Abstract
Fenobam is a negative allosteric modulator of the metabotropic glutamate receptor subtype 5 (mGluR5) with inverse agonist activity and is expected to contribute to the treatment of neuropsychiatric disorders involving dysfunction of mGluR5 including Fragile × syndrome. This study examined whether [11C]ABP688, an antagonist PET radioligand, competes with fenobam for the same binding site in the non-human primate brain and would allow examination of occupancy-plasma concentration relationships in the evaluation of the drug for target disorders in the human brain. Four paired PET studies with [11C]ABP688 were performed in baboons at a baseline condition and after intravenous treatment with fenobam at different dose levels (0.3 - 1.33 mg/kg). Total distribution volume (VT) and binding potential (BPND) using the cerebellum as a reference region were obtained by the plasma reference graphical method. Then it was examined whether occupancy follows a dose-dependent, saturating pattern that was predicted by a modified first-order Hill equation in individual regions. Baseline regional VT and BPND values agreed with previously published data. Occupancy showed dose-dependent and saturating patterns in individual regions, reaching >90% occupancy at 1.33 mg/kg dose of fenobam in the majority of regions. To our knowledge, this is the first use of PET to characterize the mGluR5 therapeutic drug fenobam. This study demonstrates a proof of principle for determining the in vivo occupancy of fenobam in primates. The results indicate that [11C]ABP688 and PET may be useful for examination of occupancy of mGluR5 by fenobam, which should prove to be useful for designing future studies and treatment of human disease states.
Keywords: [11C]ABP688, fenobam, glutamate, mGluR5, occupancy, PET
INTRODUCTION
Glutamate is considered to be the major excitatory neurotransmitter in the central nervous system, and balanced glutamatergic transmission is required for normal brain function (Pin et al., 1995). Glutamate receptors are divided into two classes, ionotropic and metabotropic. The metabotropic glutamate receptors (mGluRs) are a family of eight G-protein-coupled receptors and are divided into three groups based on their structure and function (Niswender et al., 2010). Group I mGluRs include the subtypes mGluR1 and mGluR5. These receptors are mainly expressed at postsynaptic neurons (Shigemoto et al., 1997) as well as glia cells (D’Ascenzo et al., 2007). Their role is to modulate excitatory transmission by stimulating the release of Ca2+ from intracellular stores and activation of N-methyl-d-aspartic acid (NMDA) receptors (Spooren et al., 2003; Pisani et al., 2001).
Much attention has been devoted to mGluR5 due to its possible involvement in a variety of disorders such as anxiety (Ballard et al., 2005), depression (Deschwanden et al., 2011), schizophrenia (Krystal et al., 2010), pain (Jacob et al., 2009), addiction (Olive et al., 2009), Parkinson’s disease (Morin et al., 2010), and amyotrophic lateral sclerosis (Anneser et al., 2004). Recent findings have also shown a connection between mGluR5 and the most common inherited form of mental retardation and autism, Fragile × syndrome (Yan et al., 2005; Dölen et al., 2007). Several mGluR5 antagonists are currently in clinical trials for the treatment of Fragile × syndrome (Berry-Kravis et al., 2009; Levenga et al., 2011). One of these drugs, fenobam, was identified using rodent model for anxiety and previously investigated as an anxiolytic in a number of phase II studies in the early 1980s (Itil et al., 1978; Friedman et al., 1980; Pecknold et al., 1982). The molecular mechanism of action of fenobam was discovered 25 years later by Porter et al. (2005) and shown to be a negative allosteric modulator of mGluR5. A recent study showed that combined fenobam and amantadine treatment produced robust antidyskinetic effects in a primate model of Parkinson’s disease (Ko et al., 2014).
The recent availability of mGluR5 PET radioligands has allowed in vivo investigations of the distribution and function of this receptor subtype (Hamill et al., 2005; Ametamy et al., 2006; Siméon et al., 2007). The highest expression of mGluR5 in the brain is in regions such as caudate/putamen, cortex, and hippocampus (Romano et al., 1995). Brain structures expressing this receptor are known to play important roles in emotion, motivation and motor control, leading to the hypothesis that mGluR5 may play a critical role in affective disorders, including anxiety, depression, and neurodegenerative diseases involving motor dysfunction (Spooren et al., 2003). It has been demonstrated that exaggerated signaling through mGluR5 can account for multiple cognitive and syndromic features of Fragile × syndrome (Dölen et al., 2008).
This finding combined with the potential availability of the phase II validated drug fenobam, has led to preliminary investigation of the efficacy of fenobam treatment for Fragile × syndrome (Berry-Kravis et al., 2009). Fenobam dosing in the aforementioned study ranged from 50 to 150 mg per adult subject and were selected based on doses in previous studies where CNS side effects were observed. One tool that would prove valuable for dosing determination is measurement of occupancy by PET. Therefore, we sought to determine the PET occupancy of mGluR5 by fenobam at various doses using [11C]ABP688 for target engagement. A similar study using 3-((2-methyl-1,3-thiazol-4-yl)ethynyl)pyridine (MTEP) as a blocker was recently reported for one fixed dose by DeLorenzo et al. (2011a). A more recent study was conducted in humans using AZD2066 as a blocker (Kågedal et al., 2013). The aim of this study is to demonstrate that fenobam occupies the same receptor as [11C]ABP688 in the non-human primate brain in a dose-dependent and saturable manner. Such a finding would indicate that [11C]ABP688 and PET could be a powerful tool for examining occupancy-drug plasma concentration relationships in the human brain, and to understand occupancy levels by fenobam associated with effective dose levels in a number of neuropsychiatric disorders.
MATERIALS AND METHODS
Synthesis of [11C]ABP688
[11C]ABP688 was synthesized using a procedure previously described (Ametamey et al., 2007) with slight modification. Briefly, 1 mg of desmethyl-ABP688 in 200 μL of dimethylformamide was added to 0.5 mg of sodium hydride and reacted with [11C]methyl iodide at 80°C for 5 min. The product was purified by semi-preparative high-performance liquid chromatography using a Phenomenex C18(2) Luna column (250 × 10 mm, 5 μm) and eluting with 60:40 acetonitrile:water (0.1M ammonium formate) at a flow rate of 10 mL/min. The collected fraction (tR = 5.3 min) was diluted with 50 mL of water and loaded onto a Waters C18 Sep-Pak plus. The Sep-Pak was washed with 10 mL of normal saline and eluted with 1 mL of ethanol. The ethanol solution was diluted with 10 mL of saline and filtered through a 0.22 μm Millex-FG sterile filter. The average specific activity at end-of–synthesis was 500 ± 259 GBq/μmol (13.5 ± 7.0 Ci/μmol).
Preparation of animals and PET imaging
The experimental protocol was approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. PET experiments were performed on four male baboons (Papio anubis) on a High Resolution Research Tomograph with an axial resolution ≤ 2 mm (HRRT, CPS Innovations, Inc., Knoxville, TN). Each animal underwent one baseline scan and one or two blocking scans following pretreatment with fenobam at various dose levels on separate dates (Table 1). The animals were fasted for 12 hours prior to each PET study. Anesthesia was induced with intramuscular Ketamine (5-10 mg/kg) and maintained with a continuous intravenous infusion of Propofol at 0.4-0.6 mg/kg/min throughout the PET experiment. One venous catheter was inserted for the radioligand injection, and one arterial catheter was inserted to obtain arterial blood samples for radioactivity determination in plasma. The head of the animal was immobilized with a thermoplastic mask to reduce head motion inside the PET scanner. A transmission scan was acquired using a rotating Cs-137 source for attenuation correction. Then a dynamic PET acquisition was performed in a three-dimensional list mode for 90 min following an intravenous bolus injection over 30 s of [11C]ABP688 (Radioactivity range: 492-777 MBq (13.3-21.0 mCi); Cold mass range: 0.21-1.45 μg). For the blocking scans, fenobam was dissolved in 0.5 mL of ethanol made acidic with 20 μL of acetic acid and diluted with 4.5 mL of polyethylene glycol and 5.0 mL of normal saline. After sterile filtration (0.22 μm), the 10 mL of fenobam solution was given intravenously 5 min before the start of the second emission PET scan. In all scans, arterial blood samples were collected at very short intervals (< 5 s) initially and gradually prolonging intervals throughout the 90 min PET study for plasma radioactivity determination.
Table 1.
Fenobam doses for blocking scans
| Baboon # | Fenobam dose (mg/kg) |
|---|---|
| 17436 | 0.20 |
| 17436 | 0.67 |
| 15070 | 0.90 |
| C18 | 1.33 |
Analysis of radioactive metabolites in plasma
Selected samples taken at 0, 5, 10, 20, 30, 60, and 90 min were analyzed by high-performance liquid chromatography for the presence of [11C]ABP688 and its radioactive metabolites using a column-switching technique (Hilton et al., 2000). Briefly, 3 mL of plasma in 8M urea acidified with 50 mg of citric acid was passed through a capture column (19 × 4.6 mm Strata-X, Phenomenex, Torrance, CA) at 2 mL/min followed by 1% acetonitrile in water to wash plasma proteins from the column. The effluent from the capture column, containing only highly polar components, passed quickly through a dual bismuth germanium oxide detector (Bioscan, Washington, DC). The solvent was then switched to 65% acetonitrile in 10 mM sodium phosphate buffer pH 5.5 (2 mL/min) for elution of the radiolabeled components bound to the capture column onto the analytical column (Synergi Polar-RP, 10 micron, 250 × 4.6mm, Phenomenex, Torrance, CA).
Reconstruction of PET images
Emission PET scans were reconstructed using the iterative ordered-subset expectation-maximization (OSEM) algorithm correcting for attenuation, scatter, and dead time. The radioactivity was corrected for physical decay to the injection time and re-binned to 35 dynamic PET frames of 256 (left-to-right) by 256 (nasion-to-inion) by 207 (neck-to-cranium) voxels. The frame schedules were, four 15-s, four 30-s, three 1-min, two 2-min, five 4-min, and twelve 5-min frames.
PET data analysis
Volumes of interest (VOIs)
VOIs that were previously prepared on individual animals’ T1-weighted MRIs as described in our previous publication (Kuwabara et al., 2012) were used for this study and edited manually. VOIs were transferred to the baseline PET according to co-registration parameters given by SPM5 using the mutual information theory (West et al., 1997; Maes et al., 1997), and adjusted for radioactivity distribution of the baseline scan for each animal. The customized VOI template was transferred to the PET spaces of blocking scans using PET-to-PET co-registration parameters given by the co-registration module of SPM5 (Gitelman et al., 2003). Time activity curves (TACs) of regions were obtained by applying the VOIs onto the PET frames.
Derivation of PET outcome variables
Plasma reference graphical analysis (PRGA) (Logan et al., 1990) was used to obtain regional distribution volume (VT), after confirming in a preliminary study an excellent agreement against the unconstrained two tissue compartmental model (TTCM-UC: =x) which was shown to be optimal for the baboon brain (y = 1•x + 0.2; R2 = 0.977) (DeLorenzo et al. 2011a). In PRGA, PET frames up to 60 min was used with t* (the start of asymptote) set at 10 min because a preliminary analysis showed stable estimates of VT after 40 min (VT40 = 0.96·VT60 -0.23; R2 = 0.995, where subscripts 40 and 60 indicate the circulation times). Regional binding potentials (BPND) were obtained using cerebellum (Cb) as the reference region (i.e., region-to-Cb VT ratio less 1) as previously recommended (DeLorenzo et al., 2011a; Ametamey et al., 2007), as well as after confirming negligible changes in Cb VT between baseline and post-fenobam scans in this study.
Calculation of occupancy and ED50
Occupancy of mGluR5 by fenobam was calculated using the percent change in BPND (baseline minus post-fenobam) as follows:
| … (1) |
A plot of occupancy versus fenobam dose in mg/kg, pooled for all baseline-blocking scan pairs was fitted by the following modified first-order Hill equation in each region:
| … (2) |
where ED50 stands for the inhibition dose (ED50) that caused 50% of the maximal occupancy (Omax; fixed at 100%) and D for doses to individual animals
The pharmacokinetic plasma levels of fenobam were determined for one animal, B15070 at 0.90 mg/kg by analysis of the plasma sample by LC/MS/MS using atmospheric pressure chemical ionization.
RESULTS
Radioactive metabolites and plasma TACs
[11C]ABP688 was rapidly metabolized after injection with total radioactive metabolites reaching 40% of total radioactivity at 3 min, 64% at 5 min, and 94% at 90 min. The time profiles of total radioactive metabolites did show differences between baseline and post-fenobam scans and appeared to have a dose-dependent relationship between fenobam dose and the rate of metabolism (Figure 1, Panel A).
Fig. 1.
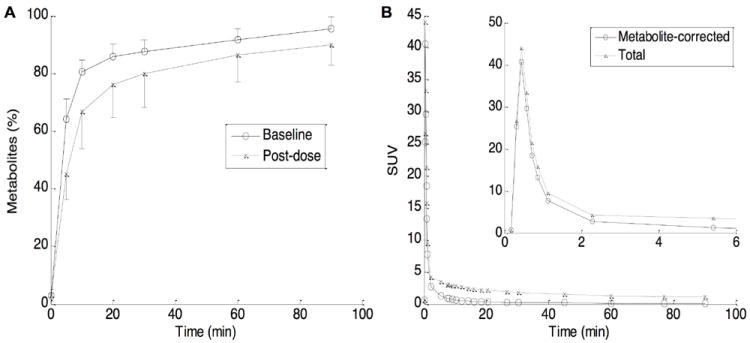
Time profiles of total radioactive metabolites in plasma (mean and SD bars) given by HPLC analysis, separately for baseline and post-Fenobam scans (panel A). Total and metabolite-corrected time-activity curves (TACs) in plasma, mean of 4 baseline scans (panel B). The inset shows TACs for early time points.
Plasma time-activity curves, expressed in standard uptake value (SUV = radioactivity (mCi/L) / (injected radioactivity dose (mCi) / body weight (kg)) × 100%) are shown in Figure 1, panel B, as mean of four baseline scans. Total and metabolite corrected radioactivity peaked within one minute of the bolus injection, decreased sharply by 2 min, and decreased slowly thereafter, while total radioactive metabolite TACs decreased uneventfully after the peak.
Concentrations of fenobam in plasma following a bolus injection are shown in Table 2 as a function of time after the radioligand injection. The time-course remained relatively stable between 30 and 90 min post-radioligand injection.
Table 2.
Plasma levels of Fenobam (0.90 mg/kg)
| Time (min) | Amount (ng/mL) | Amount (nM) |
|---|---|---|
| 5 | 1,116 | 4,184 |
| 15 | 772 | 2,896 |
| 30 | 654 | 2,453 |
| 60 | 479 | 1,798 |
| 90 | 345 | 1,292 |
Radioactivity images and tissue TACs
Figure 2 shows coronal and sagittal images of averaged PET frames for baseline and post-fenobam scans of B15070 who received 1.33 mg/kg of fenobam. On visual inspection, cingulate cortex, especially anterior cingulate cortex, caudate nucleus, putamen, and insula cortex showed the highest accumulation of radioactivity, while cerebellum showed the lowest accumulation across baseline scans. Tissue TACs for selected brain regions are shown in Figure 3. In baseline scans, the caudate nucleus showed the highest radioactivity, followed by putamen, cortical regions, and hippocampus, while the cerebellum showed the lowest radioactivity. Treatment with 1.33 mg/kg of fenobam inhibited the uptake of [11C]ABP688 in all brain regions including some reduction in the cerebellum.
Fig. 2.
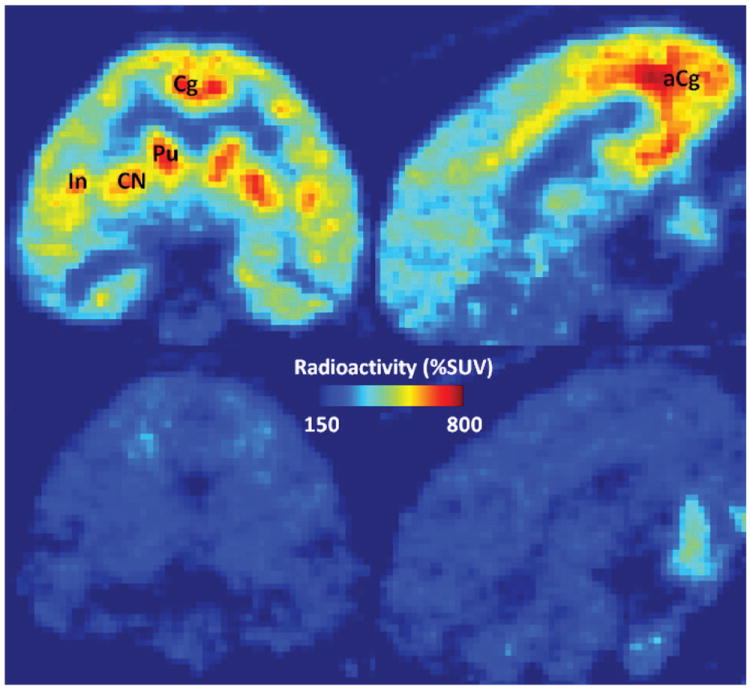
PET images, averages of 10-90 min frames of [11C]ABP688 scans, baseline (upper row) and post-fenobam (lower row; dose: 1.33 mg/kg) scans. This dose of fenobam showed almost complete occupancy of mGluR5s. Coronal view images (left column), showing high radioactivity accumulations in putamen (Pu), caudate nucleus (CN), insula (In), and cingulate cortex (Cg), while sagittal images (right column) about 4 mm from the mid-plane show high accumulations in anterior cingulate cortex (aCg).
Fig. 3.
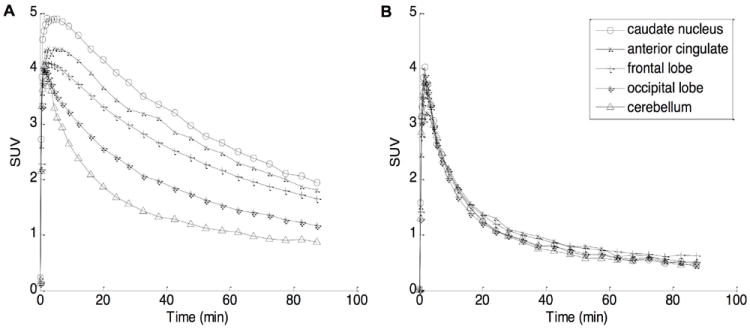
Time-activity curves (TACs) of selected brain regions following a bolus injection of [11C]ABP688, average of 4 baseline scans (panel A) and one post-fenobam scan with 1.33 mg/kg dose (B), expressed in standardized uptake value (SUV) in percentage.
Regional VT and BPND
Baseline regional VT and BPND values of PRGA are shown in Figure 4. Anterior temporal region, anterior cingulate cortex, insula, putamen, and caudate nucleus showed high VT and BPND values exceeding 1, as predicted by averaged radioactivity images, followed by posterior cingulate cortex and hippocampus. Other cortical regions and thalamus showed low VT and BPND values while Cb showed the lowest VT among examined regions and in the brain by visual inspection of radioactivity images. Although post-fenobam scans showed slightly lower VT in Cb (3.7 ± 2.2 mL/mL, mean ± SD) than baseline scans (baseline: 4.6 ± 1.5 mL/mL), no statistical differences were noted (t = 1.80; df = 3; p > 0.1, paired t-test).
Fig. 4.
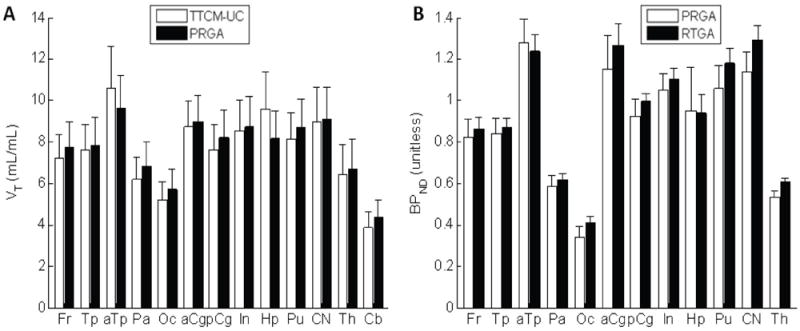
Histograms showing regional values of VT given by an unconstrained two-tissue compartmental model (TTCM-UM) and the plasma reference graphical analysis (PRGA) (panel A), and regional values of BPND given by PRGA and the reference tissue graphical analysis (RTGA) (panel B). Regions are frontal (Fr), temporal (Tp), parietal (Pa), and occipital (Oc) lobes, anterior (aCg) and posterior (pCg) cingulate cortices, insula (In), hippocampus (Hp), putamen (Pu), caudate nucleus (CN), thalamus (Th), and cerebellum (Cb).
Occupancy of mGluR5 by fenobam
Plots of occupancy versus dose followed Equation 2, as exemplified for anterior cingulate cortex and putamen in Figure 5. AIC values ranged from 16.9 in anterior cingulate cortex (the best fit) to 24.1 in thalamus (the poorest fit among 12 brain regions examined). AIC values were lower for the saturation model (Equation 2) than a no-saturation model (occupancy = α•dose) (t = -5.45, df = 11; p < 0.001, paired t-test), suggesting that the saturation model fitted the data better than a no-saturation model. ED50 estimates ranged from 0.3 mg/kg (thalamus) to 1.1 mg/kg (parietal cortex).
Fig. 5.
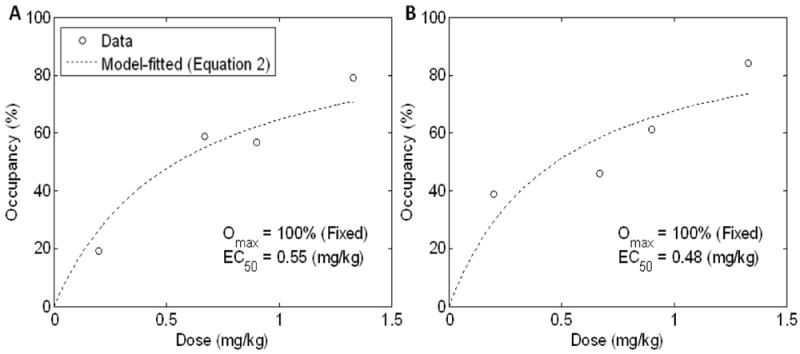
Scatter plots of occupancy versus dose for the anterior cingulate cortex (panel A) and putamen (B). Dotted lines indicate model-predicted occupancy-dose curves using Equation 2.
DISCUSSION
Although other PET tracers such as [18F]FPEB now exist, we used [11C]ABP688 because of its availability and lack of uptake in the cerebellum. Regional VT patterns and magnitudes for [11C]ABP688 agreed with published data (DeLorenzo et al., 2011a; Ametamey et al., 2007), with caudate nucleus and putamen showing high VT. Some methodology-oriented studies indicated that TTCM-UC yielded more robust estimates of VT than TTCM-C for [11C]ABP688 (Ametamey et al., 2007; Treyer et al., 2007). However, all methodology-intensive studies on [11C]ABP688 to our knowledge claimed that BPND could be obtained as the (region-to-Cb VT ratio) - 1 (DeLorenzo et al., 2011a; Burger et al., 2010) alone for [11C]ABP688 due to unstable BPND estimates given by the k3-k4 ratio as defined by Innis et al. (2007). Therefore, in order to claim that [11C]ABP688 yields BPND, it is critical to confirm negligible specific binding in Cb. In support of this requirement, Elmenhorst et al. (2010) reported Bmax values of 80 and 2300 fmol/mg tissue (about 30-fold differences) for Cb and putamen-caudate nucleus, respective in the rat brain using [3H]ABP688. In addition, this study showed no statistical differences in VT of Cb between baseline and post-fenobam scans, albeit a < 20% decrease in mean value. In a study using another specific mGluR5 antagonist MTEP at a fixed dose of 1 mg/kg, Cb (gray matter only as in this study) showed a 7% (SD: 30.0%) decrease in VT between baseline and blocking scans while other brain regions showed close to or more than 50% decreases in VT (DeLorenzo et al., 2011a). Although no statistics were provided for the aforementioned study, it is not likely to have a statistically different decrease in VT for Cb because of the relatively large SD. Lastly, mRNA for mGluR5 is reported to be negligible in Cb (Berthele et al., 1999). Therefore, it appeared to be appropriate to claim negligible displacements of [11C]ABP688 binding in Cb for fenobam and MTEP blocking experiments, and that BPND is measurable with [11C]ABP688 and PET. Regional BPND patterns were very similar to regional VT patterns using the BPND calculation formula. In addition to caudate nucleus and putamen, we noted high BPND in anterior cingulate cortex, anterior temporal cortex, and insula as previously reported for baboons (DeLorenzo et al., 2011a) and humans (Ametamey et al., 2007; Treyer et al., 2008; DeLorenzo et al., 2011b) with [11C]ABP688 and PET.
This study demonstrated that in vivo binding of [11C]ABP688 was blocked by pre-treatment with fenobam in a dose-dependent, saturable manner, approaching close to full occupancy (> 90%) at a dose of 1.33 mg/kg. Although the plasma concentration of fenobam was only measured for one study (0.90 mg/kg), the measured levels at later time points compared favorably with those observed during oral dosing in humans (Itil et al., 1978). It has been demonstrated that fenobam inhibits mGlu5 receptor activity via its actions as an inverse agonist (Porter et al., 2005). Therefore, the agreement of the observed occupancy-dose curve with the first-order (i.e., Hill coefficient = 1) Hill equation (Equation 2) suggested that [11C]ABP688 and fenobam compete for one single site of mGluR5. MTEP, another mGluR5 antagonist exhibited relatively homogenous blockade of [11C]ABP688 binding (72% - 88%) with a fixed dose (1 mg/kg) in the baboon brain (DeLorenzo et al., 2011a). In rodents, a dose-dependent, saturable blocking of [11C]ABP688 binding that followed the same saturation equation was reported by pre-treatment with MPEP, a mGluR5 antagonist (Elmenhorst et al., 2010). Lastly, a dose-dependent, saturable blockade of [3H]ABP688 was reported with CTEP, yet another highly specific mGluR5 antagonist in the mouse brain (Lindemann et al., 2011). Taken altogether, it is very likely that [11C]ABP688 may be appropriate for drug occupancy studies of above mentioned drugs and their analogues targeting mGluR5. However, it should be noted that applications of [11C]ABP688 on humans remain uncertain because a human test-retest study (DeLorenzo et al., 2011b) reported significant increases (>15%) in BPND in retest scans in a same-day scanning design. However, [18F]FPEB showed stable estimates when repeated a few days apart (Wong et al., 2013). At present, it is not clear whether the phenomenon of ’unstable VT’ is specific to [11C]ABP688. On the other hand, no specific bias toward test or retest scans were found in the baboon brain (DeLorenzo et al., 2011a). Thus, it is likely that the use of [11C]ABP688 is justified for drug occupancy studies on baboons.
One limitation of this study is that it included only 4 pairs of baseline and post-fenobam scans. However, this number appeared appropriate for the purpose of demonstrating proof of principle for determining the in vivo occupancy of a mGluR5 therapeutic drug fenobam since the distribution of doses was relatively equally spaced, and near full blockade was observed at the highest dose (1.33 mg/kg). Second, plasma concentrations of fenobam were measured in one study alone. Despite that occupancy-plasma drug concentration plots may be preferable as shown for other drug-receptor system combinations (Sparshatt et al., 2009; Bishara et al., 2013), our study showed acceptably good fit of the plot to the occupancy equation (Equation 2) compared to occupancy-dose plots of these meta-analysis articles. Thus, it is likely that occupancy-dose plots were sufficient for the purpose of demonstrating proof of principle with [11C]ABP688. It was assumed that fenobam occupies mGluR5 in a stable manner throughout the duration of PET studies as commonly assumed in preclinical drug occupancy evaluations (e.g., Kuwabara et al., 2012). Lastly, Cb VT was used as the estimate of VND in this study while DeLorenzo et al. (2011a) proposed the use of the transposed Lassen plot (i.e., scatter pots ΔVT (= baseline - post-fenobam) versus baseline VT of regions to yield VND as the x-intercept of the regression line (Lassen et al., 1995; Cunningham et al., 2010) to obtain a ’theoretically correct’ VND. Although excellent correlations were observed at high dose experiments, uncertainties arose on estimates of VND (i.e., substantially different from Cb VT) in low dose experiments because of the relatively flat arrangement of the plot (i.e., small ΔVT). For this reason, together with aforementioned line of evidence supporting negligible mGluR5 in Cb (gray matter) in the brain of non-human primate, the Lassen plot was not employed in this study.
CONCLUSIONS
This study indicates that [11C]ABP688 may be appropriate for examination of the in vivo occupancy of the mGluR5 therapeutic drug fenobam. To our knowledge, this is the first use of PET to characterize fenobam, albeit preliminary. Existing literature has indicated that [11C]ABP688 could be applicable to various candidate drugs targeting mGluR5. To be fully applicable to humans, the test-retest issues in the literature raise the question whether one [11C]ABP688 scan influences subsequent [11C]ABP688 scans in the human brain. Nevertheless, these baboon PET studies suggest that [11C]ABP688 could be a promising PET radioligand for human occupancy studies.
Acknowledgments
This work was supported by National Institutes of Health grants R33 DA016182 and K24 DA000412. We thank Dr. Michael Tranfaglia of the FRAXA Research Foundation for his suggestions regarding fenobam dosing. We would also like to thank the staff of the Johns Hopkins PET Center for their contributions to this work.
Footnotes
Conflict of Interest. Dr. Fabrizio Gasparini is an employee of Novartis Pharma AG. The authors declare that they have no other conflict of interest.
References
- Ametamey SM, Kessler LJ, Honer M, Wyss MT, Buck A, Hintermann S, Auberson YP, Gasparini F, Schubiger PA. Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med. 2006a;47:698–705. [PubMed] [Google Scholar]
- Ametamey SM, Treyer V, Streffer J, Wyss MT, Schmidt M, Blagoev M, Hintermann S, Auberson Y, Gasparini F, Fischer UC, Buck A. Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J Nucl Med. 2007;48:247–252. [PubMed] [Google Scholar]
- Anneser JM, Chahli C, Ince PG, Borasio GD, Shaw PJ. Glial proliferation and metabotropic glutamate receptor expression in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2004;63:831–840. doi: 10.1093/jnen/63.8.831. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Woolley ML, Prinssen E, Huwyler J, Porter R, Spooren W. The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison. Psychopharmacology. 2005;179:218–229. doi: 10.1007/s00213-005-2211-9. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile × syndrome. J Med Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Laurie DJ, Weis S, Sommer B, Zieglgänsberger W, Conrad B, Tölle TR. Expression of metabotropic glutamate receptor subtype mRNA (mGluR1-8) in human cerebellum. Neuroreport. 1999;10:3861–3867. doi: 10.1097/00001756-199912160-00026. [DOI] [PubMed] [Google Scholar]
- Bishara D, Olofinjana O, Sparshatt A, Kapur S, Taylor D, Patel MX. Olanzapine: a systematic review and meta-regression of the relationships between dose, plasma concentration, receptor occupancy, and response. J Clin Psychopharmacol. 2013;33:329–335. doi: 10.1097/JCP.0b013e31828b28d5. [DOI] [PubMed] [Google Scholar]
- Black KJ, Snyder AZ, Koller JM, Gado MH, Perlmutter JS. Template images for nonhuman primate neuroimaging: 1. Baboon. Neuroimage. 2001;14:736–743. doi: 10.1006/nimg.2001.0752. [DOI] [PubMed] [Google Scholar]
- Burger C, Deschwanden A, Ametamey S, Johayem A, Mancosu B, Wyss M, Hasler G, Buck A. Evaluation of a bolus/infusion protocol for 11C-ABP688, a PET tracer for mGluR5. Nucl Med Biol. 2010;37:845–851. doi: 10.1016/j.nucmedbio.2010.04.107. [DOI] [PubMed] [Google Scholar]
- Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab. 2010;30:46–50. doi: 10.1038/jcbfm.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Milak MS, Brennan KG, Kumar JS, Mann JJ, Parsey RV. In vivo positron emission tomography imaging with [11C]ABP688: binding variability and specificity for the metabotropic glutamate receptor subtype 5 in baboons. Eur J Nucl Med Mol Imaging. 2011a;38:1083–1094. doi: 10.1007/s00259-010-1723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Kumar JS, Mann JJ, Parsey RV. In vivo variation in metabotropic glutamate receptor subtype 5 binding using positron emission tomography and [11C]ABP688. J Cereb Blood Flow Metab. 2011b;31:2169–2180. doi: 10.1038/jcbfm.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, Buck A, Hasler G. Reduced Metabotropic Glutamate Receptor 5 Density in Major Depression Determined by [11C]ABP688 PET and Postmortem Study. Am J Psychiatry. 2011;168:727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile × syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile × syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Minuzzi L, Aliaga A, Rowley J, Massarweh G, Diksic M, Bauer A, Rosa-Neto P. In vivo and in vitro validation of reference tissue models for the mGluR(5) ligand [11C]ABP688. J Cereb Blood Flow Metab. 2010;30:1538–1549. doi: 10.1038/jcbfm.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann C, Davis L, Ciccone P, Rubin R. Phase II double-blind controlled study of a new anxiolytic, fenobam (McN-3377) vs placebo. Curr Ther Res. 1980;27:144–151. [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Hamill TG, Krause S, Ryan C, Bonnefous C, Govek S, Seiders TJ, Cosford ND, Roppe J, Kamenecka T, Patel S, Gibson RE, Sanabria S, Riffel K, Eng W, King C, Yang X, Green MD, O’Malley SS, Hargreaves R, Burns HD. Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse. 2005;56:205–216. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF. Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl Med Biol. 2000;27:627–630. doi: 10.1016/s0969-8051(00)00125-6. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Itil TM, Seaman BA, Huque M, et al. The clinical and quantitative EEG effects and plasma levels of fenobam (McN-3377) in subjects with anxiety: an open rising dose tolerance and efficacy study. Curr Ther Res. 1978;24:708–724. [Google Scholar]
- Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E, Malyshkin A, Greco S, Barberi C, Danysz W. The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology. 2009;57:97–108. doi: 10.1016/j.neuropharm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kågedal M, Cselényi Z, Nyberg S, Raboisson P, Ståhle L, Stenkrona P, Varnäs K, Halldin C, Hooker AC, Karlsson MO. A positron emission tomography study in healthy volunteers to estimate mGluR5 receptor occupancy of AZD2066 - estimating occupancy in the absence of a reference region. Neuroimage. 2013;82:160–169. doi: 10.1016/j.neuroimage.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Ko WK, Pioli E, Li Q, McGuire S, Dufour A, Sherer TB, Bezard E, Facheris MF. Combined fenobam and amantadine treatment promotes robust antidyskinetic effects in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate model of Parkinson’s disease. Mov Disord. 2014;29:772–779. doi: 10.1002/mds.25859. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–693. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kuwabara H, Wong DF, Gao Y, Valentine H, Holt DP, Ravert HT, Dannals RF, Horti AG. PET Imaging of nicotinic acetylcholine receptors in baboons with 18F-AZAN, a radioligand with improved brain kinetics. J Nucl Med. 2012;53:121–129. doi: 10.2967/jnumed.111.092338. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Bartenstein PA, Lammertsma AA, Prevett MC, Turton DR, Luthra SK, Osman S, Bloomfield PM, Jones T, Patsalos PN, O’Connell MTO, Duncan JS, Vanggaard Andreson J. Benzodiazepine receptor quantification in vivo in humans using [11C]flumazenil and PET: application of the steady-state principle. J Cereb Blood Flow Metab. 1995;15:152–165. doi: 10.1038/jcbfm.1995.17. [DOI] [PubMed] [Google Scholar]
- Levenga J, Hayashi S, de Vrij FM, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, Song C, Buijsen RA, Pop AS, Gomezmancilla B, Nelson DL, Willemsen R, Gasparini F, Oostra BA. AFQ056, a new mGluR5 antagonist for treatment of fragile × syndrome. Neurobiol Dis. 2011;42:311–317. doi: 10.1016/j.nbd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Jaeschke G, Michalon A, Vieira E, Honer M, Spooren W, Porter R, Hartung T, Kolczewski S, Büttelmann B, Flament C, Diener C, Fischer C, Gatti S, Prinssen EP, Parrott N, Hoffmann G, Wettstein JG. CTEP: a novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J Pharmacol Exp Ther. 2011;339:474–486. doi: 10.1124/jpet.111.185660. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, Christman DR. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- Morin N, Grégoire L, Gomez-Mancilla B, Gasparini F, Di Paolo T. Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys. Neuropharmacology. 2010;58:981–986. doi: 10.1016/j.neuropharm.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo controlled study. J Clin Psychopharmacol. 1982;2:129–133. [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Büttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Mühlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siméon FG, Brown AK, Zoghbi SS, Patterson VM, Innis RB, Pike VW. Synthesis and simple 18F-labeling of 3-fluoro-5-(2-(2-(fluoromethyl)thiazol-4-yl)ethynyl)benzonitrile as a high affinity radioligand for imaging monkey brain metabotropic glutamate subtype-5 receptors with positron emission tomography. J Med Chem. 2007;50:3256–3266. doi: 10.1021/jm0701268. [DOI] [PubMed] [Google Scholar]
- Sparshatt A, Taylor D, Patel MX, Kapur S. Amisulpride - dose, plasma concentration, occupancy and response: implications for therapeutic drug monitoring. Acta Psychiatr Scand. 2009;120:416–428. doi: 10.1111/j.1600-0447.2009.01429.x. [DOI] [PubMed] [Google Scholar]
- Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of group I and group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- Treyer V, Streffer J, Wyss MT, Bettio A, Ametamey SM, Fischer U, Schmidt M, Gasparini F, Hock C, Buck A. Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: assessment of methods. J Nucl Med. 2007;48:1207–1215. doi: 10.2967/jnumed.107.039578. [DOI] [PubMed] [Google Scholar]
- Treyer V, Streffer J, Ametamey SM, Bettio A, Bläuenstein P, Schmidt M, Gasparini F, Fischer U, Hock C, Buck A. Radiation dosimetry and biodistribution of 11C-ABP688 measured in healthy volunteers. Eur J Nucl Med Mol Imaging. 2008;35:766–770. doi: 10.1007/s00259-007-0638-4. [DOI] [PubMed] [Google Scholar]
- West J, Fitzpatrick JM, Wang MY, Dawant BM, Maurer CR, Jr, Kessler RM, Maciunas RJ, Barillot C, Lemoine D, Collignon A, Maes F, Suetens P, Vandermeulen D, van den Elsen PA, Napel S, Sumanaweera TS, Harkness B, Hemler PF, Hill DL, Hawkes DJ, Studholme C, Maintz JB, Viergever MA, Malandain G, Woods RP. Comparison and evaluation of retrospective intermodality brain image registration techniques. J Comput Assist Tomogr. 1997;21:554–566. doi: 10.1097/00004728-199707000-00007. [DOI] [PubMed] [Google Scholar]
- Wong DF, Waterhouse R, Kuwabara H, Kim J, Brašić JR, Chamroonrat W, Stabins M, Holt DP, Dannals RF, Hamill TG, Mozley PD. 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: a first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. J Nucl Med. 2013;54:388–396. doi: 10.2967/jnumed.112.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile × syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]


