Abstract
Given the emerging evidence of an association between periodontal infections and systemic conditions, the search for specific methods to detect the presence of P. gingivalis, a principal etiologic agent in chronic periodontitis, is of high importance. The aim of this study was to characterize antibodies raised against purified P. gingivalis HmuY protein and selected epitopes of the HmuY molecule. Since other periodontopathogens produce homologs of HmuY, we also aimed to characterize responses of antibodies raised against the HmuY protein or its epitopes to the closest homologous proteins from Prevotella intermedia and Tannerella forsythia. Rabbits were immunized with purified HmuY protein or three synthetic, KLH-conjugated peptides, derived from the P. gingivalis HmuY protein. The reactivity of anti-HmuY antibodies with purified proteins or bacteria was determined using Western blotting and ELISA assay. First, we found homologs of P. gingivalis HmuY in P. intermedia (PinO and PinA proteins) and T. forsythia (Tfo protein) and identified corrected nucleotide and amino acid sequences of Tfo. All proteins were overexpressed in E. coli and purified using ion-exchange chromatography, hydrophobic chromatography and gel filtration. We demonstrated that antibodies raised against P. gingivalis HmuY are highly specific to purified HmuY protein and HmuY attached to P. gingivalis cells. No reactivity between P. intermedia and T. forsythia or between purified HmuY homologs from these bacteria and anti-HmuY antibodies was detected. The results obtained in this study demonstrate that P. gingivalis HmuY protein may serve as an antigen for specific determination of serum antibodies raised against this bacterium.
Introduction
Periodontitis is a group of multifactorial, inflammatory infectious diseases, initiated by an ecological shift in the composition of subgingival biofilm, resulting in inflammation and destruction of tooth-supporting tissues, eventually leading to tooth loss [1–3]. From a clinical point of view, chronic periodontitis is characterized by deep periodontal pockets, resulting from the loss of alveolar bone and connective tissue attachment to the tooth. The severity of bleeding upon probing depends on the intensity of the gingival inflammation. Most of the tissue damage results from both direct destructive effects of the pathogenic plaque microorganisms themselves and from the exaggerated host responses to bacterial challenges.
Several studies have demonstrated that about 700 species are capable of colonizing the adult oral cavity [4,5]. Analysis of bacterial species isolated from subgingival samples has revealed the presence and relative abundance of periodontal pathogens, including the “red complex” members (Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola), associated with the clinical features of chronic periodontitis [4–7]. In addition, Prevotella intermedia, a member of the "orange complex", serves as a bridging species through binding to members of the "red complex" [8,9]. Among periodontal pathogens, P. gingivalis is considered the main etiologic agent and a key pathogen responsible for initiation and progression of chronic periodontitis [10,11].
P. gingivalis is a heme auxotroph, and therefore the uptake of this compound is essential for bacterial survival and the ability to establish an infection. To acquire iron and heme, P. gingivalis uses several sophisticated mechanisms [12], and some of them, e.g., the hmu system [13], are well characterized. One of its components, namely HmuY, is a membrane-associated heme-binding lipoprotein [14,15]. Heme uptake served by this hemophore-like protein is a novel system which was identified for the first time in P. gingivalis [13–21].
P. gingivalis can enter gingival epithelial and immune cells, remaining viable and capable of spreading among host cells, thus contributing to its survival in the oral cavity [22–25]. The bacterium produces various secreted and structural components that directly cause destruction of periodontal tissues and play a crucial role in the induction of innate immune responses [10]. It has been demonstrated that P. gingivalis can also spread systemically to other tissues [26–28]. Our data also suggest that the P. gingivalis HmuY could constitute a mechanism for stimulation of the host immune system and be of particular importance in development of chronic periodontitis [29–32]. P. gingivalis HmuY is constitutively produced, but at higher levels when bacteria grow under low-iron/heme conditions or are a biofilm constituent [14]. Importantly, the protein may be released from the bacterium in the form of outer-membrane vesicles [14,33] or may be shed from the membrane surface in the soluble form [15]. Therefore, HmuY production and its release to the surrounding environment could be of significance in periodontal pockets, where the biofilm provides persistent bacterial colonization. After entering the periodontal pocket epithelium, free-soluble bacterial products may readily gain access to the blood vascular network and spread systemically. Indeed, we have demonstrated that patients with chronic periodontitis produce higher levels of anti-HmuY antibodies compared to healthy subjects [29] and patients with gingivitis or aggressive periodontitis (S.C. Trindade, T. Olczak, unpublished data).
Given the emerging evidence of an association between periodontal infections and systemic conditions such as diabetes mellitus, rheumatoid arthritis, cardiovascular and respiratory diseases [28,34,35], as well as increasing resistance of bacteria against antibiotics, the search for methods for the specific detection of P. gingivalis and its inactivation is highly important. Previously, we developed a simple but efficient assay for specific and sensitive detection of P. gingivalis using the hmuY gene sequence and qPCR [36]. Based on our results it has emerged that the unique hmuY sequence may serve as one of molecular markers of P. gingivalis. The aim of this study was to characterize antibodies raised against purified P. gingivalis HmuY protein and selected epitopes of the HmuY molecule. Since other periodontopathogens produce homologs of HmuY, we also aimed to characterize responses of antibodies raised against the HmuY protein or its epitopes to the closest homologous proteins from P. intermedia and T. forsythia.
Materials and Methods
Ethics statement
All animal protocols and procedures used to produce custom designed polyclonal antibodies purchased from GenScript Corporation (GenScript USA Inc., Piscataway, NJ 08854) were approved by the GenScript Corporation Institutional Animal Care and Use Committee (IACUC, #ANT11-001). The Office of Laboratory Animal Welfare (OLAW) of the U.S. National Institutes of Health (NIH) provides guidance and interpretation of the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals. GenScript received OLAW's Animal Welfare Assurance. OLAW's Animal Welfare Assurance accentuates the responsibilities and procedures of GenScript regarding the care and use of laboratory animals. The GenScript developed partnership with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International, which is a private, nonprofit organization that promotes the humane treatment of animals in science through voluntary accreditation and assessment programs. GenScript has earned AAALAC accreditation, demonstrating their commitment to responsible animal care and use through ongoing voluntary participation in AAALAC programs.
Bacterial strains and growth conditions
P. gingivalis A7436 and ATCC 33277, P. intermedia 17, and T. forsythia ATCC 43037 were grown under anaerobic conditions as described previously [36,37].
Construction of expression plasmids
To construct expression plasmids containing sequences encoding P. gingivalis HmuY homologs from P. intermedia and T. forsythia, DNA sequences encoding predicted mature proteins were PCR-amplified using the genomic DNAs as templates and primers listed in Table 1. For this purpose, P. intermedia DNA sequences encoding PinA (NCBI accession number WP_014709321) and PinO (NCBI accession number WP_014708291) were used. In the case of DNA encoding T. forsythia Tfo, some discrepancies in the gene sequence compared to the DNA sequence deposited in databases (NCBI accession number YP_005014932) were found. Therefore, in this study we determined the corrected DNA sequence encoding Tfo. All amplified PCR products were ligated into the pTriEx-4 vector (Merck), resulting in sequences encoding untagged proteins.
Table 1. Primers designed and used in this study.
| Primer | 5’→3’ sequence (restriction sites are underlined) | Amplification product length (bp) | Locus ID | Description |
|---|---|---|---|---|
| FPin0009_Esp3I BsmBI | atctcgtctcgcatgagcaaggacaacaacgacgac | 645 | PIN_0009 | Amplifies sequence coding for untagged P. intermedia HmuY homolog (PinO) without signal peptide sequence and cysteine residue (20–225 aa) |
| RPin0009_STOP XhoI | gatctcgagttacttcgctttctttatgaacttatag | |||
| FPIN_A0726 BsaI | acgtggtctcgcatgagcaatgatgacccaactccaaaac | 705 | PIN_A0726 | Amplifies sequence coding for untagged P. intermedia HmuY homolog (PinA) without signal peptide sequence and cysteine residue (21–246 aa) |
| RPIN_A0726_STOP XhoI | cgtctcgagttaattcttcttgacaaatttatacttgaagc | |||
| FHmuY_Tf_NcoI | cgtccatggacaagaaagacgacgtaaaag | 605 | Bfo_2078 | Amplifies sequence coding for untagged T. forsythia HmuY homolog (Tfo) without signal peptide sequence and cysteine residue (22–216 aa) |
| RHmuY_Tf_STOP XhoI | agtctcgagttatttcggttgaaattcgtaattaaaag |
Overexpression and purification of proteins
P. gingivalis HmuY lacking the first 25 amino acid residues (NCBI accession number CAM 31898) was overexpressed using a pHmuY11 plasmid and Escherichia coli ER2566 cells (New England Biolabs) and purified from a soluble fraction of the E. coli cell lysate as described previously [13]. As the soluble protein shed from the outer membrane, the purified HmuY lacked the signal peptide and first five amino acid residues (CGKKK) of the nascent secreted protein [14,15]. P. intermedia PinA, PinO and T. forsythia Tfo were overexpressed in E. coli ER2566 and Rosetta (DE3) strains (New England Biolabs or Merck), respectively. PinA, PinO and Tfo, lacking predicted signal peptides (Table 1), were prepared using purification procedures established in our laboratory. All proteins were purified from whole E. coli cell lysates obtained after sonication and centrifugation, as described for P. gingivalis HmuY [13] with some modifications. Briefly, PinO, PinA and Tfo purification was carried out using DEAE-Sephacel chromatography (50 mM Tris/HCl buffer, pH 7.6, containing 30 mM NaCl). Then, hydrophobic chromatography using Phenyl Superose (HR 5/5, Pharmacia) was used. The proteins were bound to the chromatographic resin in 50 mM sodium phosphate buffer, pH 7.0, containing 2 M ammonium sulfate, and eluted using linear salt gradient 2.0–0.0 M. As the final step, gel filtration using Sephadex G-75 (50 mM Tris/HCl buffer, pH 7.6, containing 400 mM NaCl) was used.
Immunization of rabbits
Custom designed polyclonal antibodies were purchased from the commercial company (GenScript USA Inc.), which is a dedicated biology research service provider. P. gingivalis HmuY and three synthetic peptides derived from the amino acid sequence of HmuY, conjugated with keyhole limpet hemocyanin (KLH), were used to immunize rabbits. Each experimental animal had one ear tag with lot number and one animal was kept in one cage with cage card. Animals were maintained at 16–22°C and relative humidity 30–70%. Polyclonal antibodies were produced by subcutaneously injecting New Zealand white rabbits at weight 2.0–2.5 kg (2 rabbits per each antigen, 8 rabbits during the entire experiment). In addition, sera from 2 rabbits immunized with the purified HmuY protein in our previous study [14] were used. The rabbits received one injection with the antigen (0.2 mg per injection) emulsified 1:1 (0.25 ml per rabbit) with Freund’s complete adjuvant, and the same amount of antigen with Freund’s incomplete adjuvant on day14, 35 and 56. Before immunization and after immunization on day 21, 42, and 63 blood was collected (2.5 ml per animal) from ear margin veins. All animals were healthy trough the entire experiment and all serum samples were properly collected. Pre-immune sera (protocol #SC1088) and immune sera after test and final bleeds (protocols #SC1247 and # SC3031), containing anti-HmuY1 (purified protein), anti-HmuY1–1 (GKKKDEPNQPSTPEC), anti-HmuY1–2 (SKGEVVNVTDYKNDC), and anti-HmuY1–3 (CEMGPDGHQMEYEEQG) antibodies were used for all experiments.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting
The reactivity of respective antibodies was investigated using purified proteins or bacterial cell lysates. Bacterial cultures were centrifuged for 20 min at 20,000×g at 4°C. Bacterial pellets were washed with 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM NaCl (PBS) and suspended in PBS to OD600 = 1.0. Samples corresponding to 20 μl of the bacterial culture or protein samples (1, 10 and 100 ng or 1 and 5 μg per lane) were denatured and separated on 12% SDS-PAGE gels, and subsequently transferred onto nitrocellulose membranes (Whatman). Nonspecific binding sites were blocked with 5% skim milk in PBS with addition of 0.1% Tween (PBST). HmuY or its epitopes were visualized with respective sera and secondary goat anti-rabbit IgG antibodies conjugated with horseradish peroxidase (HRP, Sigma). All sera were used at 1:10,000 dilution. The reaction was developed using chemiluminescence reagents (Western Lightning Plus-ECL, Perkin Elmer).
Enzyme-linked immunosorbent assay (ELISA)
96-well polystyrene plates (Polysorp, Nunc) were coated for 1 h at 37°C with respective proteins (1, 2, 5, 10, 25, 50, 75 and 100 ng per well) or live bacterial cells (100 μl; OD600 = 1.0) prepared in PBS. The plates were washed three times with 200 μl of PBS prior to blocking overnight at 4°C with 200 μl of 2% bovine serum albumin (BSA) dissolved in PBS and then washed five times with 200 μl of PBS. Diluted sera were prepared in PBS and incubated for 1 h at 37°C. After washing, antibody binding was detected using goat anti-rabbit IgG conjugated with HRP at 1:10,000 dilution. After five final washes, a substrate solution (100 μl) containing 0.05% o-phenylenediamine (Sigma) with 0.01% H2O2 was added for color development at room temperature. The reaction was stopped after 15 min by adding 25 μl of 12.5% H2SO4 and the absorbance was measured at 450 nm using a Multiskan Ascent microplate reader (Thermo Electron Corporation).
Construction of three-dimensional (3D) protein structure of HmuY homologs
PinA, PinO and Tfo protein structures were modeled using the Phyre2 modeling server (http://www.sbg.bio.ic.ac.uk/phyre2) and appropriate templates (PinA—PDB ID: 3U22, crystal structure of a putative HmuY-like heme-binding protein BVU_2192 from Bacteroides vulgatus ATCC 8482; PinO—PDB IDs: 3U22, 3H8T, crystal structure of P. gingivalis heme-binding protein HmuY and PDB ID: 4GBS, crystal structure of a putative lipoprotein BF2707 from Bacteroides fragilis NCTC 9343; Tfo—PDB IDs: 3U22, 3H8T, 4GBS). The resulting protein models were refined using ModRefiner (http://zhanglab.med.umich.edu/ModRefiner/) and evaluated using the PSVS online server (PROCHECK, Verify3, MolProbity, ProSA; http://psvs-15-dev.nesg.org/). The best models were selected on the basis of overall G-factor and Ramachandran plot scores and further analyzed using Verify3D (http://nihserver.mbi.ucla.edu/Verify_3D/) and ERRAT (http://nihserver.mbi.ucla.edu/ERRATv2/). Protein models were visualized using PyMOL software (The Open-Source PyMOL Molecular Graphics System Version 0.99r6 Schrödinger, LLC, New York, NY, USA).
Results and Discussion
Infection with periodontopathogens leads to humoral immune responses with elevated serum antibodies to periodontal species in patients with periodontitis [29,38–44]. In addition to P. gingivalis, antibody responses to several other periodontopathogens have been found, including P. intermedia [45,46] and T. forsythia [47]. More importantly, recent data demonstrated that an elevated antibody level against P. gingivalis indicated advanced periodontal disease and suggested progression of atherosclerosis, hypertension and rheumatoid arthritis [48–52]. Therefore, efficient screening of patients is essential for estimation of general health and disease stage as well as subsequent treatment efficiency. In our recent study, we presented an extensive phylogenetic analysis of P. gingivalis HmuY protein and the hmuY gene [36]. The HmuY from P. gingivalis was placed at the base of other Bacteroidia sequences represented by Bacteroides, Prevotella and Tannerella. In contrast, low identity to homologous sequences found in other bacteria was observed. We also demonstrated the presence of antibodies directed against P. gingivalis HmuY in sera of patients with chronic periodontitis [29].
This study extends our previously published data and presents characterization of the HmuY protein with regard to its future application to determine specific anti-P. gingivalis antibodies in serum. The best antigen targets are bacterial components, which are constitutively expressed and secreted from the bacterium or exposed on the pathogen’s surface. The use of whole-sonicate antigens, which encompass many of the P. gingivalis proteins [53,54], would increase the possibility of detecting responses, but also increase the potential for false positive results because of cross-reacting antibodies to components of other bacteria. Therefore, to detect specific anti-P. gingivalis antibodies which do not cross react with components produced by other bacteria, we employed purified HmuY protein or its selected epitopes as antigens (Fig. 1). Since other periodontopathogens produce HmuY homologs, we aimed to characterize responses of antibodies raised against HmuY protein or its epitopes to the most homologous proteins from P. intermedia and T. forsythia. Taking into account the amino acid sequences and the theoretically modeled three-dimensional protein structures, we found the two closest HmuY homologs in P. intermedia 17, termed here PinA and PinO, and one HmuY homolog from T. forsythia ATCC 43037, termed here Tfo (Fig. 2). For the latter, we identified corrected DNA (Fig. 3A) and amino acid (Fig. 3B) sequences (EMBL accession number LN624459). It appears that previously in databases a nucleotide sequence possessing a 6-bp insertion and nucleotide substitutions has been deposited. In contrast, the amino acid sequence of Tfo identified in our laboratory contains both a two-amino-acid deletion and substitutions of nine amino acids (Fig. 3C). We did not identify the HmuY homolog in T. denticola, the third member of the "red complex" of periodontopathogens. In this study we overexpressed all proteins and established purification procedures for the PinA, PinO and Tfo proteins.
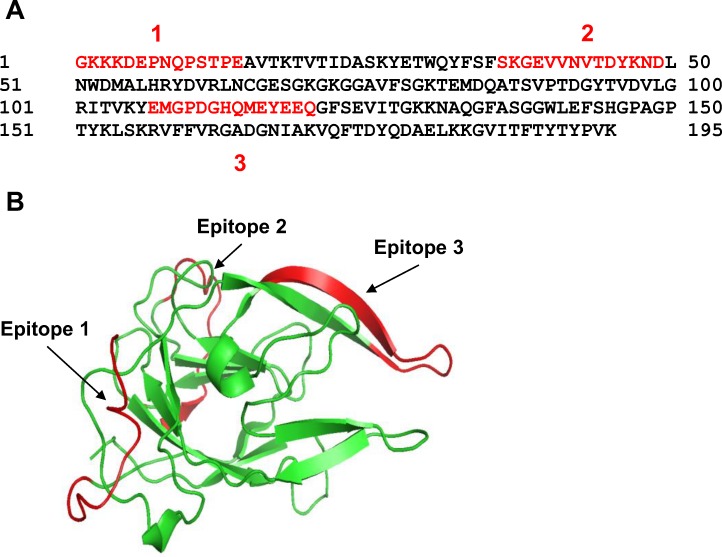
Fig 1. Presentation of P. gingivalis HmuY epitopes in amino acid sequence (A) and three-dimensional protein structure (B).
Epitopes analyzed are indicated in red.
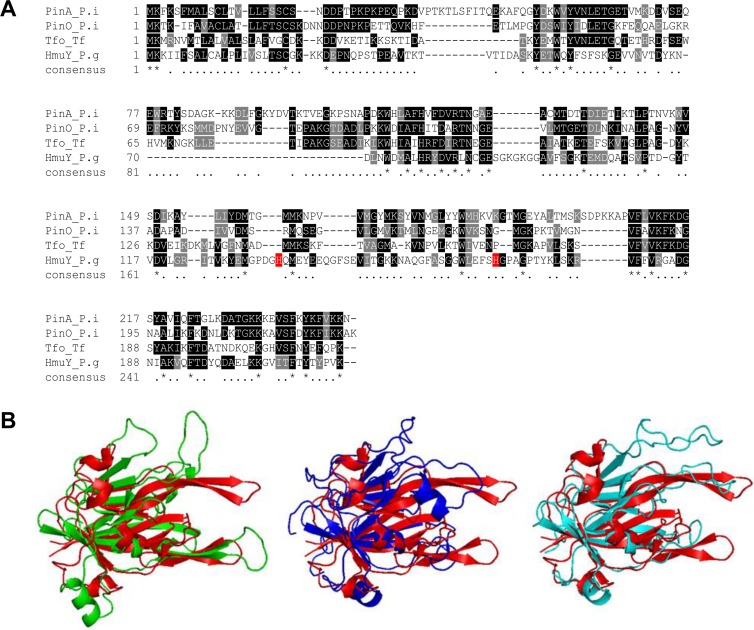
Fig 2. Comparison of P. gingivalis HmuY and its homologs from P. intermedia and T. forsythia.
Amino acid alignment (A) and approximated protein structures (B). P. intermedia PinA is shown in green, P. intermedia PinO in navy blue, T. forsythia Tfo in light blue, and P. gingivalis HmuY (PDB ID: 3H8T) in red. PinA, PinO and Tfo structures were modeled using Phyre2 modeling server and appropriate templates (PinA—PDB IDs: 3U22; PinO—PDB IDs: 3U22, 3H8T, 4GBS; Tfo—PDB IDs: 3U22, 3H8T, 4GBS).
Fig 3. Identification of DNA (A) and amino acid (B) sequences of T. forsythia Tfo protein.
Differences between the amino acid sequence deposited in the database (NCBI ID: YP_005014932) and the sequence identified in this study (EMBL accession number LN624459) are also shown (C).
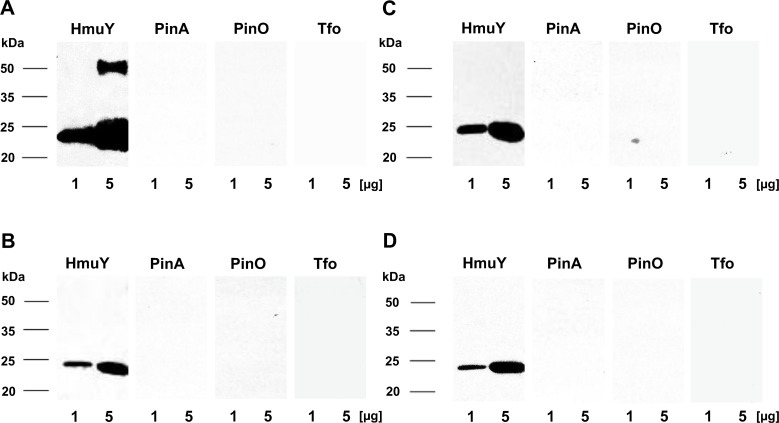
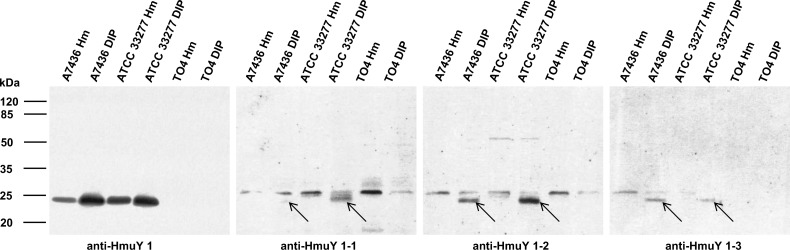
First we characterized all antibodies produced in rabbits against purified HmuY protein using Western blotting. For this purpose, we examined antibodies against the purified HmuY protein which were raised in 4 rabbits, and antibodies against each synthetic peptide which were raised in 3 pairs of rabbits. We did not observe any significant differences in reactivity between antigens tested and the sera of the different animals (data not shown). Therefore, in this study representative results from single rabbits only are shown. As shown in Fig. 4, the denatured HmuY protein reacted with antibodies directed against the HmuY protein as well as with antibodies directed against all three epitopes, with the highest affinity towards the purified protein and epitope 2. Further analyses showed that antibodies raised against the purified HmuY protein did not recognize HmuY homologs from P. intermedia and T. forsythia (Fig. 5A). This strongly demonstrates that antibodies raised against P. gingivalis HmuY recognize only this antigen, but not homologous proteins from other periodontopathogens. Also, whilst antibodies directed against epitopes 1, 2, and 3 reacted with purified HmuY protein, they did not recognize HmuY homologs examined in this study (Fig. 5B, 5C, 5D). Similar experiments performed with denatured P. gingivalis cell lysates showed that antibodies raised against purified HmuY protein (anti-HmuY 1) exhibited the highest specificity and recognized the HmuY protein only (Fig. 6). Other antibodies were less specific, and in addition to detection of HmuY protein (Fig. 6, indicated with arrows), they cross reacted with other P. gingivalis antigens. This non-specific effect was corroborated when the reactivity of pre-immune serum was tested with P. gingivalis lysates (data not shown). Based on our data we concluded that antibodies raised against HmuY protein (most sensitive and specific) and epitope 2 (less sensitive and specific) detected HmuY protein compared to antibodies raised against epitope 1 and 3. We did also not observe significant differences between two different P. gingivalis strains used. The P. gingivalis strain A7436 was originally isolated from a refractory periodontitis patient. This strain is highly similar (both in its genetic background and phenotypic properties) to strain W83. Both strains belong to a group of more virulent P. gingivalis isolates and are often found in patients with chronic periodontitis [36]. Conversely, P. gingivalis ATCC 33277 strain was used because it belongs to a less virulent group of strains and is known to exhibit a different genetic background and attenuated virulence properties.
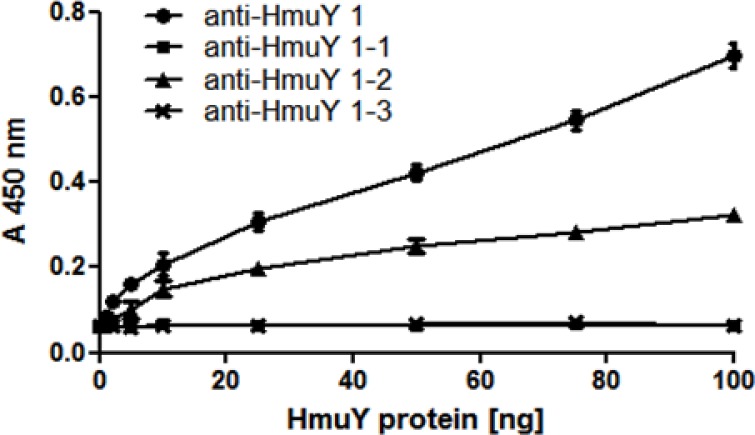
Fig 4. Reactivity of anti-HmuY antibodies with purified P. gingivalis HmuY protein.

Antibodies raised against purified HmuY protein (anti-HmuY 1) or epitope 1 (anti-HmuY 1–1), epitope 2 (anti-HmuY 1–2), and epitope 3 (anti-HmuY 1–3) were analyzed using Western blotting. In Figs 4–9, representative data for one immune serum for each antigen are shown out of 4 immune sera containing antibodies raised against HmuY protein and out of 2 immune sera containing antibodies raised against each epitope.
Fig 5. Reactivity of anti-HmuY antibodies with purified P. gingivalis HmuY or its homologs from P. intermedia and T. forsythia.
P. intermedia PinA, PinO and T. forsythia Tfo were overexpressed, purified and analyzed using Western blotting and antibodies raised against purified HmuY protein (anti-HmuY 1) (A), epitope 1 (anti-HmuY 1–1) (B), epitope 2 (anti-HmuY 1–2) (C), and epitope 3 (anti-HmuY 1–3) (D).
Fig 6. Reactivity of anti-HmuY antibodies with P. gingivalis wild-type (A7436 and ATCC 33277) and hmuY mutant (TO4) strains.
Bacteria were cultured in high iron/heme (Hm) or low-iron/heme (DIP) media. Western blotting was carried out using antibodies raised against purified HmuY protein (anti-HmuY 1), epitope 1 (anti-HmuY 1–1), epitope 2 (anti-HmuY 1–2), and epitope 3 (anti-HmuY 1–3). Arrows denote location of bands corresponding to P. gingivalis HmuY protein.
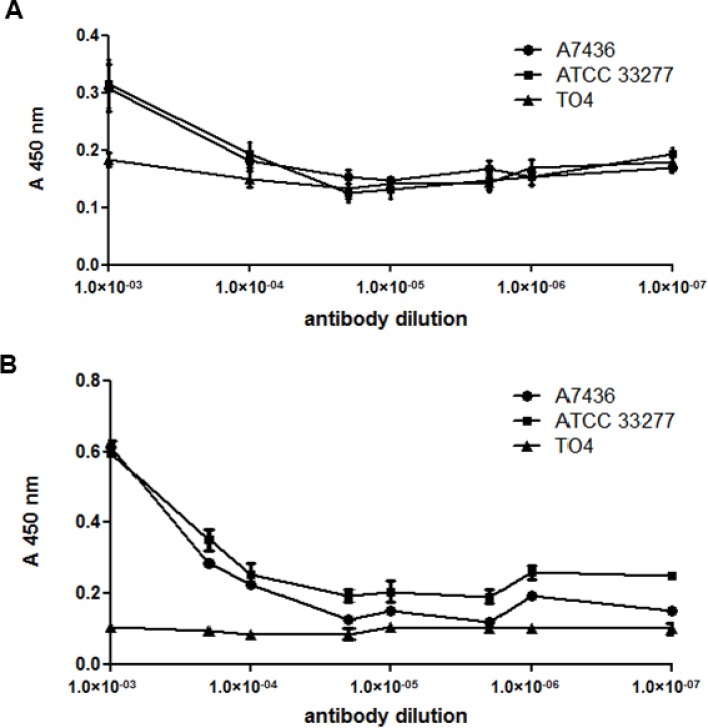
Characterization of antibodies was also carried out using native, non-denatured antigens. In agreement with Western blotting results, only antibodies raised against the purified HmuY protein or raised against epitope 2 reacted with HmuY protein used as an antigen, as determined by the ELISA assay (Fig. 7). When purified HmuY homologs from P. intermedia and T. forsythia were used as antigens, no reactivity with antibodies directed against purified HmuY protein (Fig. 8A) or epitope 2 (Fig. 8B) was observed. In addition, there was no reactivity when antibodies raised against epitope 1 and 3 were used (data not shown). When live P. gingivalis bacteria were used as antigens in the ELISA assay, only reactivity with antibodies raised against purified HmuY protein was visible (Fig. 9), especially in bacteria which were starved of iron and heme (Fig. 9B), compared with bacteria cultured under high-iron/heme conditions (Fig. 9A). As a negative control we used the hmuY mutant strain (TO4), which does not produce the HmuY protein. Importantly, no reactivity was found when live cells of P. intermedia or T. forsythia were used as antigens (data not shown).
Fig 7. Reactivity of anti-HmuY antibodies with P. gingivalis HmuY protein.
ELISA was carried out using antibodies raised against purified HmuY protein (anti-HmuY 1), epitope 1 (anti-HmuY 1–1), epitope 2 (anti-HmuY 1–2), and epitope 3 (anti-HmuY 1–3). Overexpressed and purified P. gingivalis HmuY was used as an antigen.
Fig 8. Reactivity of anti-HmuY antibodies with P. gingivalis HmuY protein or its homologs from P. intermedia and T. forsythia.
ELISA was carried out using antibodies raised against purified HmuY protein (anti-HmuY 1) (A) and epitope 2 (anti-HmuY 1–2) (B). No reactivity was observed for anti-HmuY 1–1 (epitope 1) and anti-HmuY 1–3 (epitope 3) antibodies. Overexpressed and purified HmuY (P. gingivalis), PinA, PinO (P. intermedia) and Tfo (T. forsythia) proteins were used as antigens.
Fig 9. Reactivity of anti-HmuY antibodies with bacteria.
ELISA was carried out using antibodies raised against purified HmuY protein (anti-HmuY 1) and P. gingivalis wild-type (A7436 and ATCC 33277) and hmuY mutant (TO4) strains. Bacteria were cultured in high iron/heme (A) or low-iron/heme (B) media. No reactivity was observed for anti-HmuY 1–1 (epitope 1), anti-HmuY 1–2 (epitope 2), or anti-HmuY 1–3 (epitope 3) antibodies. Live bacteria were used as antigens.
In conclusion, the results obtained in this study demonstrate that P. gingivalis HmuY protein may serve as an antigen for specific determination of antibodies raised against this bacterium. Therefore, purified HmuY protein will be used to determine levels of anti-P. gingivalis antibodies in sera of patients with chronic periodontitis and patients with other forms of periodontal diseases. These studies are underway in our laboratory.
Data Availability
Nucleotide and amino acid sequences of Tannerella forsythia Tfo protein are available from the EMBL database under accession number LN624459.
Funding Statement
This study was supported by Wroclaw Research Center EIT+ (www.eitplus.pl) under the project "Biotechnologies and advanced medical technologies - BioMed" (POIG 01.01.02-02-003/08/00) financed by the European Regional Development Fund (Operational Program Innovative Economy, 1.1.2). Publication costs were supported by Wroclaw Center of Biotechnology program “The Leading National Research Center (KNOW) for years 2014-2018”. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Graves DT, Jiang Y, Genco CA (2000) Periodontal disease: bacterial virulence factors, host response and impact on systemic health. Curr Opin Infect Dis 13: 227–232. [DOI] [PubMed] [Google Scholar]
- 2. Craig RG, Yip YK, So MK, Boylan RJ, Socransky SS, et al. (2003) Relationship of destructive periodontal disease to the acute-phase response. J Periodontol 74: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 3. Byrne SJ, Dashper SG, Darby IB, Adams GG, Hoffmann B, et al. (2009) Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol Immunol 24: 469–477. 10.1111/j.1399-302X.2009.00544.x [DOI] [PubMed] [Google Scholar]
- 4. Griffen AL, Becker MR, Lyons SR, Moeschberger ML, Leys EJ (1998) Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol 36: 3239–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, et al. (2010) The human oral microbiome. J Bacteriol 192: 5002–5017. 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haffajee AD, Cugini MA, Tanner A, Pollack RP, Smith C, et al. (1998) Subgingival microbiota in healthy, well-maintained elder and periodontitis subjects. J Clin Periodontol 25: 346–353. [DOI] [PubMed] [Google Scholar]
- 7. Abiko Y, Sato T, Mayanagi G, Takahashi N (2010) Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res 45: 389–395. 10.1111/j.1600-0765.2009.01250.x [DOI] [PubMed] [Google Scholar]
- 8. Kolenbrander PE, Anderesen RN, Blehert DS, Egland PG, Foster JS, et al. (2002) Communication among oral bacteria. Microbiol Mol Biol Rev 66: 486–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamaguchi A, Ohyama T, Sakai E, Nakamura R, Watanabe T, et al. (2003) Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for co-aggregation with Prevotella intermedia . Microbiology 149: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 10. Hajishengallis G (2011) Immune evasion strategies of Porphyromonas gingivalis . J Oral Biosci 53: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bostanci N, Belibasakis GN (2012) Porphyromonas gingivalis: an invasive and evasive opportunistic pathogen. FEMS Microbiol Lett 333: 1–9. 10.1111/j.1574-6968.2012.02579.x [DOI] [PubMed] [Google Scholar]
- 12. Olczak T, Simpson W, Liu X, Genco CA (2005) Iron and heme utilization in Porphyromonas gingivalis . FEMS Microbiol Rev 29: 119–144. [DOI] [PubMed] [Google Scholar]
- 13. Olczak T, Sroka A, Potempa J, Olczak M (2008) Porphyromonas gingivalis HmuY and HmuR: further characterization of a novel mechanism of heme utilization. Arch Microbiol 183: 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olczak T, Wojtowicz H, Ciuraszkiewicz J, Olczak M (2010) Species specificity, surface exposure, protein expression, immunogenicity, and participation in biofilm formation of Porphyromonas gingivalis HmuY. BMC Microbiol 10: 134 10.1186/1471-2180-10-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wojtowicz H, Guevara T, Tallant C, Olczak M, Sroka A, et al. (2009) Unique structure and stability of HmuY, a novel heme-binding protein of Porphyromonas gingivalis . PLoS Pathog 5(5): e1000419 10.1371/journal.ppat.1000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olczak T, Maszczak-Seneczko D, Smalley JW, Olczak M (2012) Gallium(III), cobalt(III) and copper(II) protoporphyrin IX exhibit antimicrobial activity against Porphyromonas gingivalis by reducing planktonic and biofilm growth and invasion of host epithelial cells. Arch Microbiol 194: 719–724. 10.1007/s00203-012-0804-3 [DOI] [PubMed] [Google Scholar]
- 17. Wojtowicz H, Wojaczynski J, Olczak M, Kroliczewski J, Latos-Grazynski L, et al. (2009) Heme environment of Porphyromonas gingivalis HmuY heme-binding protein. Biochem Biophys Res Commun 382: 178–182. [DOI] [PubMed] [Google Scholar]
- 18. Wojtowicz H, Bielecki M, Wojaczynski J, Olczak M, Smalley JW, et al. (2013) Porphyromonas gingivalis HmuY haemophore binds gallium(III), zinc(II), cobalt(III), manganese(III), nickel(II), and copper(II) protoporphyrin IX but in a manner different to iron(III) protoporphyrin IX. Metallomics 5: 343–351. 10.1039/c3mt20215a [DOI] [PubMed] [Google Scholar]
- 19. Wojaczynski J, Wojtowicz H, Bielecki M, Olczak M, Smalley JW, et al. (2011) Iron(III) mesoporphyrin IX and iron(III) deuteroporphyrin IX bind to the Porphyromonas gingivalis HmuY hemophore. Biochem Biophys Res Commun 411: 299–304. 10.1016/j.bbrc.2011.06.129 [DOI] [PubMed] [Google Scholar]
- 20. Smalley JW, Byrne DP, Birss AJ, Wojtowicz H, Sroka A, et al. (2011) HmuY haemophore and gingipain proteases constitute a unique synthrophic system of haem acquisition by Porphyromonas gingivalis . PLoS One 6(2): e17182 10.1371/journal.pone.0017182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Byrne DP, Potempa J, Olczak T, Smalley JW (2013) Evidence of mutualism between two periodontal pathogens: co-operative haem acquisition by the HmuY haemophore of Porphyromonas gingivalis and the cysteine protease interpain A (InpA) of Prevotella intermedia . Mol Oral Microbiol 28: 219–229. 10.1111/omi.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, et al. (1995) Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun 63: 3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vitkov L, Krautgartner WD, Hannig M (2005) Bacterial internalization in periodontitis. Oral Microbiol Immunol 20: 317–321. [DOI] [PubMed] [Google Scholar]
- 24. Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM (2006) Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect Immun 74: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guyodo H, Meuric V, Le Pottier L, Martin B, Faili A, et al. (2012) Colocalization of Porphyromonas gingivalis with CD4+ T cells in periodontal disease. FEMS Immunol Med Microbiol 64: 175–183. 10.1111/j.1574-695X.2011.00877.x [DOI] [PubMed] [Google Scholar]
- 26. Iida Y, Honda K, Suzuki T, Matsukawa S, Kawai T, et al. (2004) Brain abscess in which Porphyromonas gingivalis was detected in cerebrospinal fluid. Br J Oral Maxillofac Surg 42: 180 [DOI] [PubMed] [Google Scholar]
- 27. Kozarov E, Sweier D, Shelburne C, Progulske-Fox A, Lopatin D (2006) Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect 8: 687–693. [DOI] [PubMed] [Google Scholar]
- 28. Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, et al. (2007) Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol 78: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 29. Trindade SC, Olczak T, Gomes-Filho IS, Moura-Costa LF, Cerqueira EM, et al. (2012) P. gingivalis HmuY-induced IL-1β, IL-10, IL-8 and IgG in humans. J Periodontal Res 47: 27–32. 10.1111/j.1600-0765.2011.01401.x [DOI] [PubMed] [Google Scholar]
- 30. Trindade SC, Olczak T, Gomes-Filho IS, Moura-Costa LF, Vale VL, et al. (2012) Porphyromonas gingivalis antigens participate differently in the proliferation and cell death of human PBMC. Arch Oral Biol 57: 314–320. 10.1016/j.archoralbio.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 31. Trindade SC, Olczak T, Gomes-Filho IS, de Moura-Costa LF, Vale VC, et al. (2013) P. gingivalis HmuY-induced production of interleukin-6 and IL-6 polymorphism in chronic periodontitis. J Periodontol 84: 650–655. 10.1902/jop.2012.120230 [DOI] [PubMed] [Google Scholar]
- 32. Carvalho-Filho PC, Trindade SC, Olczak T, Sampaio GP, Oliveira-Neto MG, et al. (2013) Porphyromonas gingivalis HmuY stimulates expression of Bcl-2 and Fas by human CD3+ T cells. BMC Microbiol 13(1): 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veith PD, Chen YY, Gorasia DG, Chen D, Glew MD, et al. (2014) Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res 13: 2420–2432. 10.1021/pr401227e [DOI] [PubMed] [Google Scholar]
- 34. Meurman JH, Sanz M, Janket SJ (2004) Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med 15: 403–413. [DOI] [PubMed] [Google Scholar]
- 35. Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G (2004) Hypothesis: The humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation 28: 311–318. [DOI] [PubMed] [Google Scholar]
- 36. Gmiterek A, Wojtowicz H, Mackiewicz P, Radwan-Oczko M, Kantorowicz M, et al. (2013) The unique hmuY gene sequence as a specific marker of Porphyromonas gingivalis infection. PLoS One 8(7): e67719 10.1371/journal.pone.0067719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roy S, Douglas CW, Stafford GP (2010) A novel sialic acid utilization and uptake system in the periodontal pathogen Tannerella forsythia . J Bacteriol 192: 2285–2293. 10.1128/JB.00079-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo S, Takahashi K, Kokeguchi S, Takashiba S, Kinane DF, et al. (2000) Antibody responses against Porphyromonas gingivalis infection in patients with early-onset periodontitis. J Clin Periodontol 27: 769–777. [DOI] [PubMed] [Google Scholar]
- 39. Papapanou PN, Neiderud AM, Papadimitriou A, Sandros J, Dahlen G (2000) "Checkerboard" assessments of periodontal microbiota and serum antibody responses: a case-control study. J Periodontol 71: 885–897. [DOI] [PubMed] [Google Scholar]
- 40. Papapanou PN, Neiderud AM, Sandros J, Dahlen G (2001) Checkerboard assessments of serum antibodies to oral microbiota as surrogate markers of clinical periodontal status. J Clin Periodontol 28: 103–106. [DOI] [PubMed] [Google Scholar]
- 41. Albandar JM, DeNardin AM, Adesanya MR, Diehl SR, Winn DM (2001) Associations between serum antibody levels to periodontal pathogens and early-onset periodontitis. J Periodontol 72: 1463–1469. [DOI] [PubMed] [Google Scholar]
- 42. Dye BA, Herrera-Abreu M, Lerche-Sehm J, Vlachojannis C, Pikdoken L, et al. (2009) Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol 80: 634–647. 10.1902/jop.2009.080474 [DOI] [PubMed] [Google Scholar]
- 43. Choi J, Lee SY, Kim K, Choi BK, Kim MJ (2011) Identification of mono- or poly-specific monoclonal antibody to Poprhyromonas gingivalis heat-schock protein 60. J Periodontol Implant Sci 41: 54–59. 10.5051/jpis.2011.41.2.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skottrup PD, Leonard P, Kaczmarek JZ, Veillard F, Enghild JJ, et al. (2011) Diagnostic evaluation of a nanobody with picomolar affinity toward the protease RgpB from Porphyromonas gingivalis . Anal Biochem 415: 158–167. 10.1016/j.ab.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ogrendik M, Kokino S, Ozdemir F, Bird PS, Hamlet S (2005) Serum antibodies to oral anaerobic bacteria in patients with rheumatoid arthritis. Med Gen Med 7: 2. [PMC free article] [PubMed] [Google Scholar]
- 46. Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, et al. (2010) Antibodies to Porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol 37:1105–1112. 10.3899/jrheum.091323 [DOI] [PubMed] [Google Scholar]
- 47. Ebersole JL, Novak MJ, Michalowicz BS, Hodges JS, Steffen MJ, et al. (2009) Systemic immune responses in pregnancy and periodontitis: relationship to pregnancy outcomes in the Obstetrics and Periodontal Therapy (OPT) study. J Periodontol 80: 953–960. 10.1902/jop.2009.080464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, et al. (2009) Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol 9: 38–42. 10.1016/j.intimp.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bohnstedt S, Cullinan MP, Ford PJ, Palmer JE, Leishman SJ, et al. (2010) High antibody levels to P. gingivalis in cardiovascular disease. J Dent Res 89: 938–942. 10.1177/0022034510370817 [DOI] [PubMed] [Google Scholar]
- 50. Holmlund A, Hedin M, Pussinen PJ, Lerner UH, Lind L (2011) Porphyromonas gingivalis (Pg) a possible link between impaired oral health and acute myocardial infarction. Int J Cardiol 148: 148–153. 10.1016/j.ijcard.2009.10.034 [DOI] [PubMed] [Google Scholar]
- 51. Hanaoka Y, Soejima H, Yasuda O, Nakayama H, Nagata M, et al. (2013) Level of serum antibody against a periodontal pathogen is associated with atherosclerosis and hypertension. Hypertens Res 36: 829–833. 10.1038/hr.2013.46 [DOI] [PubMed] [Google Scholar]
- 52. Arvikar SL, Collier DS, Fisher MC, Unizony S, Cohen GL, et al. (2013) Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther 15: R109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Franca M, Moura-Costa L, Meyer RJ, Trindade SC, Tunes Uda R, et al. (2007). Humoral immune response to antigens of Porphyromonas gingivalis ATCC 33277 in chronic periodontitis. J Appl Oral Sci 15: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Honma Y, Sugita N, Kobayashi T, Abiko Y, Yoshie H (2008) Lower antibody response to Porphyromonas gingivalis associated with immunoglobulin G Fc gamma receptor IIB polymorphism. J Periodontal Res 43: 706–711. 10.1111/j.1600-0765.2007.01078.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nucleotide and amino acid sequences of Tannerella forsythia Tfo protein are available from the EMBL database under accession number LN624459.