Abstract
Background
Mammographic density is a strong risk factor for breast cancer and is highly variable, but, to date, few studies have examined density in Asian women, particularly those in low and middle-income Asian countries where genetic and lifestyle determinants may be significantly different.
Methods
A total of 1,240 women who attended an opportunistic mammogram screening programme were eligible for analysis. Mammographic density was estimated using a fully-automated thresholding method and differences across ethnic groups were examined using linear regression in 205 randomly selected Chinese women, 138 Malay and 199 Indian women.
Results
Percent density was significantly higher in Chinese women (28.5%; 95% CI 27.0%, 30.0%) compared to Malay (24.2%; 95% CI 22.5%, 26.0%) and Indian (24.3%; 95% CI 22.8%, 25.7%) women (p<0.001), after adjustment for age, BMI, menopausal status, parity and age at first full term pregnancy. Correspondingly, adjusted nondense area was significantly lower in Chinese (72.2cm2; 95% CI 67.9cm2, 76.5cm2) women compared to Malay (92.1cm2; 95% CI 86.9cm2, 97.2cm2) and Indian (97.7cm2; 95% CI 93.4cm2, 101.9cm2) women (p<0.001), but dense area did not differ across the three ethnic groups.
Conclusions
Our study shows that higher percent density and lower nondense area reflect the higher incidence of breast cancer in Chinese compared to Malay and Indian women in Malaysia. Known lifestyle determinants of mammographic density do not fully account for the ethnic variations observed in mammographic density in this Asian cohort.
Introduction
Mammographic density is the area on a mammogram that is white and represents the radiodense connective and epithelial tissue in the breast. Mammographic density is expressed either as dense area, which in cm2, is the total area that is radiodense, or percent mammographic density, which is the proportion of dense tissue to the total amount of breast tissue. Notably, percent mammographic density and absolute dense area have been shown to be strong risk factors for breast cancer, where women with highest densities have an increased risk of up to six-fold compared to those with little or no densities [1,2,3,4,5]. Nondense areas of the mammogram, which represent adipose tissue, on the other hand have been suggested to be protective against breast carcinogenesis [6].
Although ethnic differences in mammographic density have been studied, the majority of these have been amongst multi-ethnic cohorts in Western countries, such as the United States and the United Kingdom. These studies show that percent density and absolute dense area reflect the differences in breast cancer incidence rates in Asian compared to Caucasian women [7,8,9]. Few studies have examined mammographic density in Asians, particularly those living in low and middle income Asian countries. The majority of Asian countries practice opportunistic screening [10] and organised population-based screening are only available in high income Asian countries such as Singapore, Japan and Korea [11].
In Malaysia, the incidence of breast cancer differs across the three major ethnic groups. Chinese women have the highest age standardised incidence rate, with 59.7 per 100 000 followed by the Indians and Malays at 55.8 and 33.9 respectively [12,13]. Mammographic density has been described previously in Chinese populations in Asia [14,15] but not yet in Malays and Indians living in Asia. Malay and Indian women constitute a significant proportion of women in the Malay Archipelago and the Indian subcontinent and make up 51% and 8% of the Malaysian population, respectively. This gives us the opportunity to study ethnic differences and lifestyle determinants of mammographic density in these ethnic groups, especially in the current generation before the disease risk approaches that of Caucasian populations through Westernisation and urbanisation. We sought to investigate whether the differences in percent density, dense area and nondense area across these three ethnicities reflect the variation in breast cancer incidence rates.
Methods
Study population and data collection
A cross-sectional study was conducted among participants of the Malaysian Mammogram Study (MyMammo), one of the several subsidised opportunistic mammogram screening programmes in the country. Women between 40 and 74 years of age with no personal history of breast cancer were eligible to participate. Participants were recruited using flyers, posters and articles in the mainstream media. Although information on the subsidised programme was provided equally across the English, Chinese and Malay media, the majority of participants were Chinese. All women provided written informed consent and this study was approved by the Sime Darby Medical Centre Independent Ethics Committee.
The questionnaire included information on anthropometric factors, menstrual and reproductive history, family history of cancer and dietary practices. Anthropometric information was self-declared for about 30% of the subjects and measured by the interviewer for the remaining 70%. Analyses conducted by stratifying the subjects by self-reported and measured height and weight showed no impact on the results of this study.
Mammograms were available for 1603 of the 1646 women who participated in MyMammo between October 2011 and October 2013. Women were excluded from this analysis if they were of mixed ethnicities or ethnicities other than Malay, Indian and Chinese (n = 84). Where women were known to be related, the oldest participant was included in the analysis. Of the 1356 unrelated participants, we excluded women who have had silicone injections to their breasts (n = 3), were symptomatic (n = 91) or were diagnosed with cancer (n = 8), had missing BMI data (n = 16) or never had menstrual periods (n = 1). Of the 1240 eligible women for analysis, there were 138 Malays, 199 Indian and 903 Chinese.
Mammographic density assessment
Mammograms were performed with the Hologic Selenia full field digital mammography (FFDM) system. Mammographic density measurements of the processed images were performed using a fully-automated thresholding method based on the ImageJ software, as described in [16]. Measurements in pixels were converted into cm2 using the conversion factor obtained from the images. Nondense areas were calculated by subtracting dense area from total breast area.
Correlation coefficients revealed a strong positive correlation between percent mammographic density estimated by the ImageJ method and Cumulus measurements performed by a trained reader (JL) for 50 randomly selected validation images. Concordance correlation and Bland-Altman plots are shown in S1 and S2 Figs. A validation study was also performed for randomly selected images within each ethnicity. Bland-Altman plots for the ethnicity stratified validation study are shown in S3, S4 and S5 Figs. Concordance correlation and Bland-Altman plots were created using R.
The CC and MLO view measurements were strongly correlated but the means were significantly different. There was no difference between measurements from the left and right mammograms. Statistical analyses were performed using randomly selected left or right CC view mammograms.
Statistical Analyses
BMI was calculated by dividing weight (kg) by the square of the height (m). Subjects were categorised as parous if they have had at least one full term pregnancy (live or still births). Menopausal status was divided into two groups i.e. premenopausal or perimenopausal and postmenopausal. Postmenopausal status was defined as no menses for the past one year. For subjects who did not provide date of last menses, the difference between the age at menopause and age at consent was calculated and those with a difference of 1 or more were categorised as postmenopausal. There were 19 women who have had bilateral oophorectomies, of whom 17 had the surgery before and 2 after menopause. Ever use of oral contraceptives and hormone replacement therapy was defined as at least one month of usage. The analyses for green tea and black tea consumption excluded those who consumed different types of tea regularly. Soy intake included consumption of soy milk, tofu, fermented soy beans and other soy products. Family history of breast cancer in a first degree relative included affected mothers and sisters.
Differences in the subject characteristics across ethnicities were determined using chi-square and F tests. We identified the determinants of percent density, dense area and nondense area using linear regression. In the regression models, we assessed the relationships of a priori determinants of mammographic density and the known risk factors for breast cancer. The regression coefficients for weight and height in the multivariable adjusted models were calculated after excluding BMI from the models. We used methods described previously [15] to recode variables which are applicable only to parous subjects in the multivariable adjusted model. Briefly, the regression coefficient for age at first full term pregnancy represents the effect of a one year difference in age at first full term pregnancy among parous subjects. The regression coefficient for number of full term pregnancies estimates the effect of additional full term pregnancies for parous subjects who have had more than one full term pregnancy. Variables significantly associated with density (p<0.05) in the multivariable ethnicity adjusted analysis were adjusted for in subsequent analyses. We compared the unadjusted and adjusted estimated marginal means of percent density, dense area and nondense area across ethnicities using linear regression and analysis of covariance models, respectively. Covariates were selected based on the determinants of density that were identified in previous regression analyses. Pairwise comparisons of ethnic groups were made using the Sidak test. We repeated the analysis after stratifying the cohort by menopausal status. We also repeated the age and BMI adjusted analyses upon stratifying the subjects into two groups; self-reported and measured anthropometric measurements.
All regression models were checked for normality of residuals and no data transformations were necessary. Linearity, homoscedasticity and independence of residuals were inspected for all continuous independent variables. Models were inspected for multicollinearity among the independent variables. The homogeneity of variance assumption for the analysis of covariance was affected by unequal sample sizes across the three ethnic groups [138 Malays, 199 Indian and 903 Chinese]. We therefore repeated all analyses using a randomly selected sample of 205 Chinese women for meaningful comparisons across ethnicities [<50% greater than the smallest cohort i.e. 138 Malays]. Selected characteristics of the random sample compared to all Chinese women are presented in S1 Table. All statistical analyses were performed using Statistical Package and Service Solutions (SPSS) Version 16.0.
Results
Study cohort characteristics
The characteristics of all subjects used in this study stratified by ethnicity are presented in Table 1. The mean age was youngest for Malays (48.4 years) compared to Chinese (50.8 years) and Indians (51.4 years). Malays and Indians had 14% higher BMI than the Chinese. The majority of women (86.9%) were parous and Malay women had the most pregnancies and breastfed the most frequently and for the longest duration. Malay women were more likely to have used oral contraceptives [40.2% compared to 25.6% Chinese and 23.6% Indian], whereas Indian women were more likely to have used hormone replacement therapy. Consistent with the population risk to breast cancer, more Chinese women, followed by Indian women, had a family history of breast cancer compared to Malay women [13%, 12.2% and 4.4% respectively]. Few women were current smokers and there was a higher proportion of ever and current smokers among Malay women. More Chinese and Indian women consumed alcohol. Women in our cohort were more affluent than the national average (48% versus 66% earning less than RM5000 per month) and more likely to have at least secondary education (93% versus 62%) compared to the general Malaysian population [17,18]. In particular, the Malay women who participated in the opportunistic screening programme were more highly educated and affluent than the Indian or Chinese women.
Table 1. Characteristics of subjects.
| Characteristic | All (N = 542) | Malay (n = 138) | Indian (n = 199) | Chinese (n = 205) | p value a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Age (years) | 50.4 (7.4) | 48.4 (6.3) | 51.4 (7.2) | 50.8 (7.9) | <0.001 | ||||
| Anthropometric | |||||||||
| Height (cm) | 157.1 (5.9) | 156.0 (5.5) | 157.7 (6.2) | 157.2 (5.9) | 0.032 | ||||
| Weight (kg) | 63.6 (12.4) | 65.9 (13.0) | 67.3 (12.5) | 58.6 (10.2) | <0.001 | ||||
| BMI (kg/m2) | 25.8 (4.9) | 27.0 (4.5) | 27.2 (5.3) | 23.7 (4.0) | <0.001 | ||||
| Menstrual and reproductive | |||||||||
| Age at menarche (years), n = 539 | 12.9 (1.4) | 12.9 (1.4) | 12.9 (1.4) | 12.8 (1.3) | 0.896 | ||||
| Parity (Parous) | 86.9 | 87.7 | 89.9 | 83.4 | 0.143 | ||||
| Number of FTP | 2. 4 (1.4) | 2.8 (1.7) | 2.4 (1.3) | 2.1 (1.3) | <0.001 | ||||
| Age at FFTP b (years) | 27.4 (4.8) | 26.6 (4.4) | 27.0 (4.9) | 28.2 (5.0) | 0.012 | ||||
| Breastfeeding b , n = 465 | |||||||||
| Ever | 82.8 | 94.9 | 87.1 | 69.8 | <0.001 | ||||
| Duration (months) | |||||||||
| 0 | 17.2 | 5.1 | 13.0 | 30.2 | |||||
| >0–12 | 53.7 | 34.7 | 60.5 | 59.7 | |||||
| >12 | 29.1 | 60.2 | 26.6 | 10.1 | |||||
| Multiple 12 month breastfeeding b | 12.3 | 32.2 | 9.0 | 1.8 | <0.001 | ||||
| Menopausal status (post) | 42.1 | 30.4 | 50.8 | 41.5 | 0.001 | ||||
| Age at menopause c (years), n = 219 | 49.1 (4.8) | 49.5 (4.0) | 48.3 (5.4) | 50.0 (4.1) | 0.043 | ||||
| Bilateral oophorectomy (yes), n = 541 | 3.5 | 2.2 | 3.5 | 4.4 | |||||
| Exogenous hormones | |||||||||
| Oral contraceptives use | |||||||||
| Ever, n = 534 | 28.5 | 40.2 | 23.6 | 25.6 | 0.003 | ||||
| Current, n = 526 | 1.3 | 3.0 | 0 | 1.5 | 0.067 | ||||
| Duration of use (months), n = 534 | |||||||||
| 0 | 71.5 | 59.8 | 76.4 | 74.4 | |||||
| 1–11 | 7.5 | 5.3 | 6.5 | 9.9 | |||||
| ≥12 | 21.0 | 34.9 | 17.1 | 15.7 | |||||
| Hormone therapy use | |||||||||
| Ever, n = 537 | 7.6 | 4.4 | 11.2 | 6.4 | 0.050 | ||||
| Current, n = 540 | 3.5 | 1.4 | 6.6 | 2.0 | 0.013 | ||||
| Duration of use (months), n = 537 | 0.105 | ||||||||
| 0 | 92.4 | 95.6 | 88.8 | 93.6 | |||||
| 1-<6 | 2.6 | 0.7 | 3.6 | 3.0 | |||||
| 6-<24 | 0.9 | 0.7 | 1.0 | 1.0 | |||||
| ≥24 | 4.1 | 2.9 | 6.6 | 2.5 | |||||
| Lifestyle variables | |||||||||
| Smoking | |||||||||
| Ever | 7.7 | 15.9 | 2.0 | 7.8 | <0.001 | ||||
| Current | 2.0 | 5.1 | 0.5 | 1.5 | 0.011 | ||||
| Alcohol (>once a month), n = 540 | 18.7 | 5.1 | 22.1 | 24.6 | <0.001 | ||||
| Coffee (≥one cup a day), n = 541 | 61.0 | 55.1 | 67.3 | 58.8 | 0.055 | ||||
| Green tea (≥one cup a day), n = 422 | 7.1 | 7.8 | 7.7 | 6.1 | 0.812 | ||||
| Black tea (≥one cup a day), n = 422 | 28.4 | 37.3 | 38.1 | 13.9 | <0.001 | ||||
| Soy products (daily), n = 538 | 10.0 | 10.2 | 11.1 | 8.9 | 0.754 | ||||
| Other variables | |||||||||
| FHBC 1st degree relative, n = 525 | 10.5 | 4.4 | 12.2 | 13.0 | 0.029 | ||||
| Educational level, n = 535 | <0.001 | ||||||||
| Primary or less | 6.9 | 0.7 | 8.6 | 9.5 | |||||
| Secondary | 48.8 | 32.1 | 57.4 | 51.7 | |||||
| Tertiary | 44.3 | 67.2 | 34.0 | 38.8 | |||||
| Average monthly household income, n = 536 | <0.001 | ||||||||
| <RM5000 | 47.9 | 32.6 | 57.1 | 49.5 | |||||
| RM5000-10 000 | 29.5 | 30.4 | 30.3 | 28.0 | |||||
| >RM10 000 | 22.5 | 36.9 | 12.6 | 22.5 | |||||
Abbreviations: BMI body mass index, FFTP first full term pregnancy, FTP full term pregnancy, FHBC family history of breast cancer
a p value from F test for continuous variables and χ2 for categorical variables, comparing ethnic groups.
b Restricted to parous women
c Restricted to postmenopausal women
Determinants of percent density, dense area and nondense area
Older age at mammogram was significantly associated with a decrease in percent density in all ethnicities. In the age and mutually adjusted regression analysis, we found that percent density decreased with BMI, parity status, earlier age at first full term pregnancy, multiple 12 month breastfeeding and postmenopausal status (S2 Table). After adjusting for ethnicity, all variables mentioned above remained significantly associated with percent density in the same direction, with the exception of multiple 12 month breastfeeding. Taken together, age, BMI, menopausal status, parity, age at first full term pregnancy and ethnicity explained 45.4% of the variation in percent density.
Dense area was found to reduce with increasing BMI, parity status and postmenopausal status in the age and mutually adjusted analysis before and after adjusting for ethnicity (S3 Table). Current use of HRT in postmenopausal women was associated with smaller dense area in the ethnicity adjusted analysis, although it is to be noted that there are only few current HRT users in the study. Taken together, age, BMI, menopausal status and parity accounted for 16.1% of the variation in dense area.
Nondense area was positively associated with BMI and postmenopausal status in the fully adjusted regression analysis (S4 Table). Taken together, age, BMI and menopausal status explained 45.8% of variation in nondense area.
Variables associated with socioeconomic status i.e. education level and average household income were not associated with percent density and dense area in all the adjusted models. We did not find an association between education level and nondense area after adjusting for age, BMI and menopausal status but did find a significant negative association after including ethnicity into this model, suggesting that this association is mediated by the association between ethnicity and nondense area. Income level was not associated with nondense area in all adjusted analyses.
None of the dietary variables i.e. soy, alcohol, coffee and tea intake, were associated with mammographic density. Similarly, we did not observe an association between density and ages at menarche and menopause, having a bilateral oophorectomy, breastfeeding (among parous women), use and duration of use of oral contraceptives and hormone replacement therapy, smoking and having an affected first degree relative (S2, S3 and S4 Tables).
Ethnic differences in mammographic density
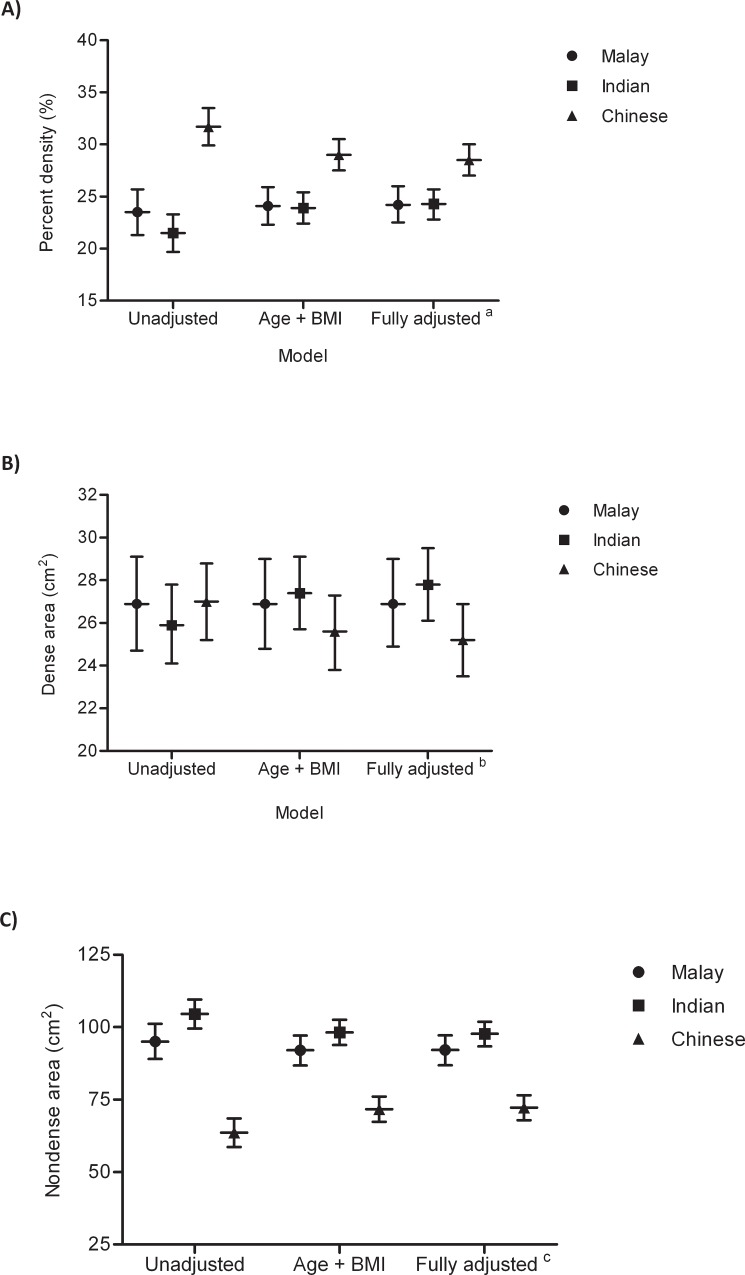
Fig. 1 shows that percent density is higher and non-dense area is lower in Chinese compared to Malay and Indian women [distribution of percent density by ethnicity is shown in S6 Fig.]. In order to determine whether the ethnic differences in mammographic density were mediated by differences in lifestyle variables, we conducted an analysis by adjusting for factors associated with each measure of mammographic density [Table 2]. Notably, the variables associated with each measure of mammographic density is different, with only BMI and post-menopausal status associated with all 3 measures of mammographic density. Chinese women had 35% and 47% higher percent density compared to Malay and Indian women respectively. This difference was partly accounted for by the lower BMI in Chinese women and reduced to 20% and 21% higher percent density respectively after adjusting for age and BMI. Further adjustment for menopausal status, parity and age at first full term pregnancy marginally reduced this difference to 18% and 17%. Percent density did not differ between Malay and Indian women in the unadjusted (p = 0.42) and fully adjusted (p = 1.00) models. Chinese women had 33% and 39% smaller nondense area compared to Malay and Indian women respectively. This difference was partly explained by the lower BMI observed in Chinese women compared to women of Malay and Indian ethnicities. After adjusting for age and BMI Chinese women had 22% and 27% less nondense area compared to Malay and Indian women respectively and further adjustment for menopausal status only reduced these differences by less than 1%. Nondense areas were similar in Malay and Indian women. There were no significant differences in dense areas across the three ethnic groups in both unadjusted and adjusted analyses.
Fig 1. Unadjusted and adjusted A) mean percent density, B) mean dense area and C) mean nondense area with 95% confidence intervals by ethnicity.
Note: aAdjusted for age, BMI, menopausal status, parity and mean adjusted age at first full term pregnancy; bAdjusted for age, BMI, menopausal status and parity; cAdjusted for age, BMI and menopausal status.
Table 2. Unadjusted and adjusted mean percent mammographic density, dense area and nondense area in Malay, Indian and Chinese women, Malaysia, 2011–2013.
| Model | Malay (n = 138) | Indian (n = 199) | Chinese (n = 205) | Overall p value | Multiple comparisons p value a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Malay vs. Indian | Malay vs. Chinese | Indian vs. Chinese | ||
| Percent density (%) | ||||||||||
| Unadjusted | 23.5 | 21.3, 25.7 | 21.5 | 19.7, 23.3 | 31.7 | 29.9, 33.5 | <0.001 | 0.415 | <0.001 | <0.001 |
| Adjusted for age + BMI | 24.1 | 22.3, 25.9 | 23.9 | 22.4, 25.4 | 29.0 | 27.5, 30.5 | <0.001 | 0.995 | <0.001 | <0.001 |
| Fully adjusted b | 24.2 | 22.5, 26.0 | 24.3 | 22.8, 25.7 | 28.5 | 27.0, 30.0 | <0.001 | 1.000 | <0.001 | <0.001 |
| Dense area (cm2) | ||||||||||
| Unadjusted | 26.9 | 24.7, 29.1 | 25.9 | 24.1, 27.8 | 27.0 | 25.2, 28.8 | 0.680 | 0.893 | 0.999 | 0.791 |
| Adjusted for age + BMI | 26.9 | 24.8, 29.0 | 27.4 | 25.7, 29.1 | 25.6 | 23.8, 27.3 | 0.350 | 0.981 | 0.714 | 0.401 |
| Fully adjusted c | 26.9 | 24.9, 29.0 | 27.8 | 26.1, 29.5 | 25.2 | 23.5, 26.9 | 0.131 | 0.898 | 0.534 | 0.132 |
| Nondense area (cm2) | ||||||||||
| Unadjusted | 95.0 | 89.0, 101.1 | 104.5 | 99.5, 109.5 | 63.6 | 58.6, 68.5 | <0.001 | 0.055 | <0.001 | <0.001 |
| Adjusted for age + BMI | 92.0 | 86.8, 97.1 | 98.2 | 93.9, 102.5 | 71.7 | 67.4, 76.0 | <0.001 | 0.186 | <0.001 | <0.001 |
| Fully adjusted d | 92.1 | 86.9, 97.2 | 97.7 | 93.4, 101.9 | 72.2 | 67.9, 76.5 | <0.001 | <0.001 | <0.001 | <0.001 |
Abbreviations: BMI body mass index, CI confidence interval, FFTP first full term pregnancy
a p value from Sidak test.
b Adjusted for variables associated with percent density [age, BMI, parity, age at first full term pregnancy and menopausal status]
c Adjusted for variables associated with dense area [age, BMI, parity, menopausal status]
d Adjusted for variables associated with non-dense area [age, BMI, menopausal status]
We found results that were similar to those described above when we analysed only premenopausal women (Table 3). Chinese women had significantly higher percent density and smaller nondense area compared to Malay and Indian women; percent density and nondense area did not differ between Malay and Indian women; and there were no differences in absolute density across all three ethnicities. In postmenopausal women (Table 4), Chinese women had higher percent density compared to women of other ethnicities but this difference was no longer significant after further adjusting for variables associated with percent density [parity and age at first full term pregnancy]. As observed among premenopausal women, dense area did not differ across the ethnic groups and nondense areas were significantly smaller in the Chinese compared to Malays and Indians among postmenopausal women.
Table 3. Unadjusted and adjusted mean percent mammographic density, dense area and nondense area in premenopausal Malay, Indian and Chinese women, Malaysia, 2011–2013.
| Model | Malay (n = 90) | Indian (n = 98) | Chinese (n = 120) | Overall p value | Multiple comparisons p value a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Malay vs. Indian | Malay vs. Chinese | Indian vs. Chinese | ||
| Percent density (%) | ||||||||||
| Unadjusted | 25.2 | 22.7, 27.7 | 25.2 | 22.7, 27.7 | 36.0 | 33.7, 38.2 | <0.001 | 1.000 | <0.001 | <0.001 |
| Adjusted for age + BMI | 27.3 | 25.1, 29.5 | 27.3 | 25.2, 29.4 | 32.6 | 30.6, 34.6 | <0.001 | 1.000 | 0.002 | 0.002 |
| Fully adjusted b | 27.6 | 25.5, 29.8 | 27.3 | 25.2, 29.4 | 32.3 | 30.3, 34.3 | 0.002 | 0.994 | 0.010 | 0.003 |
| Dense area (cm2) | ||||||||||
| Unadjusted | 28.7 | 26.1, 31.3 | 29.2 | 26.6, 31.8 | 30.2 | 27.8, 32.6 | 0.690 | 0.988 | 0.784 | 0.931 |
| Adjusted for age + BMI | 29.9 | 27.4, 32.5 | 30.5 | 28.0, 33.0 | 28.2 | 25.8, 30.5 | 0.392 | 0.982 | 0.701 | 0.461 |
| Fully adjusted c | 30.1 | 27.6, 32.7 | 30.5 | 28.0, 33.0 | 28.0 | 25.7, 30.3 | 0.323 | 0.995 | 0.564 | 0.409 |
| Nondense area (cm2) | ||||||||||
| Unadjusted | 92.5 | 85.5, 99.6 | 95.8 | 88.8, 102.8 | 56.6 | 50.3, 62.9 | <0.001 | 0.887 | <0.001 | <0.001 |
| Adjusted for age + BMI d | 86.5 | 80.3, 92.8 | 90.5 | 84.4, 96.7 | 65.8 | 60.0, 71.5 | <0.002 | 0.747 | <0.001 | <0.001 |
Abbreviations: BMI body mass index, CI confidence interval, FFTP first full term pregnancy
a p value from Sidak test.
b Adjusted for variables associated with percent density [age, BMI, parity and age at first full term pregnancy]
c Adjusted for variables associated with dense area [age, BMI and parity]
d Adjusted for variables associated with non-dense area [age and BMI]
Table 4. Unadjusted and adjusted mean percent mammographic density, dense area and nondense area in postmenopausal Malay, Indian and Chinese women, Malaysia, 2011–2013.
| Model | Malay (n = 42) | Indian (n = 101) | Chinese (n = 85) | Overall p value | Multiple comparisons p value a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Malay vs. Indian | Malay vs. Chinese | Indian vs. Chinese | ||
| Percent density (%) | ||||||||||
| Unadjusted | 19.7 | 16.0, 23.3 | 17.9 | 15.6, 20.3 | 25.6 | 23.1, 28.2 | <0.001 | 0.808 | 0.025 | <0.001 |
| Age + BMI | 19.8 | 16.8, 22.9 | 19.7 | 17.7, 21.7 | 23.5 | 21.2, 25.7 | 0.038 | 1.000 | 0.172 | 0.048 |
| Fully adjusted b | 19.5 | 16.5, 22.5 | 20.0 | 18.0, 21.9 | 23.3 | 21.1, 25.5 | 0.053 | 0.992 | 0.138 | 0.092 |
| Dense area (cm2) | ||||||||||
| Unadjusted | 22.7 | 19.0, 26.4 | 22.7 | 20.4, 25.1 | 22.5 | 19.9, 25.1 | 0.993 | 1.000 | 1.000 | 0.999 |
| Adjusted for age + BMI | 22.7 | 19.1, 26.3 | 23.6 | 21.3, 26.0 | 21.5 | 18.9, 24.1 | 0.493 | 0.965 | 0.927 | 0.552 |
| Fully adjusted c | 22.0 | 18.5, 25.6 | 24.2 | 21.9, 26.6 | 20.9 | 18.4, 23.5 | 0.183 | 0.668 | 0.947 | 0.198 |
| Non-dense area (cm2) | ||||||||||
| Unadjusted | 100.7 | 89.8, 111.7 | 112.9 | 105.9, 120.0 | 73.4 | 65.7, 81.1 | <0.001 | 0.186 | <0.001 | <0.001 |
| Adjusted for age + BMI d | 99.9 | 90.9, 108.9 | 107.1 | 101.2, 113.0 | 80.7 | 74.2, 87.2 | <0.001 | 0.464 | 0.003 | <0.001 |
Abbreviations: BMI body mass index, CI confidence interval, FFTP first full term pregnancy
a p value from Sidak test.
b Adjusted for variables associated with percent density [age, BMI, parity and age at first full term pregnancy]
c Adjusted for variables associated with dense area [age, BMI, parity and current use of hormone replacement therapy]
d Adjusted for variables associated with non-dense area [age and BMI]
Discussion
Our study shows that percent mammographic density and nondense area, but not dense area, vary amongst Asians of different ethnicities living in the same region. Chinese women have significantly higher percent mammographic density and lower nondense area compared to Malays and Indians. Notably, in post-menopausal women, age, BMI and parity explained the differences in percent density across ethnic groups. By contrast, in pre-menopausal women, age, BMI and parity did not account for ethnic differences, suggesting that the mechanism by which percent density is modulated in post-menopausal women may be different from that in premenopausal women. These results suggest that genetic or other hitherto undetermined factors may explain the remaining variation observed across these ethnic groups. To the best of our knowledge, our study is the first to investigate ethnic variations in mammographic density measured as a continuous variable and with adjustment for lifestyle variables among Asian women living in Asia.
Other studies have shown that ethnic differences in percent mammographic density and dense area reflect variations in breast cancer risk [9,19,20]. Consistent with this, we found that both breast cancer risk and percent density are highest amongst Chinese women compared to the Indians and Malays, reflecting the higher breast cancer risk in Chinese. However, although Indian women in Malaysia have higher age standardised incidence rates of breast cancer compared to Malay women, we did not find any difference between percent densities of Indians compared to Malays in our study. One possible explanation is that the Malay women in our study, who were more affluent and well-educated compared to the other two ethnicities and to the Malaysian population, probably had lower BMI and higher percent density compared to the rest of the Malays in Malaysia. Indeed, other studies have reported that poorer socioeconomic status is associated with decreasing mammographic density, which is driven by the negative association between BMI and socioeconomic status [21]. Further studies in population-based cohorts of Malay and Indian women are needed.
The lifestyle factors we analysed explained 44.7% of variation in percent density, 16.2% in dense areas and 45.5% in nondense areas respectively. This is consistent with a study in Singaporean Chinese women reported that lifestyle factors explained 13% of the variation in dense areas and 45% in nondense areas respectively [15], but larger than the 25% of variation in percent density reported in that study. One plausible explanation is that there is a wider variation in percent density in our study as it is a multi-ethnic study compared to a Chinese only study.
Of the lifestyle variables that were reported to be associated with mammographic density, our results were not consistent with published literature for hormone replacement therapy and intake of green tea and soy. Hormone replacement therapy, particularly when estrogen is used in combination with progestin, has been consistently shown to be associated with increased density [22,23,24], but this was not observed in our cohort. This may because there were few hormone therapy users in this study [<8%] and the combination that was used was not taken into consideration in our analyses. Intake of soy or green tea was associated with decreased density among Singaporean Chinese women [25], but this was not found in our cohort, perhaps because our cohort was smaller with a lower reported prevalence of daily green tea [7% compared to 10%] and daily soy intake was not quantitated.
There are several limitations to this study. The MyMammo subsidised mammogram programme is an opportunistic screening programme and may not be representative of the population of Malaysia. Subang Jaya, where the recruitment centre is located, is one of the most populous cities in Malaysia and is part of the largest urban agglomeration in Malaysia, the Klang Valley. Therefore the cohort is likely to be more affluent, well-educated and westernised compared to the general Malaysian population. Another limitation is that the cohort does not represent the ethnic distribution of Malaysia and therefore extrapolation to other Asians must be interpreted with care. Malays form the largest proportion (51%) of the Malaysian population, compared to 30% Chinese and 8% Indian [26], but only make up 10% of the cohort. Indeed, few Malays come forward for opportunistic screening mammograms in Malaysia [27] or organised mammography screening in Singapore [28].
In conclusion, our data show that established modifiers of density such as age, BMI, menopausal status and parity do not fully account for the variations observed in percent mammographic density and nondense area among Chinese, Malay and Indian women living in Malaysia. Larger studies investigating genetic and other possible determinants of mammographic density are therefore required in this population.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank participants for taking part in this study, all staff at the Breast Care Centre, Health Screening Centre, and Imaging Department of Sime Darby Medical Centre for assistance in mammographic screening; Maheswari Jaganathan, Kavitta Sivanandan, Kang In Nee, Phuah Sze Yee, Daphne Lee Shin Chi, Andrew Khoo, Hanis Nazihah Hasmad, Afiqah Maiden, Ng Jin Tong, Rohaya Mohd Kasim, Malkit Kaur Dhillon, Liu Thin Chai, Alex Tang Ah Lak, Azubah Awang Abd Rahman and Tan Sook Pei, for assistance with patient recruitment, data entry and mammographic reporting.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is supported by the donors of Cancer Research Initiatives Foundation, particularly funds raised through the Sime Darby LPGA Tournament Malaysia. Work on the automated thresholding method was supported by the Agency for Science, Technology and Research (A-STAR), Singapore under the 2nd Joint Council Office (JCO) Career Development Grant (13302EG065). Jingmei Li is a UNESCO-L’OREAL International Fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Saftlas AF, Szklo M (1987) Mammographic parenchymal patterns and breast cancer risk. Epidemiol Rev 9: 146–174. [DOI] [PubMed] [Google Scholar]
- 2. Oza AM, Boyd NF (1993) Mammographic parenchymal patterns: a marker of breast cancer risk. Epidemiol Rev 15: 196–208. [DOI] [PubMed] [Google Scholar]
- 3. Warner E, Lockwood G, Tritchler D, Boyd NF (1992) The risk of breast cancer associated with mammographic parenchymal patterns: a meta-analysis of the published literature to examine the effect of method of classification. Cancer Detect Prev 16: 67–72. [PubMed] [Google Scholar]
- 4. Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ (1998) Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 7: 1133–1144. [PubMed] [Google Scholar]
- 5. McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 6. Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, et al. (2011) Nondense mammographic area and risk of breast cancer. Breast Cancer Res 13: R100 10.1186/bcr3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maskarinec G, Meng L, Ursin G (2001) Ethnic differences in mammographic densities. Int J Epidemiol 30: 959–965. [DOI] [PubMed] [Google Scholar]
- 8. Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, et al. (2004) Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol 159: 140–147. [DOI] [PubMed] [Google Scholar]
- 9. McCormack VA, Perry N, Vinnicombe SJ, Silva Idos S (2008) Ethnic variations in mammographic density: a British multiethnic longitudinal study. Am J Epidemiol 168: 412–421. 10.1093/aje/kwn169 [DOI] [PubMed] [Google Scholar]
- 10. Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA (2005) Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol 34: 405–412. [DOI] [PubMed] [Google Scholar]
- 11. Shin HR, Boniol M, Joubert C, Hery C, Haukka J, et al. (2010) Secular trends in breast cancer mortality in five East Asian populations: Hong Kong, Japan, Korea, Singapore and Taiwan. Cancer Sci 101: 1241–1246. 10.1111/j.1349-7006.2010.01519.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(2004) Cancer Incidence in Malaysia 2003. Kuala Lumpur.
- 13. Yip CH, Taib NA, Mohamed I (2006) Epidemiology of breast cancer in Malaysia. Asian Pac J Cancer Prev 7: 369–374. [PubMed] [Google Scholar]
- 14.Dai H, Yan Y, Wang P, Liu P, Cao Y, et al. (2014) Distribution of mammographic density and its influential factors among Chinese women. Int J Epidemiol. [DOI] [PMC free article] [PubMed]
- 15. Heng D, Gao F, Jong R, Fishell E, Yaffe M, et al. (2004) Risk factors for breast cancer associated with mammographic features in Singaporean chinese women. Cancer Epidemiol Biomarkers Prev 13: 1751–1758. [PubMed] [Google Scholar]
- 16. Li J, Szekely L, Eriksson L, Heddson B, Sundbom A, et al. (2012) High-throughput mammographic-density measurement: a tool for risk prediction of breast cancer. Breast Cancer Res 14: R114 10.1186/bcr3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(2012) Household income and basic amenities survey report 2012 In: Malaysia DoS, editor: Department of Statistics Malaysia.
- 18.(2011) Quick Facts 2011 Malaysia Educational Statistics. Education Data Sector, Education Planning and Research Division, Ministry of Education Malaysia. pp. 39.
- 19. Maskarinec G, Pagano I, Lurie G, Wilkens LR, Kolonel LN (2005) Mammographic density and breast cancer risk: the multiethnic cohort study. Am J Epidemiol 162: 743–752. [DOI] [PubMed] [Google Scholar]
- 20. Maskarinec G, Pagano I, Chen Z, Nagata C, Gram IT (2007) Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res Treat 104: 47–56. [DOI] [PubMed] [Google Scholar]
- 21. Aitken Z, Walker K, Stegeman BH, Wark PA, Moss SM, et al. (2010) Mammographic density and markers of socioeconomic status: a cross-sectional study. BMC Cancer 10: 35 10.1186/1471-2407-10-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carmona-Sanchez E, Cuadros Lopez JL, Cuadros Celorrio AM, Perez-Roncero G, Gonzalez Ramirez AR, et al. (2013) Assessment of mammographic density in postmenopausal women during long term hormone replacement therapy. Gynecol Endocrinol 29: 1067–1070. 10.3109/09513590.2013.831831 [DOI] [PubMed] [Google Scholar]
- 23. Colacurci N, Fornaro F, De Franciscis P, Palermo M, del Vecchio W (2001) Effects of different types of hormone replacement therapy on mammographic density. Maturitas 40: 159–164. [DOI] [PubMed] [Google Scholar]
- 24. Persson I, Thurfjell E, Holmberg L (1997) Effect of estrogen and estrogen-progestin replacement regimens on mammographic breast parenchymal density. J Clin Oncol 15: 3201–3207. [DOI] [PubMed] [Google Scholar]
- 25. Wu AH, Ursin G, Koh WP, Wang R, Yuan JM, et al. (2008) Green tea, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol Biomarkers Prev 17: 3358–3365. 10.1158/1055-9965.EPI-08-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(2012) Statistics Yearbook Malaysia 2011.
- 27. Parsa P, Kandiah M, Mohd Zulkefli NA, Rahman HA (2008) Knowledge and behavior regarding breast cancer screening among female teachers in Selangor, Malaysia. Asian Pac J Cancer Prev 9: 221–227. [PubMed] [Google Scholar]
- 28. Sim HL, Seah M, Tan SM (2009) Breast cancer knowledge and screening practices: a survey of 1,000 Asian women. Singapore Med J 50: 132–138. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.