Abstract
Purpose
Propofol injection pain, despite various strategies, remains common and troublesome. This study aimed to test the hypothesis that pretreatment with the combination of intravenous lidocaine and magnesium would have an additive effect on reducing propofol injection pain.
Methods
After IRB approval and informed consent, we performed a prospective, double-blind, placebo-controlled, randomized trial. Subjects were randomly assigned to pretreatment with either lidocaine (50 mg), magnesium sulfate (0.25 mg), lidocaine (50 mg) plus magnesium sulfate (0.25 mg), or 0.9% sodium chloride. Following pretreatment, propofol (50 mg) was administered and subjects were questioned regarding injection site pain and observed for behavioral signs of pain.
Results
Two hundred subjects were enrolled and 158 subjects (39 placebo, 38 lidocaine, 44 magnesium sulfate, and 37 lidocaine plus magnesium sulfate) received their assigned pretreatment intervention. Intergroup baseline characteristics were similar. The proportion of subjects reporting propofol injection pain was highest in those pretreated with magnesium sulfate (57%), followed by those pretreated with placebo (46%), lidocaine plus magnesium sulfate (41%), and then lidocaine (29%; p=0.011). When adjusted for age, gender, diabetes mellitus, chronic pain, tobacco use, and selective-serotonin reuptake inhibitor use, the pain response scale scores were significantly reduced by lidocaine pretreatment compared to magnesium sulfate and placebo (p=0.031 and p=0.0003, respectively).
Conclusions
In this double-blind, placebo-controlled, randomized trial, the combination of intravenous magnesium sulfate and lidocaine offered no additional benefit for the relief of propofol injection pain compared to intravenous lidocaine. An improved, receptor-based understanding of the mechanism of propofol injection pain remains needed.
Keywords: propofol, magnesium sulphate, injection pain
Introduction
Propofol is commonly used for the induction and maintenance of general anesthesia and for sedation during monitored anesthesia care. Pain with its injection, however, has been reported to occur in 28–90% of patients1–4 and has been identified as a troubling concern for anesthesiologists.5 Various factors, including the site of injection, speed of injection, vein size, aqueous phase propofol concentration, propofol temperature, blood buffering, and the concomitant use of various drugs appear to influence this pain,3–4 while activation of the kallikrein-kinin system has been implicated mechanistically.7 Yet, despite more than 175 randomized trials attempting to discover an intervention to alleviate this pain, no intervention has consistently effected its complete relief. Current evidence suggests propofol injection in an antecubital vein versus a hand vein is most effective; however, this is not always practical in clinical practice. Alternatively, lidocaine pretreatment with and without venous occlusion and mixing lidocaine with propofol also consistently provide a relative risk reduction.3–4
Magnesium is a noncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor ion channel8 and plays a role in the regulation of calcium influx into cells at different voltage-gated channels.9 These actions, respectively, have been implicated in pain modulation and antinociceptive effects.10–11 Accordingly, magnesium sulfate has also been tested with promising results as a pretreatment intervention to reduce propofol injection pain.12–15 With this in mind, clinical experience at our institution suggested lidocaine plus magnesium sulfate may have an increased effect on propofol injection pain; however, this combination had not been studied.
Therefore, the purpose of this study was to test the hypothesis that pretreatment with a combination of magnesium sulfate and lidocaine would have an increased effect on reducing propofol injection pain in patients during the intravenous induction of general anesthesia. The primary endpoint of this study was the presence of pain during propofol injection. Secondarily, pain severity was assessed.
Methods
The University of Wisconsin Health Sciences Institutional Review Board (800 University Bay Drive, Madison, WI, USA; Protocol#: H-2010-0139; approved 2/23/2011) approved this prospective, randomized, double-blind, placebo controlled trial and it was registered at ClinicalTrials.gov (NCT 01342510). After written informed consent, adult patients considered ASA physical status 1 or 2 by the investigators, presenting to the outpatient surgery center and scheduled to receive general anesthesia, were randomly assigned to one of four pretreatment study groups (group L = lidocaine 50 mg; group M = magnesium sulfate 0.25 mg; group LM = lidocaine 50 mg plus magnesium sulfate 0.25 mg; and group C = 0.9% sodium chloride). Magnesium sulfate and lidocaine doses were based on prior studies.3, 12, 14, 15 Exclusion criteria included age less than 18 years, allergy to local anesthetics or magnesium sulfate, end stage renal disease, pregnancy, incarceration, patients requiring a rapid sequence induction and intubation, refusal to participate, and current participation in another clinical study.
Subjects were randomized by computer-generated numbers and study drugs were prepared in identical syringes by our institution’s Pharmaceutical Research Center (PRC). All drugs were diluted to 10 mL using 0.9% normal saline. For each subject, study personnel received a syringe and a data collection sheet each labeled with the study subject number. A 20 gauge Angiocatheter (BD, Franklin Lakes, NJ, USA) was inserted into the dorsum of the hand for intravenous (IV) fluids and medication administration. After applying standard monitors (electrocardiogram, non-invasive arterial blood pressure, and pulse oximetry) and providing pre-oxygenation in the operating room, the assigned study drug was injected by bolus over two-three seconds. The intravenous line containing lactated ringers solution was then allowed to flow freely. Twenty seconds later, 50 mg of propofol was injected by bolus over two-three seconds, followed again by free flow of the lactated ringers solution. Ten seconds following the injection of propofol, the subjects were asked a standard question about pain on injection (“Are you having pain at your IV site?”) and their response was noted. Injection pain severity was assessed using the following four point pain response scale: 0 = no pain; 1 = mild pain (pain reported only in response to questioning and without behavioral signs); 2 = moderate pain (pain reported in response to questioning and accompanied by a behavioral sign, or pain reported spontaneously without questioning); and 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears).12 Two of the investigators (JH and JG) performed the pain response assessments on all subjects. Following the pain response assessment, the induction of general anesthesia was then completed with the administration of an additional amount of propofol, deemed appropriate by the anesthesiologist responsible for the subject’s care. Throughout the study and data analysis periods, all study personnel, the subjects, and the anesthesia providers involved in the subject’s care remained unaware of each subject’s group assignment. The group assignments were revealed only after the data analyses were complete.
The study sample size was calculated based on a hypothesized 65% incidence of propofol injection pain with no intravenous pretreatment and a 35% incidence with treatment.3 With these assumptions, 48 subjects were required per group to detect a significant difference with 80% power (one-sided, α = 0.05/3). We choose to enroll 50 subjects per group to control costs, but to still allow for an anticipated small number of dropouts. Baseline characteristics were analyzed using one-way ANOVA and chi-square tests for continuous and categorical data, respectively. Pain occurrence and scaled responses were assessed using logistic and linear regression models, respectively. Statistical analyses were performed using R (Version 2.13.1, R Foundation for Statistical Computing, Austria). A p-value < 0.05 was considered significant.
Results
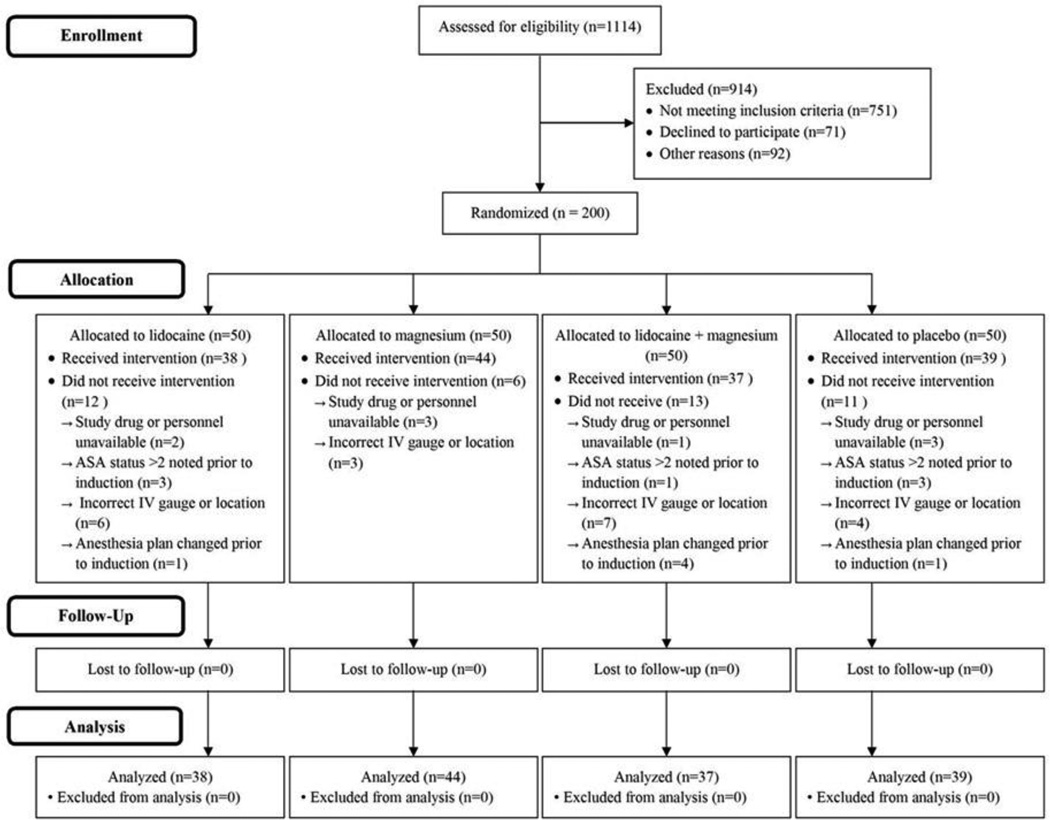
Subject enrollment and analysis is illustrated in Figure 1. After IRB approval, 200 subjects as planned provided written consent, were enrolled in the study, and randomized to a specific study group between May 1, 2011 and July 1 2011. Forty-two subjects did not receive the assigned intervention and were lost to follow-up for several reasons, including study drug or personnel unavailable, American Society of Anesthesiologists (ASA) physical status greater than 2 noted prior to induction, incorrect IV gauge or location, and anesthesia plan changed after enrollment, but prior to induction. As such, 158 enrolled subjects received the assigned intervention and were included in the analyses on a per protocol basis.
Figure 1.
Study and Data Analysis Flowchart
Thirty-nine, 38, 44, and 37 subjects were randomized to and administered placebo, lidocaine, magnesium sulfate, and lidocaine plus magnesium sulfate solutions, respectively. Baseline characteristics were similar amongst the groups (Table 1).
Table 1.
Group Demographics
| Placebo (n = 39) |
Lidocaine (n = 38) |
Magnesium (n = 44) |
Lidocaine + Magnesium (n = 37) |
||
|---|---|---|---|---|---|
| Age, yrs | 45 (19) | 45 (15) | 45 (16) | 44 (16) | |
| Gender, n (%) | |||||
| Male | 25 (64) | 16 (42) | 23 (52) | 23 (62) | |
| Female | 14 (36) | 22 (58) | 21 (48) | 14 (38) | |
| Height, cm | 174 (10) | 173 (11) | 175 (10) | 173 (12) | |
| Weight, kg | 83 (17) | 85 (25) | 83 (15) | 83 (16) | |
| *BMI, kg.m−2 | 27 (4) | 28 (6) | 27 (4) | 27 (5) | |
| †ASA, n (%) | |||||
| 1 | 10 (26) | 9 (24) | 11 (25) | 12 (32) | |
| 2 | 29 (74) | 29 (76) | 32 (75) | 25 (68) | |
| Comorbidities, n (%) | |||||
| ‡DM | 2 (5) | 1 (3) | 1 (2) | 3 (8) | |
| Chronic Pain | 2 (5) | 3 (8) | 3 (7) | 3 (8) | |
| Tobacco Use | 7 (18) | 4 (11) | 7 (16) | 4 (11) | |
| §SSRI Use | 5 (13) | 4 (11) | 7 (16) | 7 (19) | |
Data are mean (SD) unless otherwise noted. Age, height, weight, and BMI compared using one-way ANOVA (all p-values > 0.05). ASA class and comorbidities compared using chi-square test (all p-values > 0.05).
BMI = body mass index.
ASA = American Society of Anesthesiologists.
SSRI = selective serotonin re-uptake inhibitor.
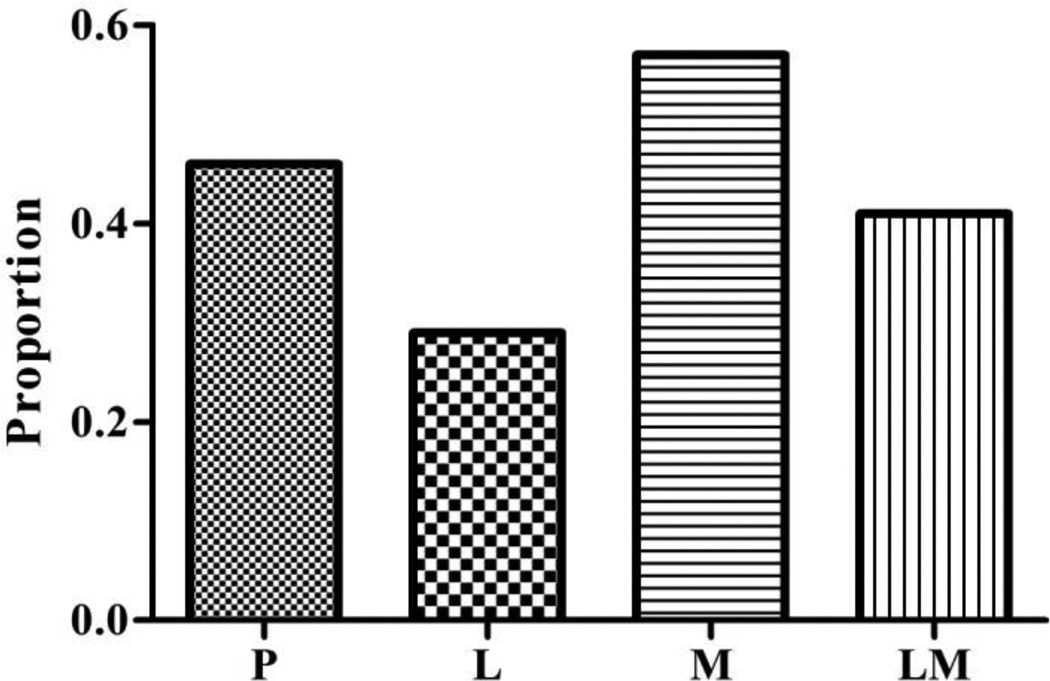
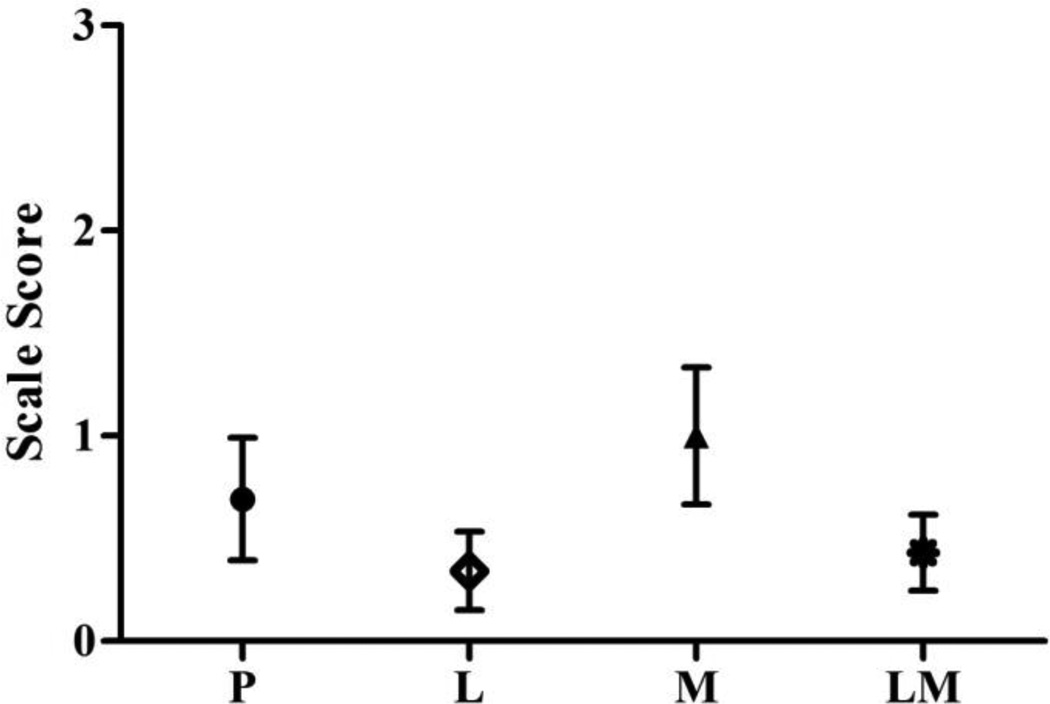
Pain rates and pain response scale score frequency distributions amongst the study groups are shown in Figures 2 and 3, respectively. The percent of subjects reporting pain with propofol injection was highest in those pretreated with magnesium sulfate (57%; 95% CI 0.42 – 0.70), followed in descending order by those pretreated with placebo (46%; 95% CI 0.32 – 0.61), lidocaine plus magnesium sulfate (41%; 95% CI 0.26 – 0.56), and then lidocaine (29%; 95% CI 0.17 – 0.45) (p = 0.010). The differences between these rates were statistically significant when comparing the lidocaine and magnesium sulfate groups (p = 0.011) and marginal when comparing the lidocaine and placebo groups (p = 0.083), but did not reach or near statistical significance amongst the remaining pairwise comparisons. In the logistic regression analysis, age, gender, diabetes mellitus, tobacco use, chronic pain, and SSRI use were not associated with the probability of having propofol injection pain. Similarly, the pain response scale scores were significantly reduced by lidocaine pretreatment compared to magnesium sulfate and placebo (p = 0.031 and p = 0.0003, respectively) and by the combination of lidocaine and magnesium sulfate versus magnesium sulfate alone (p = 0.005). In the linear regression analysis, female versus male gender was associated with higher pain response scale scores (p = 0.03). The differences in these scores between the study groups, however, persisted when adjusted for this gender effect.
Figure 2.
The group proportions of subjects experiencing propofol injection pain are shown (P = placebo, L = lidocaine, M = magnesium, LM = lidocaine plus magnesium).
Figure 3.
Mean (95% CI) group pain response scale scores are shown (P = placebo, L = lidocaine, M = magnesium, LM = lidocaine plus magnesium). Pain score scale: 0 = no pain; 1 = mild pain (pain reported only in response to questioning and without behavioral signs); 2 = moderate pain (pain reported in response to questioning and accompanied by a behavioral sign, or pain reported spontaneously without questioning); and 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears.).
Discussion
The main finding of this study is that the addition of magnesium sulfate (0.25 mg) to lidocaine (50 mg) for pretreatment does not, unfortunately, have an additive effect on reducing the pain associated with the injection of propofol. Further, magnesium sulfate may lessen the effect of lidocaine pretreatment and may increase propofol injection pain when given alone as pretreatment, which is in contradistinction to prior results that suggest magnesium sulfate pretreatment imparts a beneficial effect.12–15
Excepting our findings regarding the effects of magnesium sulfate, our study results are consistent with prior studies investigating the effect of lidocaine pretreatment on propofol injection pain, supporting our study’s internal validity. Jalota et al recently performed a quantitative meta-analysis on various techniques used to reduce the incidence and severity of propofol injection pain, including lidocaine pretreatment.3 In this study, they found lidocaine pretreatment to be associated with a relative risk (95% CI) of propofol injection pain of 0.47 (0.40 – 0.56) compared to no treatment. In our study, lidocaine pretreatment produced a relative reduction in the incidence of propofol injection pain compared to placebo of 37%. Further, the incidences of pain in our placebo and lidocaine pretreatment groups (46% and 29%, respectively) were not incongruent with prior study results comparing pretreatment with 50 mg or more of lidocaine with placebo. In these prior studies, the incidence of pain in the lidocaine pretreatment groups ranged from 9–30%, while 23–83% of subjects receiving placebo pretreatment injections experienced propofol injection pain.16–20
The etiology of our findings with respect to the effect of magnesium pretreatment may in part reflect pain from the magnesium sulfate pretreatment injection itself, which we did not assess individually apart from pain from the propofol injection; or more likely, an incomplete understanding of the receptor mediation of propofol injection pain and its modification by magnesium interactions.
Propofol is generally believed to cause pain via phenol related venous intima irritation or activation of the kallikrein-kinin system.7 The analgesic effect of magnesium pretreatment for reducing propofol injection pain has been ascribed to NMDA receptor antagonism, intracellular calcium antagonism, and/or nitric oxide mediated vasodilation.12–13, 15 However, our results, combined with prior contradictory reports describing the failure of magnesium and other agents to substantially impact propofol injection pain and more recent reports of propofol and magnesium effects on peripheral nociceptors, suggest that the etiology and regulation of propofol injection pain may be more complex, which warrants questioning of these models. Recent basic science evidence suggests propofol may effect peripheral sensitization to nociceptive endogenous inflammatory mediators through activation or interaction with capsaicin stimulated transient receptor potential (TRP) vanilloid receptor subtypes.21–22 Magnesium and other protons have also been found to stimulate vanilloid receptors,23 while millimolar increases in magnesium concentration have been shown to produce a four-fold increase in capsaicin-evoked currents.24 In contrast to magnesium, local anesthetics have been found to inhibit capsaicin-induced vanilloid receptor activation, which may account for their apparent increased efficacy when utilized for decreasing propofol injection pain.25 In addition to vanilloid receptors, prostanoid receptors may also be involved. Ando et al recently demonstrated that propofol injection pain is also initiated by prostanoids (specifically PGE2).26 This, combined with prior work demonstrating magnesium significantly increases prostaglandin-receptor interactions,27–28 provides another potential mechanism to explain the increased pain associated with magnesium pretreatment in our study.
Unfortunately, the strength of our conclusions are limited by the study’s relatively high dropout rate, which resulted from the various logistical issues described above; many of which occurred in the early stages of the study. We were unable to expand the study to accommodate this dropout rate due to funding limitations. Nonetheless, our results in many ways are consistent with prior reports on the efficacy of lidocaine pretreatment on propofol injection pain, and our study is the first to test the efficacy of the combination of magnesium sulfate and lidocaine pretreatment on propofol injection pain.
In conclusion, we performed a double-blind, placebo-controlled, randomized trial and found the addition of magnesium sulfate to lidocaine for pretreatment does not have an additive effect on reducing the pain associated with the injection of propofol. The results of this study, in combination with prior clinical and recent basic science data suggest further investigations into the underlying receptor mechanisms associated with propofol injection pain and their regulation are necessary.
Acknowledgements
This study was supported by (1) intradepartmental funding, Department of Anesthesiology, University of Wisconsin School of Medicine and Public Health, Madison, WI, and (2) a National Institutes of Health, National Center for Advancing Translational Sciences, Clinical Translational Science Award Program, Grant UL1TR000427. The authors would like to thank Luke Hattenhauer, CRNA, MS APNP, James Albrecht, CRNA, MS APNP, Peter Shearer, MMSc, AA-C, Guangde Chen, MS and Brooke Anderson, RN, MSN for their assistance with initiating, conducting and evaluating this clinical trial.
Footnotes
Prior Presentation: American Society of Anesthesiologists 2012 Annual Meeting, Washington, DC
The authors have no commercial or non-commercial affiliations that are or may be perceived to be a conflict of interest.
References
- 1.Stark RD, Binks SM, Dutka VN, O’Connor KM, Arnstein MJ, Glen JB. A review of the safety and tolerance of propofol (‘Diprivan’) Postgrad Med J. 1985;61(Suppl):152–156. [PubMed] [Google Scholar]
- 2.Mangar D, Holak EJ. Tourniquet at 50 mm Hg followed by intravenous lidocaine diminishes hand pain associated with propofol injection. Anesth Analg. 1992;74:250–252. doi: 10.1213/00000539-199202000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Jalota L, Kalira V, George E, Shi YY, Homuss C, Radke O, Pace NL, Apfel CC. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011;342:d1110. doi: 10.1136/bmj.d1110. [DOI] [PubMed] [Google Scholar]
- 4.Picard P, Tramer MR. Prevention of pain on injection of propfol: a quantitative systemic review. Anesth Analg. 2000;90:963–969. doi: 10.1097/00000539-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998;53:468–476. doi: 10.1046/j.1365-2044.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- 7.Scott RPF, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia. 1988;43:492–494. doi: 10.1111/j.1365-2044.1988.tb06641.x. [DOI] [PubMed] [Google Scholar]
- 8.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 9.Iseri LT, French JH. Magnesium: Nature’s physiologic calcium blocker. Am Heart J. 1984;108:188–193. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 10.Begon S, Pickering G, Eschalier A, Mazur A, Rayssiguier Y, Dubray C. Role of spinal NMDA receptors, protein kinase C and nitric oxide synthase in the hyperalgesia induced by magnesium deficiency in rats. Br J Pharmacol. 2001;134:1227–1236. doi: 10.1038/sj.bjp.0704354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantyh PW, Allen CJ, Rogers S DeMaster E, Ghilardi JR, Mosconi T, Kruger L, Mannon PJ, Taylor IL, Vigna SR. Some sensory neurons express neuropeptide Y receptors: potential paracrine inhibition of primary afferent nociceptors following peripheral nerve injury. J Neurosci. 1994;14:3958–3968. doi: 10.1523/JNEUROSCI.14-06-03958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Memis D, Turan A, Karamanlioglu B, Sut N, Pamukcu Z. The use of Magnesium Sulfate to Prevent Pain on Injection of Propofol. Anesth Analg. 2002;95:606–608. doi: 10.1097/00000539-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Dhiraj S, Raza M, Pandey R, Pandey CK, Singh PK, Singh U, Gupta D. Vein pretreatment with magnesium sulfate to prevent pain on injection of propofol is not justified. Can J Anesth. 2004;51:130–133. doi: 10.1007/BF03018771. [DOI] [PubMed] [Google Scholar]
- 14.Honarmand A, Safavi M. Magnesium sulfate pretreatment to alleviate pain on propofol injection: a comparison with ketamine or lidocaine. Acute Pain. 2008;10:23–29. [Google Scholar]
- 15.Singh DK, Jindal P, Singh G. Comparitive study of attenuation of the pain caused by propofol intravenous injection, by granisetron, magnesium sulfate and nitroglycerine. Saudi J Anaesth. 2011;5:50–54. doi: 10.4103/1658-354X.76511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok MS, Pang WW, Hwang MH. The analgesic effect of tramadol, metoclopramide, meperidine and lidocaine in ameliorating propofol injection pain: a comparative study. J Anaesth Clin Pharmacol. 1999;15:37–42. [Google Scholar]
- 17.Pang WW, Huang PY, Chang DP, Huang MH. The peripheral analgesic effect of tramadol in reducing propofol injection pain: a comparison with lidocaine. Reg Anesth Pain Med. 1999;24:246–249. doi: 10.1016/s1098-7339(99)90136-0. [DOI] [PubMed] [Google Scholar]
- 18.Wong WH, Cheong KF. Role of tramadol in reducing pain on propofol injection. Singapore Med J. 2001;42:193–195. [PubMed] [Google Scholar]
- 19.Reddy MS, Chen FG, Ng HP. Effect of ondansetron pretreatment on pain after rocuronium and propfol injection: a randomised, double-blind controlled comparison with lidocaine. Anaesthesia. 2001;56:902–905. doi: 10.1046/j.1365-2044.2001.02059-6.x. [DOI] [PubMed] [Google Scholar]
- 20.Madenoglu H, Yildiz K, Dogru K, Boyaci A. Efficacy of different doses of lidocaine in the prevention of pain due to propofol injection: a randomized, open-label trial in 120 patients. Curr Ther Res. 2003;64:310–316. doi: 10.1016/S0011-393X(03)00066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Wickley PJ, Sinha S, Bratz IN, Damron DS. Propofol restores Transient Receptor Vanilloid Receptor Subtype-1 sensitivity via activation of Transient Receptor Potential Ankyrin Receptor Subtype-1 in sensory neurons. Anesthesiology. 2011;114:1169–1179. doi: 10.1097/ALN.0b013e31820dee67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumacher MA. Transient receptor potential channels in pain and inflammation: therapeutic opportunities. Pain Pract. 2010;10:185–200. doi: 10.1111/j.1533-2500.2010.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Poon K, Oswald RE, Chuang H. Distinct modulations of human Capsaicin receptor by protons and magnesium through different domains. J Biol Chem. 2010;285:11547–11556. doi: 10.1074/jbc.M109.058727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahern GP, Brooks IM, Miyares RL, Wang X. Extracellular cations sensitize and gate Capsaicin receptor TRPV1 modulating pain signaling. J Neurosci. 2005;25:5109–5116. doi: 10.1523/JNEUROSCI.0237-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirota K, Smart D, Lambert DG. The effects of local and intravenous anesthetics on recombinant rat VR1 Vanilloid Receptors. Anesth Analg. 2003;96:1656–1660. doi: 10.1213/01.ANE.0000061580.89627.91. [DOI] [PubMed] [Google Scholar]
- 26.Ando R, Watanabe C. Characteristics of propofol-evoked vascular pain in anaesthetized rats. Br J Anaesth. 2005;95:384–392. doi: 10.1093/bja/aei184. [DOI] [PubMed] [Google Scholar]
- 27.Blair IA, Cresp TM, MacDermot J. Divalent cations increase [3H]-Prostacyclin binding to membranes of neuronal somatic hybrid cells. Br J Pharmac. 1981;73:691–694. doi: 10.1111/j.1476-5381.1981.tb16804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams LT, Mullikin D, Lefkowitz RJ. Magnesium dependence of agonist binding to Adenylate Cyclase-coupled hormone receptors. J Biol Chem. 1978;253:2984–2989. [PubMed] [Google Scholar]