10.1016/j.jad.2014.03.038. [DOI] [PubMed] [Google Scholar]
- Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional Impairment, Disability, and Frailty in Adults Aging with HIV-Infection. Current HIV/AIDS reports. 2014 doi: 10.1007/s11904-014-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15 (5–6):360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialho RM, Pereira M, Mendonca N, Ouakinin S. Depressive symptoms and neurocognitive performance among HIV-infected women. Women & health. 2013;53 (2):117–134. doi: 10.1080/03630242.2013.767301. [DOI] [PubMed] [Google Scholar]
- Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, Mason K, Castellon S, Hinkin CH. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin Neuropsychol. 2010;24 (2):265–285. doi: 10.1080/13854040903482830. 919290253 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NS, Moxley RT, Creighton J, Roosa HV, Skolasky RL, Selnes OA, McArthur J, Sacktor N. Comparison of scales to evaluate the progression of HIV-associated neurocognitive disorder. HIV Ther. 2010;4 (3):371–379. doi: 10.2217/hiv.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24 (3):275–283. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175 (3):1148–1159. doi: 10.2353/ajpath.2009.081067. S0002-9440(10)60624-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A, Correia S, Devlin K, Dunsiger S, Ross S, Navia B, Tashima K, DeLaMonte S, Cohen R. Clinical Factors and Plasma Cytokine Markers Are Related to Brain Volumes in HIV+ Individuals. Paper presented at the 19th Conference on Retroviruses and Opportunistice Infections; Seattle, WA. March 5–8.2012. [Google Scholar]
- Haddow LJ, Floyd S, Copas A, Gilson RJ. A systematic review of the screening accuracy of the HIV Dementia Scale and International HIV Dementia Scale. PloS one. 2013;8 (4):e61826. doi: 10.1371/journal.pone.0061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153 (9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75 (23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17 (1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130S1355617704102130. [pii] [DOI] [PubMed] [Google Scholar]
- Herndier BG, Kaplan LD, McGrath MS. Pathogenesis of AIDS lymphomas. AIDS. 1994;8 (8):1025–1049. doi: 10.1097/00002030-199408000-00003. [DOI] [PubMed] [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, Levin J, Miotti PG, Munk RJ, Nass H, Rinaldo CR, Jr, Shlipak MG, Tracy R, Valcour V, Vance DE, Walston JD, Volberding P. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. Journal of acquired immune deficiency syndromes. 2012;60(Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13 (2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H. Clinical Neuropsychology. Med Clin North Am. 1963;47:1647–1658. [PubMed] [Google Scholar]
- Lamers SL, Fogel GB, Singer EJ, Salemi M, Nolan DJ, Huysentruyt LC, McGrath MS. HIV-1 Nef in macrophage-mediated disease pathogenesis. International reviews of immunology. 2012;31 (6):432–450. doi: 10.3109/08830185.2012.737073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70 (5):539–545. doi: 10.1097/PSY.0b013e3181777a5f. PSY.0b013e3181777a5f [pii] [DOI] [PubMed] [Google Scholar]
- Levine AJ, Hardy DJ, Miller E, Castellon SA, Longshore D, Hinkin CH. The effect of recent stimulant use on sustained attention in HIV-infected adults. J Clin Exp Neuropsychol. 2006;28 (1):29–42. doi: 10.1080/13803390490918066. Q7715T7808L62491 [pii] [DOI] [PubMed] [Google Scholar]
- Li C, Zhang X, Komery A, Li Y, Novembre FJ, Herndon JG. Longitudinal diffusion tensor imaging and perfusion MRI investigation in a macaque model of neuro-AIDS: a preliminary study. Neuroimage. 2011;58 (1):286–292. doi: 10.1016/j.neuroimage.2011.05.068. S1053-8119(11)00576-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, Grinspoon SK. Effects of TNF-alpha neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. American journal of physiology Endocrinology and metabolism. 2007;293 (1):E102–109. doi: 10.1152/ajpendo.00089.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan A, Marquie-Beck J, FitzSimons C, Letendre S, Ellis R, Heaton RK, Wolfson T, Marra C, Ances B, Grant I. Role of Central Obesity, Diabetes, and Metabolic Variables in HIV-associated Neurocognitive Disorder. Paper presented at the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. March 5–8.2012. [Google Scholar]
- Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. J Behav Med. 2011;34 (2):128–138. doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanini B, Wendelken LA, Esmaeili-Firidouni P, Chartier M, Crouch PC, Valcour V. The Montreal Cognitive Assessment to Screen for Cognitive Impairment in HIV Patients Older Than 60 Years. Journal of acquired immune deficiency syndromes. 2014;67 (1):67–70. doi: 10.1097/QAI.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C, Grant I, Singer EJ. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27(4):326–335. doi: 10.1046/j.0305-1846.2001.00334.x. nan334 [pii] [DOI] [PubMed] [Google Scholar]
- Morgello S, Holzer CE, 3rd, Ryan E, Young C, Naseer M, Castellon SA, Frol AB, Atkinson JH, Gelman BB, Grant I, Singer EJ. Interrater reliability of the Psychiatric Research Interview for Substance and Mental Disorders in an HIV-infected cohort: experience of the National NeuroAIDS Tissue Consortium. Int J Methods Psychiatr Res. 2006;15 (3):131–138. doi: 10.1002/mpr.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41(6):778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8 (3):273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27 (1):24–31. doi: 10.1016/j.it.2005.11.006. S1471-4906(05)00288-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [pii] [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, Shikuma C, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol. 2007;13 (3):203–209. doi: 10.1080/13550280701258423. 779949428 [pii] [DOI] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19 (13):1367–1374. [PubMed] [Google Scholar]
- Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS. 2011;25 (5):561–575. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13 (3):348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- Shapshak P, Kangueane P, Fujimura RK, Commins D, Chiappelli F, Singer E, Levine AJ, Minagar A, Novembre FJ, Somboonwit C, Nath A, Sinnott JT. Editorial neuroAIDS review. AIDS. 2011;25 (2):123–141. doi: 10.1097/QAD.0b013e328340fd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Ananworanich J, Chalermchai T, Siangphoe U, Troelstrup D, Shikuma C, De Grutolla V, Sithinamsuwan P, Praihirunkit P, Rattanamanee S, Valcour V. Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol. 2011 doi: 10.1007/s13365-011-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Shah A, Weemhoff J, Kumar S, Singh DP, Kumar A. HIV-1 gp120 and drugs of abuse: interactions in the central nervous system. Current HIV research. 2012;10 (5):369–383. doi: 10.2174/157016212802138724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, van Gorp WG, Ryan ER, Ferrando SJ, Rabkin J. Screening subtle HIV-related cognitive dysfunction: the clinical utility of the HIV dementia scale. J Acquir Immune Defic Syndr. 2003;33 (1):116–118. doi: 10.1097/00126334-200305010-00018. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Inteligence Scale-III. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wiens AN, Fuller KH, Crossen JR. Paced Auditory Serial Addition Test: adult norms and moderator variables. J Clin Exp Neuropsychol. 1997;19 (4):473–483. doi: 10.1080/01688639708403737. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [pii] [DOI] [PubMed] [Google Scholar]
- Yang SJ. Hyperlipidemia and liver diseases. Hepatobiliary Pancreat Dis Int. 2004;3(1):10–11. 162 [pii] [PubMed] [Google Scholar]
- Zipursky AR, Gogolishvili D, Rueda S, Brunetta J, Carvalhal A, McCombe JA, Gill MJ, Rachlis A, Rosenes R, Arbess G, Marcotte T, Rourke SB. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: a systematic review of the literature. AIDS. 2013;27 (15):2385–2401. doi: 10.1097/QAD.0b013e328363bf56. [DOI] [PMC free article] [PubMed] [Google Scholar]