Summary
Chronic sleep fragmentation (SF), common in patients with sleep apnea, correlates with the development of obesity. We hypothesized that SF differentially affects neurobehavior in lean wildtype (WT) and obese pan-leptin receptor knockout (POKO) mice fed the same normal diet. First we established an SF paradigm by interrupting sleep every 2 min during the inactive light span. The maneuver was effective in decreasing sleep duration and bout length, and in increasing sleep state transition and waking, without significant rebound sleep in the dark span. Changes of sleep architecture were evident in the light span and consistent across days 1–10 of SF. There was reduced NREM, shortened sleep latency, and increased state transitions. During the light span of the first day of SF, there also was reduction of REM and increased delta power of slow wave sleep. Potential effects of SF on thermal pain threshold, locomotor activity, and anxiety were then tested. POKO mice had a lower circadian amplitude of pain latency than WT mice in the hot plate test, and both groups had lowest tolerance at 4 pm (ZT 10) and longest latency at 4 am (ZT 22). SF increased the pain threshold in WT but not POKO mice when tested at 8 am (ZT 2). Both the POKO mutation and SF resulted in reduced physical activity and increased anxiety, but there was no additive effect of these two factors. Overall, SF and the POKO mutation differentially regulate mouse behavior. The results suggest that obesity can blunt neurobehavioral responses to SF.
Keywords: sleep fragmentation, sleep architecture, obesity, pain, anxiety
Introduction
We have shown that obese mice with pan leptin receptor knockout (POKO) (Hsuchou et al., 2013) have hypersomnolence. There is major increase of non-rapid eye movement (NREM) sleep in the light span correlating with the extent of obesity (Wang et al., 2013). This raises a question how obesity affects the response of mice to sleep disruption. Clinically, many obese patients do not seek sleep treatment until after many years of sleep apnea. We propose that tolerance to chronic sleep disturbance may be perceived differently in obese and lean subjects as a result of altered neural circuitry that modifies neurobehavior. Although it is known that chronic sleep restriction contributes to weight gain, the spectrum of behavioral changes when obese subjects experience chronic sleep fragmentation (SF) has not been fully addressed.
Both sleep disturbance and obesity result in changes of biopotentials, metabolic activity, and neurobehavior. Insufficient sleep duration increases caloric intake (Calvin et al., 2013; Markwald et al., 2013), decreases the amount and intensity of physical activity (Bromley et al., 2012), and changes sensory gating, leading to hyperalgesia (Onen et al., 2001; Roehrs et al., 2006). Increased nociceptive sensitivity is also seen after rapid eye movement (REM) sleep deprivation (Lautenbacher et al., 2006). In rats subjected to sleep deprivation or sleep fragmentation, there is reduced anxiety but no change in general locomotor activity (Tartar et al., 2009). Experimental studies used paradigms of sleep deprivation or restriction (Zager et al., 2007; Matos et al., 2012), but few studies have addressed how SF affects physiological function in obesity other than feeding and metabolism.
Here we hypothesized that SF has a greater impact on lean subjects than obese mice in regard to behavioral changes, as obese mice have undergone chronic neuroendocrine adaptation. SF is a common consequence of many sleep disorders in humans, including sleep apnea (Kimoff, 1996). SF might have more severe adverse effects than chronic sleep restriction because of its greater interference with slow wave sleep and REM sleep. Experimentally induced short-term SF leads to excessive daytime sleepiness and cognitive impairment in humans, even in the absence of any reduction in total sleep duration (Bonnet, 1987). To determine the relationships among chronic SF, obesity, and neurobehavior in rodents, we established an effective SF paradigm and used it to determine the differential effect of SF on lean and obese mice on the same normal diet.
Material and Methods
1. Mice, headmount surgery, and sleep recording
The animal studies were approved by the Institutional Animal Care and Use Committee. Mice were group-housed with a 12 h light-dark cycle, lights on at 6 am (zeitgeber time ZT 0) and lights off at 6 pm (ZT 12), and fed freely available normal LabDiet 5001 (LabDiet, St. Louis, MO). The mice were single-housed only when being placed in the sleep recording or fragmentation chambers.
To validate that the SF protocol induced adequate changes of sleep architecture, 5 male C57 mice (Jackson Laboratory, Bar Harbor, ME) were used for sleep recording. The mice were subjected to surgery at age 14–16 weeks, received a prefabricated headmount containing 2 channels of electroencephalographic (EEG) and 1 channel of electromyographic (EMG) on cervical paraspinal muscles as described previously (Wang et al., 2013). The mice were allowed to recover for at least 10 days before initiation of sleep recording.
Sleep recording used a repeated measures design, with each mouse serving as its own control. After 3 days of adaptation single-housed with tethered EEG preamplifier and amplifier connected to the headmount, sleep was sampled starting at the lights-on period and continued for 14 consecutive days (24 h periods) without interruption. The recording consisted of 48 h of baseline recording, 10 consecutive days of SF during the light span, and another 48 h of recovery sleep. Data were acquired with the Serenia Acquisition software (Pinnacle Technology) during continuous recording.
For the analysis of sleep data, digitized EEG (band pass, 0.5–40 Hz) and EMG (10 – 100 Hz) were processed in 10 second (sec) epochs. Manual scoring was performed by experienced researchers blinded to the group, with each of the 10 sec epochs assigned as wake, rapid eye movement sleep (REM), or non- rapid eye movement sleep (NREM), following standard criteria (Radulovacki et al., 1984). Hypnograms, the percent of each sleep stage, sleep bouts, bout duration, and delta power were analyzed with Sleep Pro software (Pinnacle Technology). Bout was defined as a minimum of 3 consecutive epochs at a length of 10 sec/epoch for a given state; a bout was considered to end by a state change of even one epoch. Delta power was determined as the average of power spectrum from 0.5 to 4 Hz. Mean and standard error of all mice were calculated for each day of 24 h continuous recording (ZT 0 – 24).
2. Sleep fragmentation procedure
The SF maneuver consisted of random rotation of a metal bar slightly shorter than the inner diameter of the round cage and positioned above the corncob bedding. The bar rotation disturbs sleep in a manner similar to gentle handling. SF was achieved by a computer-controlled schedule, repetitive on a 120 s cycle (30 s on, 90 s off) during the light span (6 am to 6 pm), at an intermediate bar rotation speed (scale of 5 out of 10). The direction of bar rotation was randomly reversed at a time interval of 10 - 30 sec. The choice of 30 event/h of SF was based on clinical evidence in severe sleep apnea, and shown to be effective in compromising sleep architecture in mouse and rat (Tartar et al., 2009; Sinton et al., 2009).
3. Group design and behavioral testing
To determine how SF and obesity could interact with each other to modulate mouse behavior, we subjected POKO and wildtype (WT) mice to the SF maneuver or left them unperturbed. Four groups of mice were studied (n = 12 /group): naïve WT, naïve POKO, SF WT, SF POKO. Each group contained male (6 mice) and female (6 mice). POKO mice are obese and have reduced metabolic activity as a result of the production of a membrane-bound mutant leptin receptor in all cells, without leptin signaling function (Hsuchou et al., 2013). The POKO mice also show hypersomnolence and reduced basal locomotor activity (Wang et al., 2013). At 13–15 weeks of age, the mice were subjected to baseline behavioral tests, including hot plate test to determine the circadian rhythm of pain, open field test for locomotor activity and anxiety-like behavior, and elevated plus maze test for anxiety-like behavior. The mice were given two days of recovery between each test. One week later, SF was applied to these mice for 20 consecutive days. The hot plate test was performed on SF day 15, the open field test was performed on SF day 17, and the elevated plus maze test was performed on SF day 19.
To test potential changes of pain threshold and its circadian rhythmicity after SF, the hot plate test was performed by use of a hot plate analgesic test meter (Columbus Instruments, Columbus, OH). The temperature was set at 55 °C, and a cutoff time of 30 sec was used to avoid burn injury. At time 0, the mouse was placed at the center of the hot plate. The latency to lick a hindpaw or jump was recorded, and the mouse was removed from the hot plate immediately.
The open field test, a standard neurobehavioral measurement, detects changes of locomotor activity and level of anxiety. The test was performed as described previously (Wu et al., 2010; Wang et al., 2013). The open field was an evenly illuminated square arena (50 × 50 cm) with level surface surrounded by walls 30 cm high, and divided into 16 equal squares. The 4 squares in the center were defined as the central region (inner grids), and the remaining 12 squares adjacent to the walls were defined as the peripheral region (outer grids). At the beginning of the test, the mouse was placed in the center. For the subsequent 5 min, the number of inner and outer grids crossed, rearing, grooming, and defecation were counted.
To determine anxiety-like behavior, the elevated plus maze test involved a standard device that had two open arms and two enclosed arms, each being 30 cm long × 5 cm wide. The enclosed arms had walls 15.25 cm high. The arms were joined at a squared central region 5 cm diameter and placed 40 cm above the floor. The mouse was placed in the center of the maze facing an open arm of the maze at the beginning of the test and observed for 5 min. The time spent and entries made on the open and enclosed arms were recorded. The mouse was returned to the home cage after 5 min.
5. Statistical analysis
Means are presented with their standard errors. For the analyses of sleep architectural changes in the course of SF and recovery sleep, one-way analysis of variance (ANOVA) was performed if there were more than two groups, followed by Turkey’s post-hoc test. When only 2 groups were compared, a 2 tailed t-test was performed. For the analysis of neurobehavioral changes, the effects of SF and obesity were determined by 2-way ANOVA. A p value of < 0.05 was considered statistically significant. Prism GraphPad 5 statistical and graphic program (GraphPad, San Diego, CA) was used to conduct statistics and generate graphics.
Results
1. SF was effective during the light span, and it blunted the circadian rhythm of sleep
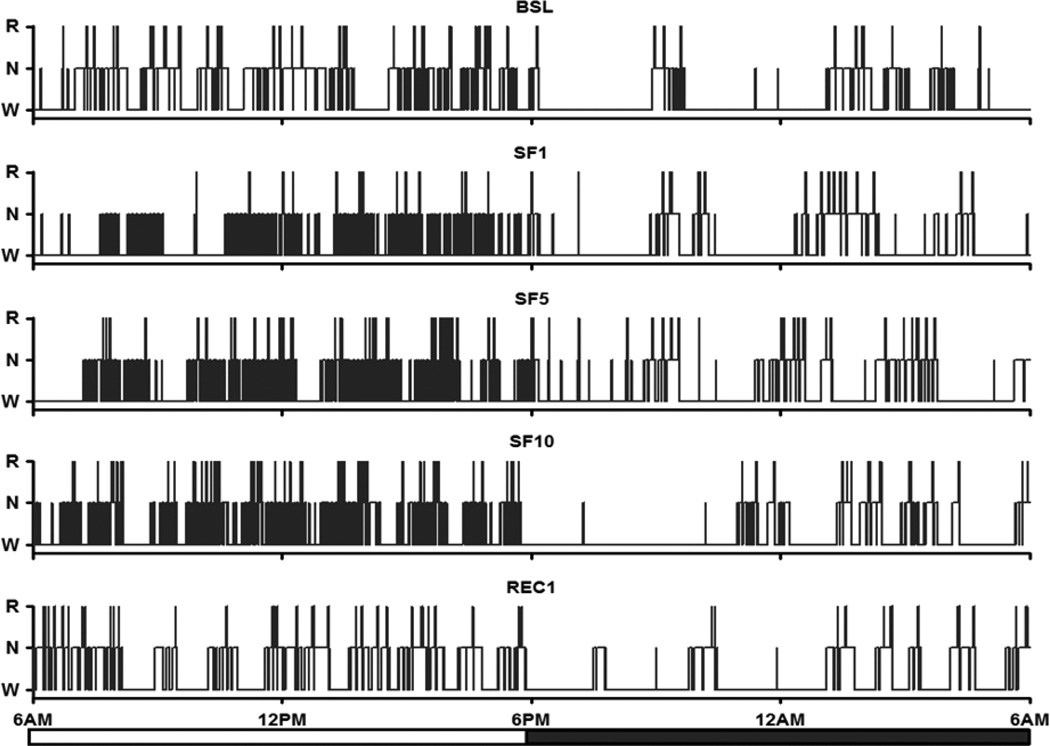
An effective chronic SF protocol should induce adequate amounts of sleep disruption during the light span without a dramatic increase of hypersomnolence in the dark span. As shown in the representative hypnograms of a mouse at baseline (BSL), SF day1 (SF1), SF day5 (SF5), SF Day 10 (SF10), and recovery day1 after SF (REC1), there were more frequent transitions of sleep stages and shorter sleep bout length during the light span of SF days, in comparison with either BSL or REC1 (Fig.1). This indicates the presence of fragmented sleep during the SF period.
Fig.1.
Representative 24 h hypnograms of baseline (BSL), sleep fragmentation day 1, day 5, and day 10 (SF1, SF5 and SF10), and recovery day 1 (REC1). During the SF periods, frequent sleep state transitions and shorter sleep bouts were seen in the light span, in comparison with those in BSL and REC.
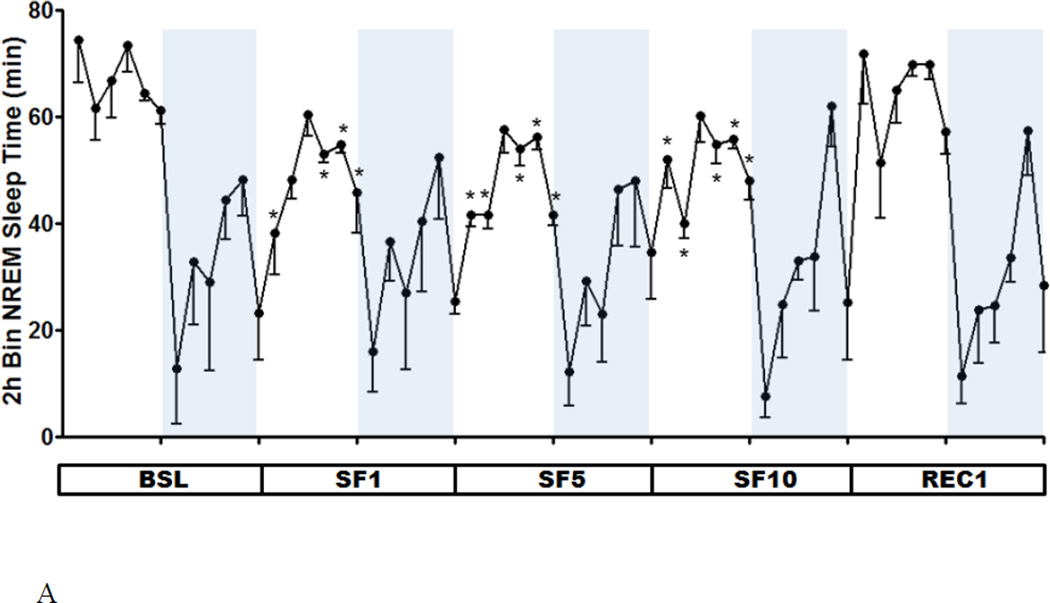
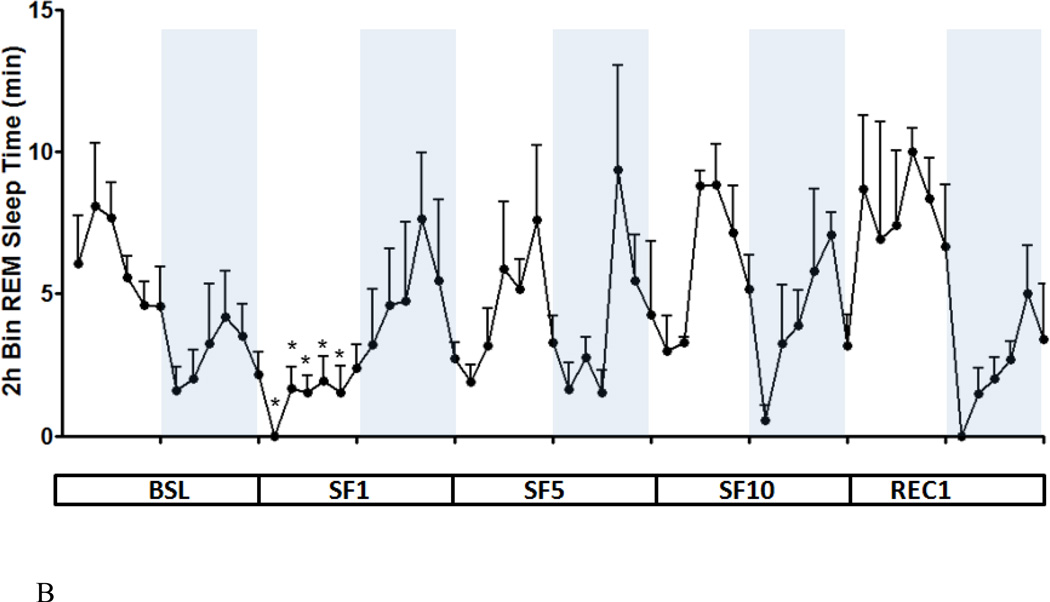
As shown by the 2 h segmental plot, the duration of NREM during SF manipulation decreased throughout the light span, except for ZT 4–6 h. This significant reduction contrasts with the lack of change of NREM sleep time during the dark span. Overall, the circadian amplitude of NREM sleep was blunted during the 10 days of SF (Fig.2A). REM sleep was only attenuated on the first day, after which a normal pattern resumed by SF5 (Fig.2B).
Fig.2.
Two h segmental plot of NREM (A) and REM (B) sleep time at BSL, SF1, SF5, SF10, and REC1. As NREM sleep time decreased during the light span, the circadian amplitude of NREM sleep was decreased throughout the SF period. The circadian amplitude of REM sleep time was only decreased at SF1, but returned to baseline at SF5 and SF10. *: p < 0.05 compared with BSL at the same ZT.
2. SF increased wake time and wake bouts, and decreased wake bout length
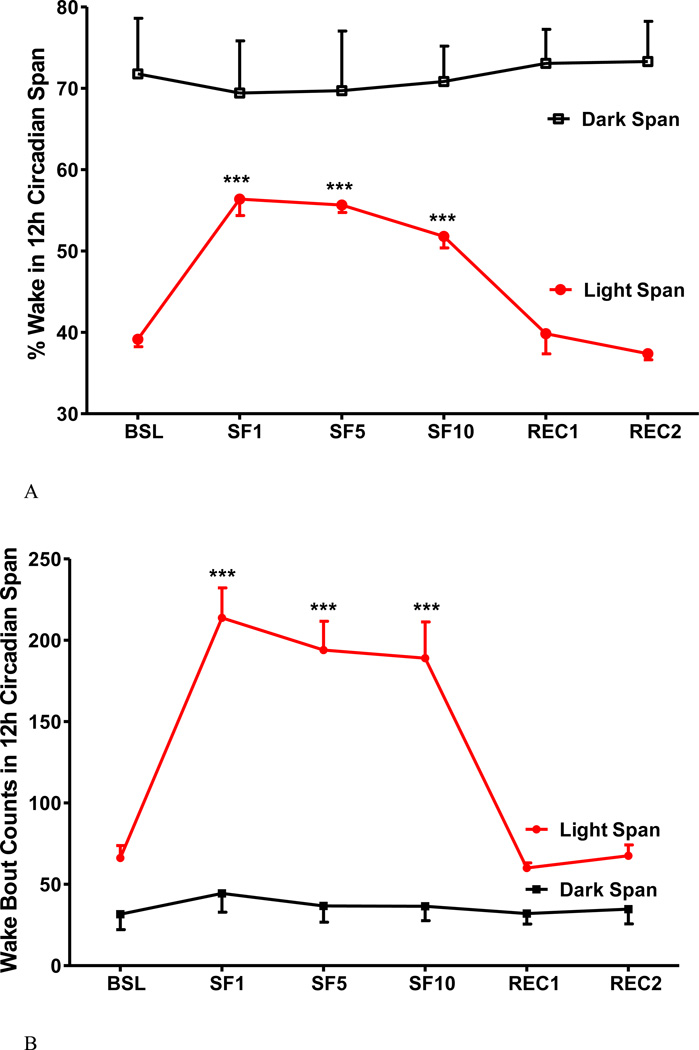
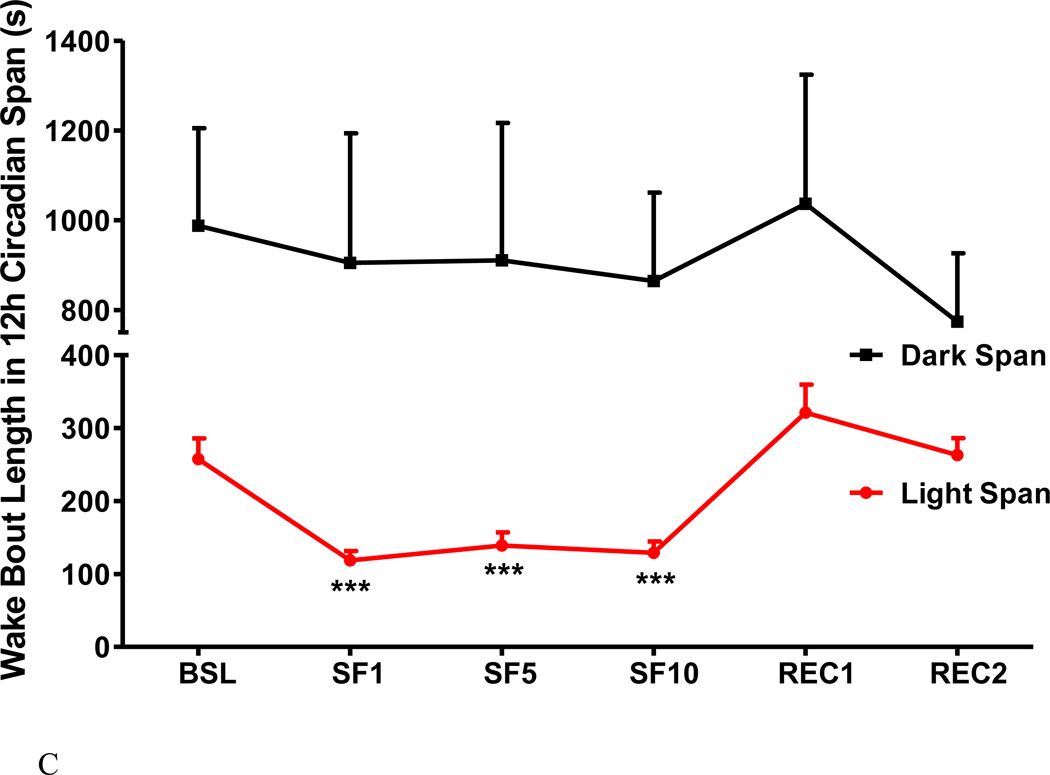
The SF procedure increased the percent of wake time in the 12 h light span, with the percent of sleep ranging from 51.8 - 56.4 % among SF days in comparison with a baseline of 39.2 % (Fig.3A). The change mainly occurred during the light span with only a mild and non-significant decrease in the dark span. There were more wake bouts during the 12 h light span, without changes in the dark span (Fig.3B). The wake bout length was decreased in the light span and remained unchanged in the dark span, resulting in a decrease over the 24 h period (Fig.3C).
Fig.3.
The effects of SF on percent of wake time (A), number of wake bouts (B), and length of wake bouts (C) shown at 12 h intervals. During the light span, SF increased the percent of wake time and the number of wake bouts, and decreased wake bout length. During the dark span and the recovery days, there was no significant change. ***: p < 0.001 compared with BSL.
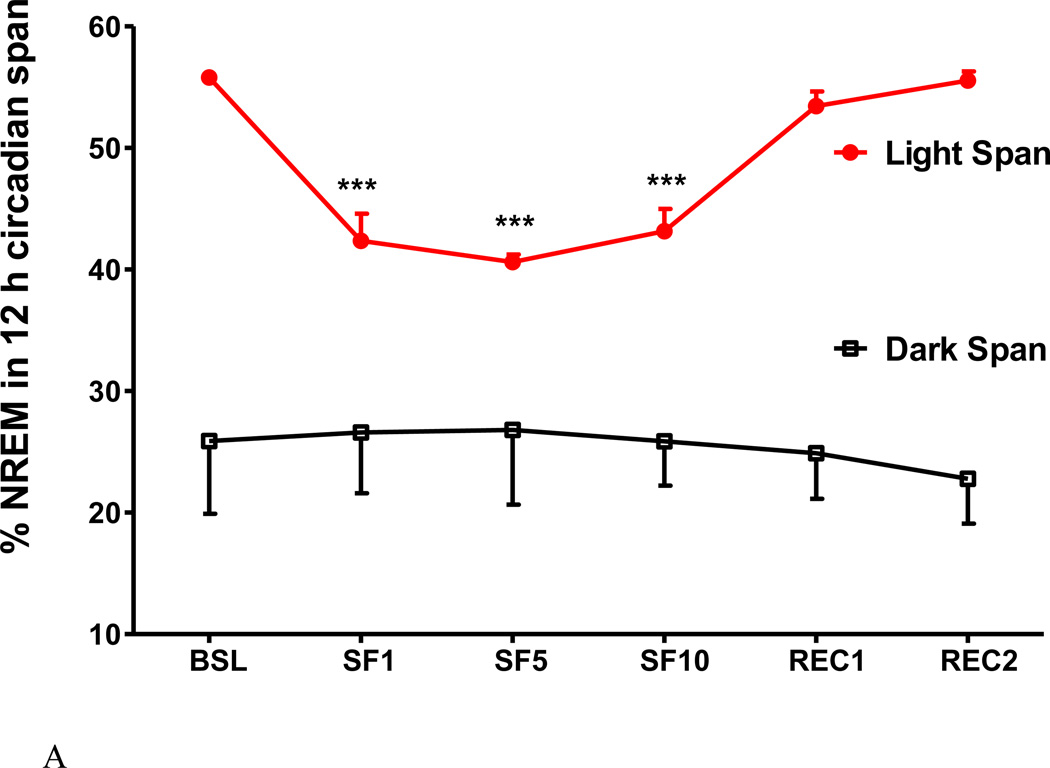
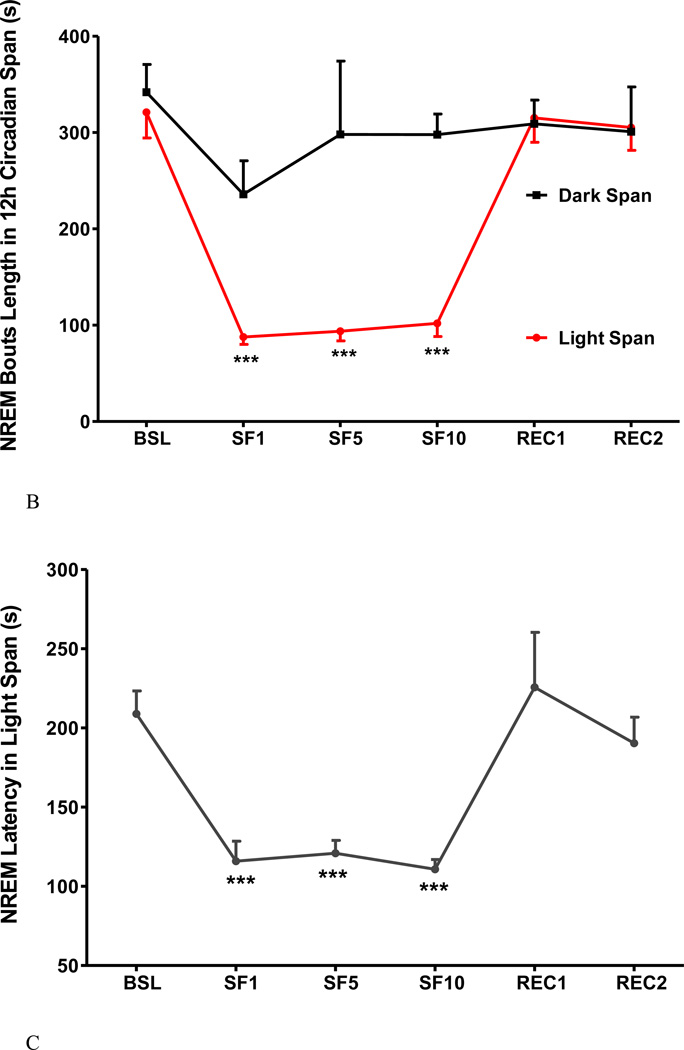
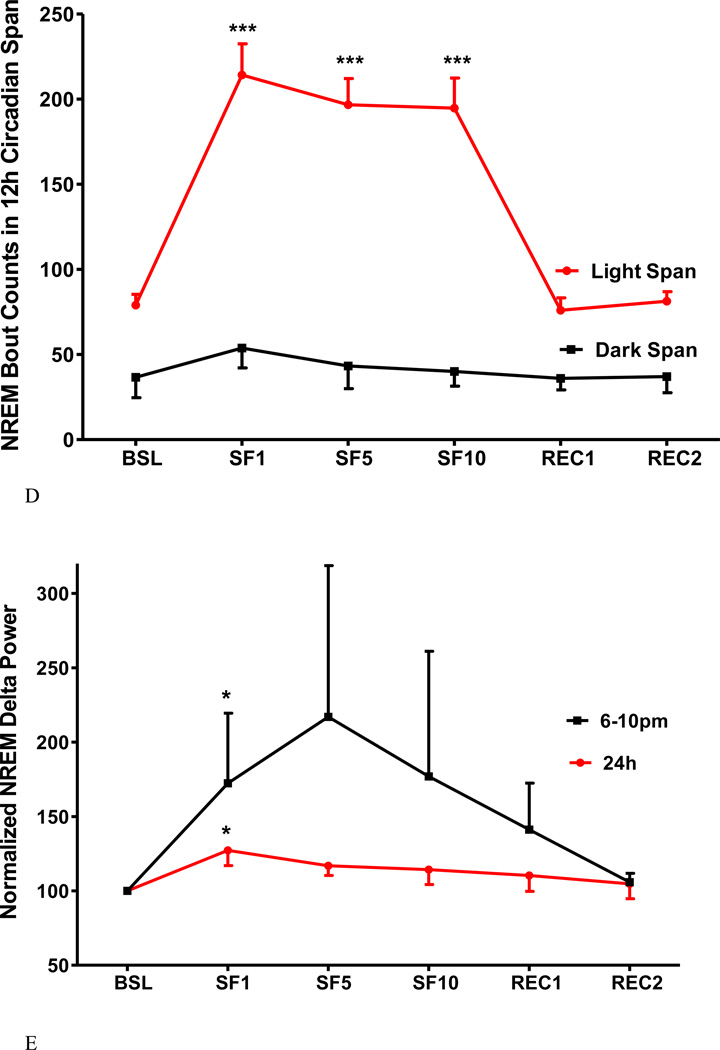
3. SF decreased NREM sleep time, bout length, and sleep latency and increased NREM sleep bouts and delta power
SF decreased the percent of time spent in NREM sleep in the light span, and the reduction was consistent across the SF period from SF1 to SF10 (Fig.4A). The average length of NREM bouts also showed a significant decrease (Fig.4B). The NREM sleep latency was shortened during the light span (Fig.4C) but did not change during the dark span (data not shown).The average number of NREM bouts increased from 79 ± 6.3 s at baseline to 214 ± 18 s at SF1, and then stabilized at 197 ± 15 s on SF5 and 195 ± 18 s on SF10 (Fig.4D). NREM sleep returned to baseline at REC1 and REC2 and it did not show rebound at the dark span. It is noteworthy that the delta power spectrum indicative of sleep pressure was only increased on SF1, and was most pronounced at the onset of the dark span. Changes of NREM delta power at SF5, SF10, REC1, and REC2 were not significant in comparison with BSL (Fig. 4E).
Fig.4.
The effects of SF on percent of NREM time (A), NREM bout length (B), NREM sleep latency (C), NREM bout counts (D) are shown at 12 h intervals. During the light span, SF decreased the percent of time spent in NREM, NREM bout length, and NREM sleep latency while it increased NREM bout counts. During the dark span and the recovery days, there was no significant change. NREM sleep delta power (E) showed an increase at SF1, and was prominent at the onset of dark span (6–10 pm). At SF5, SF10, and recovery day, there was no significant change of delta power. *: p < 0.05, ***: p < 0.001 compared with BSL.
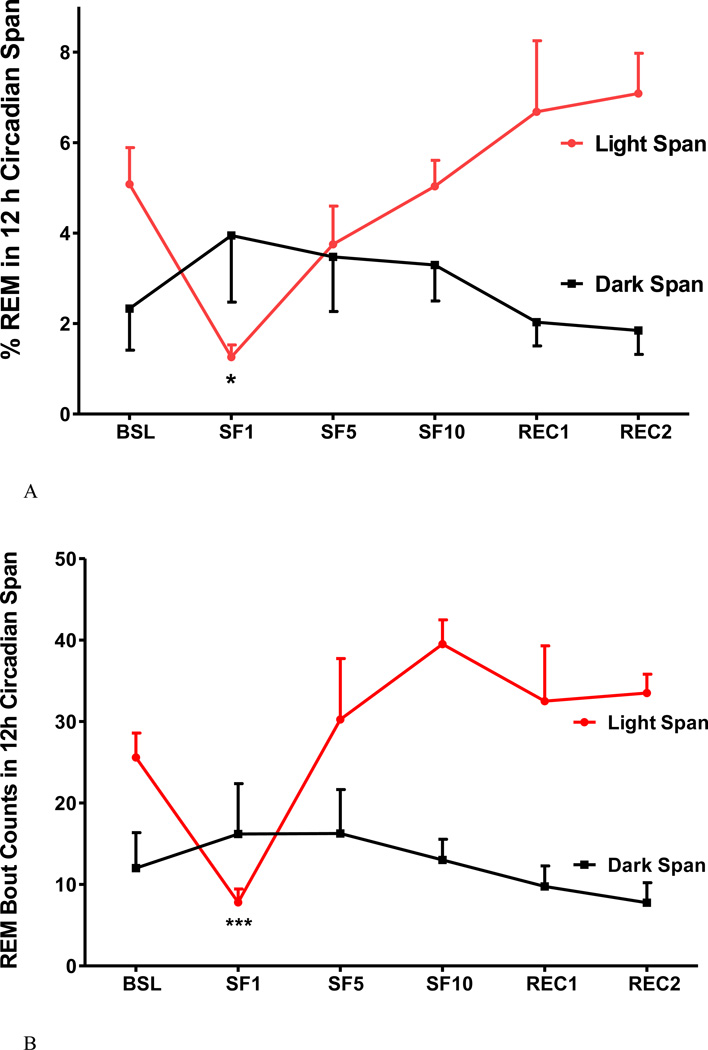
4. Acute (SF1) and chronic SF (SF5, and SF10) had differential effects on REM sleep
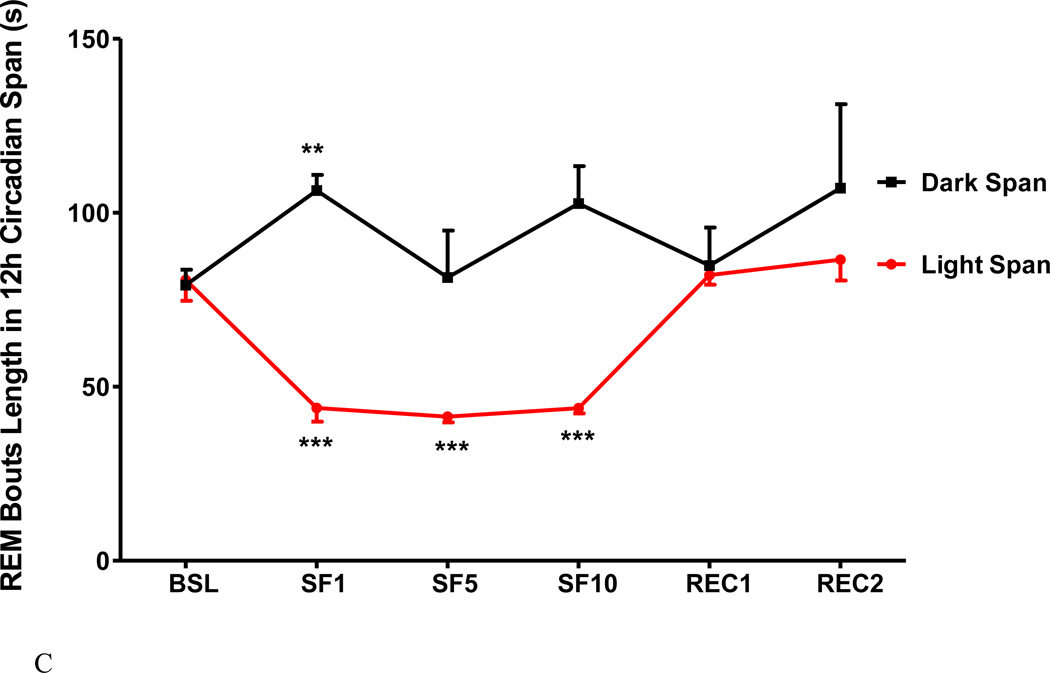
SF induced greater changes of REM sleep, as shown when SF1 was compared with SF5 and SF10 (Fig 5A–C). At baseline, there was 5.1±0.8 % REM in the light span, composed of 26±3.0 bouts at an average bout length of 80.7±6 s. On SF1, there was a reduction of % REM in the light span with fewer and shorter bouts. There was an increase of REM bout length in the dark span (Fig.5C), indicating the presence of rebound sleep on SF1 (Fig.5A). On subsequent days when SF was in the chronic stage, the percent of REM remained stable and did not differ from the baseline. No REM rebound in the dark span was seen on SF5 and SF10 (Fig 5A–C).
Fig.5.
The effects of SF on percent of REM time (A), REM bout counts (B), and REM bout length (C), shown at 12 h intervals. During the light span of SF1, % REM and REM bout length was decreased, but the number of REM bouts were increased. The subsequent 12 h of recovery sleep (dark span of SF1) showed increased REM bout length and REM sleep time. At SF5 and SF10, REM sleep time had no significant change, as REM bouts increased but bout length decreased. *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with BSL.
5. Effect of obesity in the POKO mice on circadian patterns of pain threshold and its interaction with SF
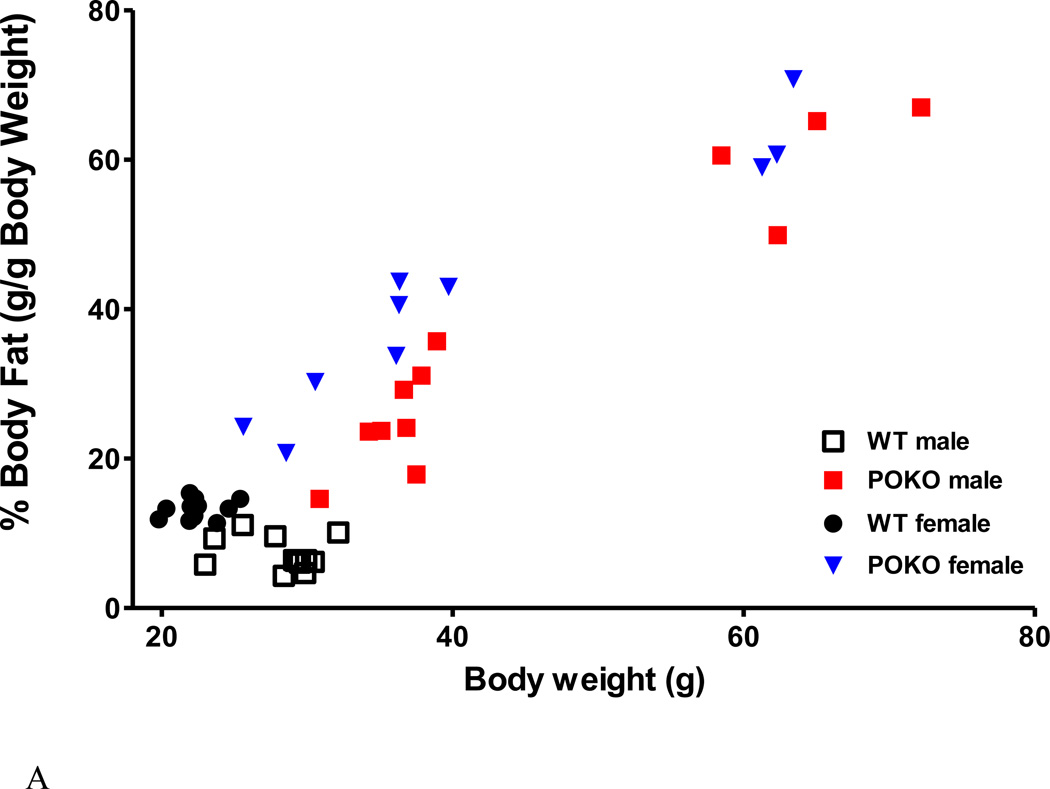
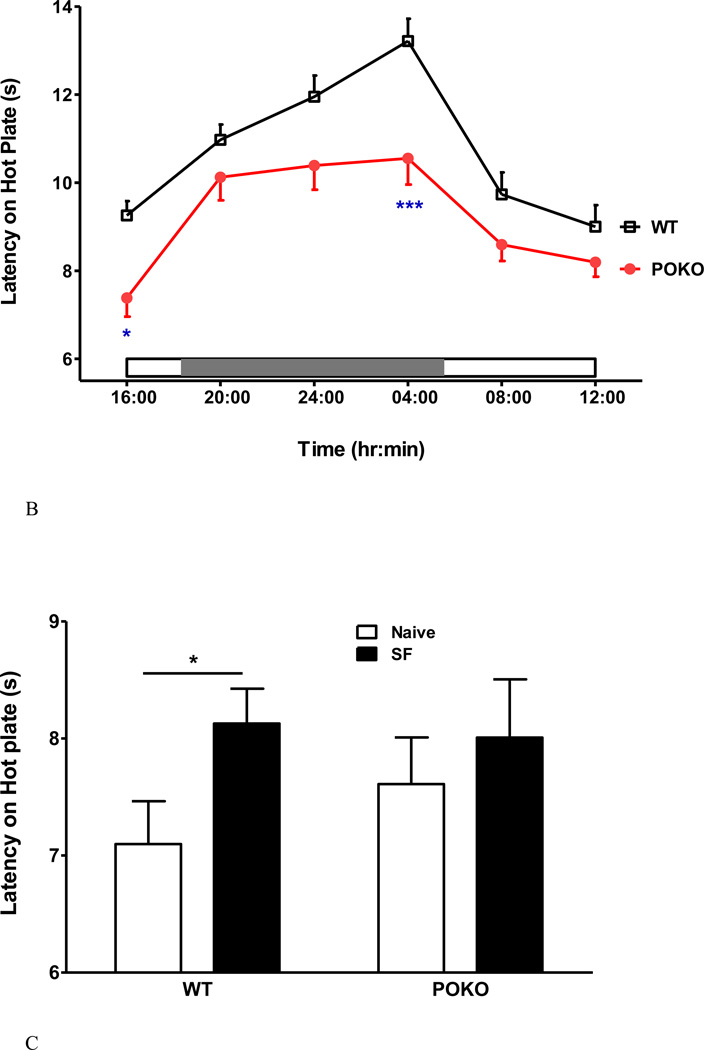
Two strains of mice were studied: POKO mice and their WT littermate controls on a C57/B6 background (n = 6 /group for each sex). The obese POKO mice with a mutant leptin receptor in all cells without signaling functions have been shown to sleep more and have more NREM sleep (Wang et al., 2013). The distribution of % fat and body weight among the mice used in this study is shown in figure 6A. The excessively overweight POKO male and female mice in the right upper quadrants were homozygotes with complete mutation of the leptin receptor in all cells. The clusters of POKO mice that were heavier and had a higher percent of fat than the respective same sex WT mice represented heterozygosity with incomplete deletion of the signaling leptin receptor. When the POKO mice were considered as a whole group and compared with the WT mice at baseline, there was a reduction of the circadian amplitude of thermal pain threshold in the hot plate test. Significant reduction of pain latency was seen at 4 pm (nadir, and first time point of the test) and 4 am (circadian peak) (Fig.6B).
Fig.6.
Metabolic phenotype and neurobehavioral performance of POKO mice. (A) There was a correlation of percent of body fat and body weight of the POKO and WT mice at age of 22–25 weeks. POKO mice had higher adiposity than WT mice (p < 0.0001). (B) POKO mice showed reduced circadian amplitude of thermal pain latency, with significant decrease at 4 pm and 4 am. (C) Potential interactions of SF and obesity were determined in 4 groups of mice. SF had a trend toward increased pain threshold (p = 0.07) by 2-way ANOVA. SF increased pain threshold in the WT mice, whereas the POKO mice did not show a significant change.
When the WT and POKO mice were tested either with or without chronic SF, there was a trend toward an effect of SF on pain threshold (F1, 41=3.37, p = 0.07). There was no effect of strain, or significant interactions. Nonetheless, SF increased the pain threshold in the WT mice as shown by unpaired two-tailed t test (p < 0.05), whereas the POKO mice did not show a significant change (Fig.6C).
6. The interactions of SF and obesity on locomotor activity and anxiety-like behavior
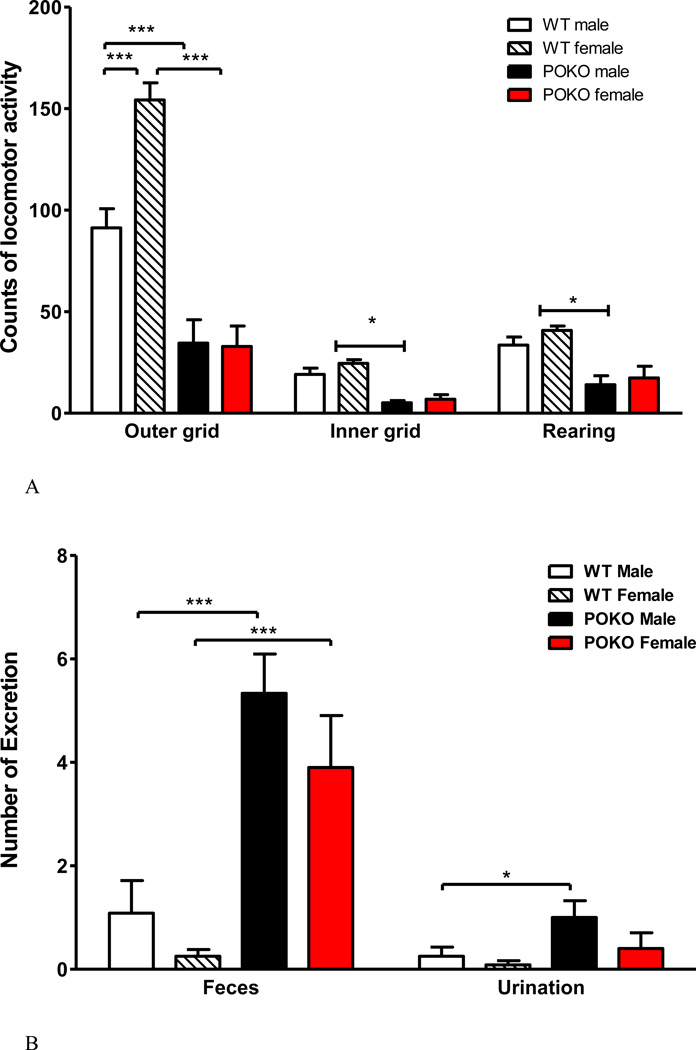
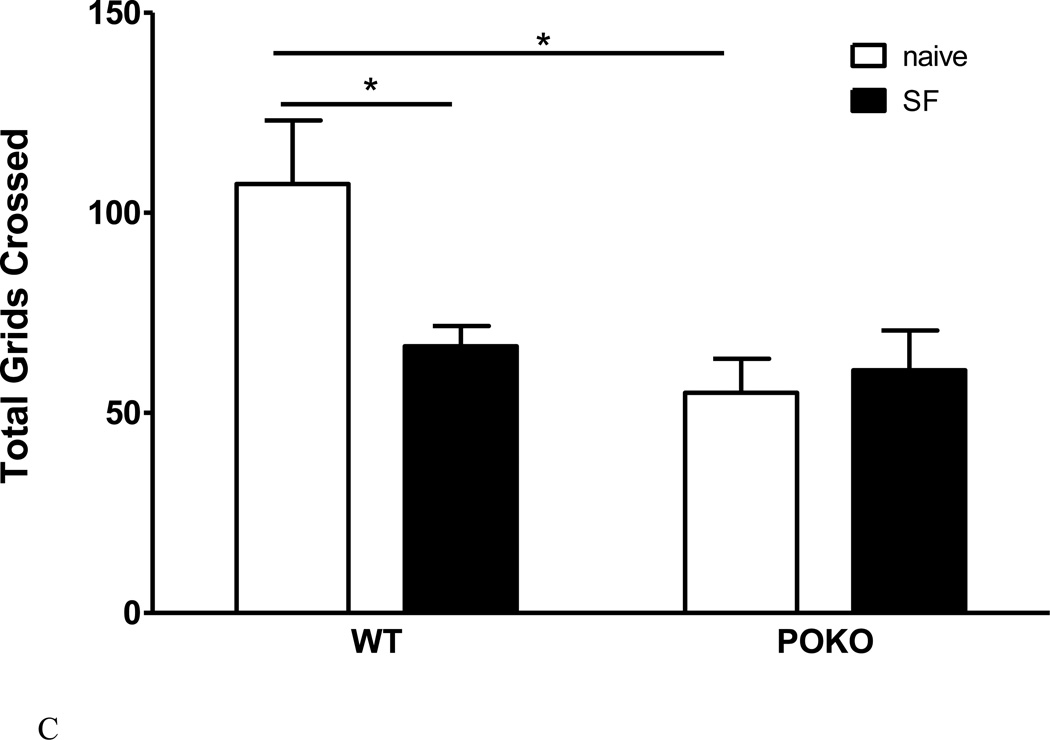
We have shown that the obese POKO mice have reduced basal locomotor activity in the open field (Wang et al., 2013). This was confirmed in both sexes in the mice that were divided into 4 groups for SF studies. All male and female POKO mice had fewer total grids crossed in the 5 min test duration than their respective WT mice. Female WT mice also showed more outer grids crossed than the male WT mice, indicating a higher level of activity (Fig.7A). There was also increased fecal and urine production by the POKO mice in the open field (Fig.7B), suggesting either an increase of metabolic activity or heightened autonomic nervous system activation resulting from changes in anxiety-like behavior. The former was ruled out by the presence of the hypometabolic state of the POKO mice in metabolic chambers (Hsuchou et al., 2013). After SF, a significant strain effect was seen (F1, 20 = 10.84, p < 0.005), as POKO mice had fewer total grids crossed than the WT mice. Post-hoc analysis showed that SF reduced activity in the WT mice (p < 0.05) but not the POKO mice that showed a lower baseline (Fig.7C). The change of excretion was no longer significant (data not shown). Overall, the results from the open field test suggest that both the POKO mutation and SF attenuated activity.
Fig.7.
The interaction of SF and obesity on locomotor activities. (A) Effect of POKO and sex difference on open field performance. All male and female POKO mice had fewer outer and inner grids crossed and less rearing than their respective WT mice. Female WT mice also showed more outer grids crossed than the male WT mice. (B) There was also an increase of fecal and urine production in the POKO mice. (C) In the SF study, a significant strain effect was seen (p < 0.005), as POKO mice crossed fewer total grids than the WT mice. Post-hoc analysis showed that SF reduced activity in the WT mice (p < 0.05) but not the POKO mice which showed a lower baseline.
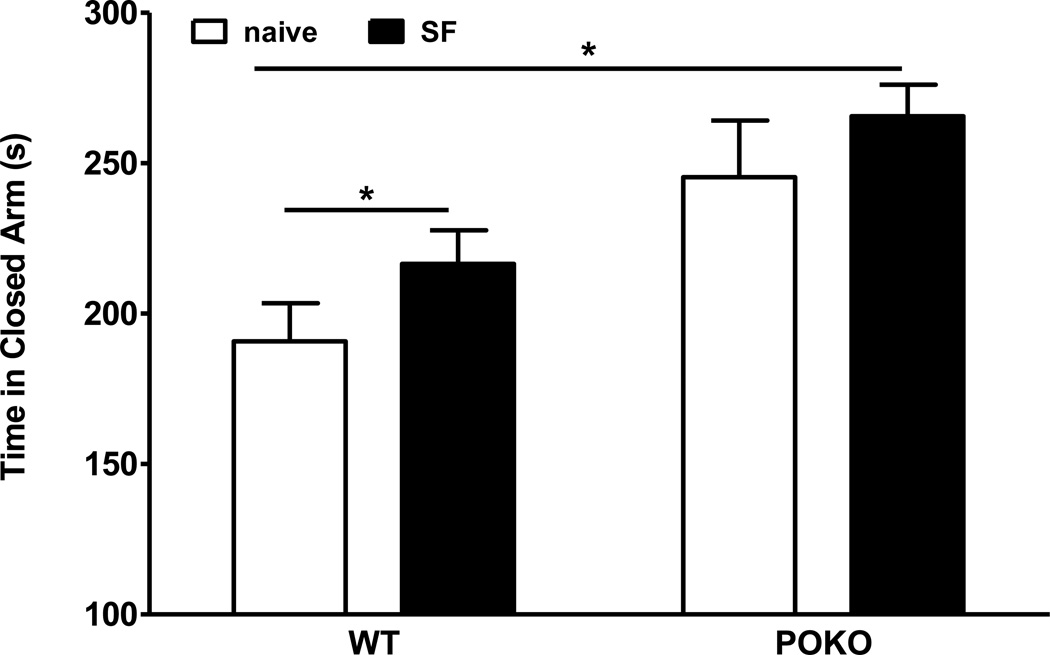
To determine whether SF had differential effects on anxiety-like behavior as suggested by the increased basal fecal excretion in the open field test, we used the elevated plus maze test. There was a significant effect of SF (F1, 29 = 4.65, p < 0.05), but not strain, or their interactions. However, post-hoc analysis showed that SF increased the time WT mice spent in the closed arm. The POKO mice with SF also spent more time in the closed arm than the WT mice without SF. SF had no additional effect on POKO mice that had a somewhat higher baseline (Fig.8). The results support the prediction that both obesity and SF increased anxiety-like behavior.
Fig.8.
The interaction of SF and obesity on the anxiety-like behavior in elevated plus maze test. SF, which had a significant effect (p < 005) by 2-way ANOVA, increased the time that WT mice spent in the closed arm, but had no additional effect on POKO mice that had a somewhat higher baseline.
Discussion
The results show that SF had differential effects on lean WT and obese POKO mice in their thermal pain threshold, activity level, and anxiety-like behavior. Contrary to what would be considered a synergistic effect of sleep disruption and obesity, the POKO mice were more resilient to change after equivalent SF challenge.
We first established and tested the validity of a chronic SF paradigm for mice housed in round Pinnacle sleep recording cages, with sleep manipulation only during the light span when most sleep occurs. SF effectively increased the number of NREM and wake episodes and decreased their duration. Increased sleep drive was reflected by shortened NREM sleep latency and an increase of delta power spectrum of slow wave sleep on the first day of SF. Chronic SF for 10 consecutive days induced a consistent increase of the percent of waking time and decreased that of NREM time. REM sleep was stable after transient induction on the first day (SF1, acute phase). Recovery after 10 days of SF was rapid, as recovery day 1 was no longer associated with significant rebound sleep.
There are a variety of techniques for interrupting sleep in rodents on a repetitive basis, intended to induce sleep disruption without total sleep loss (Tartar et al., 2006; Guzman-Marin et al., 2007; Sinton et al., 2009; Nair et al., 2011; Kaushal et al., 2012; Baud et al., 2013). Different devices used include an activity wheel, orbital shaker, rotating cage, and a treadmill cage. There have been a variety of schedules, such as disruption for 3 seconds (s) in every 30 s, 4 s in every 56 s, 30 s in every 90 s, or 9 s in every 2 min, for a period of 1 - 18 days, either throughout the entire day (24 h) or the light span (12 h) only. Here we chose a schedule of 30 s sleep disruption at 2 min intervals during the 12 h light span in which most sleep occurs. This is to better mimic the changes in patients with severe sleep apnea, in which periods of hypopnea or apnea often last 10 – 60 sec at 1–2 min intervals, and frequently terminate in arousals.
Both obesity and sleep disturbance have been shown to impair concentration, memory, metabolic activity, and overall level of physical activity. Studies of the effects of chronic SF on locomotion, pain, mood, and metabolic activities have reported inconsistent findings, probably related to the model system and the circadian time of testing. The POKO mice have a mutant, membrane-bound leptin receptor that retains its ability to bind leptin but fails to exert intracellular signaling. As a result, homozygote mice are obese and have reduced metabolic activity (Hsuchou et al., 2013). Despite the presence of hyperleptinemia, POKO mice do not respond to leptin with any increase of phosphorylated Signal Transducer and Activator of Transcription-3. Although SF protocols show variable effects on leptin concentrations (Pan and Kastin, 2014), leptin does not appear to play a major role in the increased sleep and attenuated physical activity of the POKO mice. Rather, the decreased locomotion may be mainly explained by increased hypersomnolence (Wang et al., 2013). Further, obesity induces a state of mild neuroinflammation. Region-specific reactive astrogliosis is seen in mice with adult-onset obesity, such as agouti-viable yellow mice (Pan et al., 2008) and those fed with a high-fat diet (Hsuchou et al., 2009). SF also causes chronic stress and inflammation (Balbo et al., 2010; Besedovsky et al., 2012). Thus, one would expect that SF and obesity play an additive role to promote changes in neurobehavior.
However, contrary to this expectation, obese POKO mice had fewer changes than lean WT mice in response to chronic SF. In WT mice, there was an increase of pain threshold with longer pain latency and lower locomotor activity after SF. The POKO mice had reduction of the basal pain threshold, but the hot plate test at 8 am did not show major changes. They also had lower levels of activity that were not further decreased by SF. Although there was no clear change of anxiety-like behavior besides increased fecal production, the increase of time spent in the closed arm was only higher than the WT baseline but not than the group of POKO without SF, indicating minimal increase of anxiety-like behavior. Although hypoxia might be a cause of anxiety, murine models of obstructive sleep apnea are rare even in the presence of obesity. This is mainly related to a protective upper airway anatomy that is not present in obese human beings or other primates. To our knowledge, there have not been studies in mice with hypoxia probes for positron emission tomography imaging in obese mice, or oxygen desaturation analysis showing intermittent hypoxemia, a hallmark of sleep apnea. Even the db/db mice with extreme obesity and type II diabetes do not appear to be hypoxic. For example, kidney blood oxygen level-dependent magnetic resonance imaging does not show differences between the db/db and control mice (Prasad P et al., 2010). We are confident that hypoxia is not a contributing factor to the increase of anxiety in either POKO or lean mice after sleep fragmentation. We also ruled out the confounding effect of stress by single housing that might have contributed to behavioral changes (or lack of changes). Mice used for sleep recording were adapted for three days before baseline recording before SF, and those used for neurobehavioral testing were group housed while undergoing SF. Overall, the obese POKO mice appear to be more resistant to changes of sensory gating and activity than the lean WT mice after SF.
In summary, we devised and validated a chronic SF regimen that resulted in sleep fragmentation mainly during the light span without significant rebound but with recovery sleep during the dark span. With this SF protocol, WT mice showed a decrease of thermal pain sensitivity and locomotor activity when tested in the light span. The obese POKO mice were unexpectedly resilient to SF without additional deterioration of their neurobehavior. The results suggest complex adaptive mechanisms for the obese animals to respond to chronic SF.
Acknowledgement
Grant support for the BBB Group was provided by NIH (DK54880, DK92245, NS62291).
References
- Balbo M, Leproult R, Van CE. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:759234. doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud MO, Magistretti PJ, Petit JM. Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice. J Sleep Res. 2013;22:3–12. doi: 10.1111/j.1365-2869.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep. 1987;10:364–373. doi: 10.1093/sleep/10.4.364. [DOI] [PubMed] [Google Scholar]
- Bromley LE, Booth JN, III, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep. 2012;35:977–984. doi: 10.5665/sleep.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin AD, Carter RE, Adachi T, Macedo PG, Albuquerque FN, van der Walt C, Bukartyk J, Davison DE, Levine JA, Somers VK. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013;144:79–86. doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–333. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Wang Y, Cornelissen-Guillaume GG, Kastin AJ, Jang E, Halberg F, Pan W. Diminished leptin signaling can alter circadian rhythm of metabolic activity and feeding. J Appl Physiol. 2013;115:995–1003. doi: 10.1152/japplphysiol.00630.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Ramesh V, Gozal D. TNF-alpha and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS One. 2012;7:e45610. doi: 10.1371/journal.pone.0045610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoff RJ. Sleep fragmentation in obstructive sleep apnea. Sleep. 1996;19:S61–S66. doi: 10.1093/sleep/19.suppl_9.s61. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos G, Ribeiro DA, Alvarenga TA, Hirotsu C, Scorza FA, Le Sueur-Maluf L, Noguti J, Cavalheiro EA, Tufik S, Andersen ML. Behavioral and genetic effects promoted by sleep deprivation in rats submitted to pilocarpine-induced status epilepticus. Neurosci Lett. 2012;515:137–140. doi: 10.1016/j.neulet.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, Gozal D. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184:1305–1312. doi: 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte Leptin Receptor (ObR) and Leptin Transport in Adult-Onset Obese Mice. Endocrinology. 2008;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Leptin: a biomarker for sleep disorders? Sleep Med Rev. 2014;18:283–290. doi: 10.1016/j.smrv.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad P, LI LP, Halter S, Cabray J, Ye M, Batlle D. Evaluation of renal hypoxia in diabetic mice by BOLD MRI. Invest Radiol. 2010;45:819–822. doi: 10.1097/RLI.0b013e3181ec9b02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228:268–274. [PubMed] [Google Scholar]
- Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Kovakkattu D, Friese RS. Validation of a novel method to interrupt sleep in the mouse. J Neurosci Methods. 2009;184:71–78. doi: 10.1016/j.jneumeth.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, Cordeira JW, Legare SL, Blanchette AJ, McCarley RW, Strecker RE. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav Brain Res. 2009;197:450–453. doi: 10.1016/j.bbr.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, He J, Kastin AJ, Hsuchou H, Pan W. Hypersomnolence and reduced activity in pan-leptin receptor knockout mice. J Mol Neurosci. 2013;51:1038–1045. doi: 10.1007/s12031-013-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kastin AJ, He Y, Hsuchou H, Rood JC, Pan W. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav Immun. 2010;24:1340–1346. doi: 10.1016/j.bbi.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R504–R509. doi: 10.1152/ajpregu.00105.2007. [DOI] [PubMed] [Google Scholar]