Abstract
Background
The WHO ‘Global Strategy for Dengue Prevention and Control, 2012–2020’ addresses the growing need for the treatment of dengue, and targets a 25% reduction in morbidity and 50% in mortality (using 2010 estimates as baseline). Achieving these goals requires future dengue prevention strategies that will employ both potential vaccines and sustainable vector-control measures. Maternally transferred dengue antibody is an important factor in determining the optimal age for dengue vaccination.
Objectives
To estimate the seroprevalence of dengue antibodies among mothers living in an area of high endemicity – Ban Pong, Ratchaburi Province – and to assess maternal dengue antibodies transferred to cord blood.
Materials & Methods
A cross-sectional study was conducted with 141 pregnant women who delivered at Ban Pong Hospital, Ratchaburi, Thailand. Maternal-cord paired sera were tested for dengue neutralizing (NT) antibody by PRNT50 assay. A ratio of ≥ 1:10 NT titer to dengue serotype was considered seropositive.
Results
Most mothers (137/141, 97.2%) had NT antibodies to at least one dengue serotype in their sera. At birth, the proportion of cord sera with NT antibodies to DEN-1, DEN-2, DEN-3, and DEN-4, were high and similar to the sera of their mothers, at 93.6%, 97.2%, 97.9%, and 92.2%, respectively. The dengue geometric mean titers (GMT) in cord blood were significantly higher than the maternal antibodies (p<0.001): highest in DEN-2, followed by DEN-3, and then DEN-1. The GMT of DEN-4 was the lowest among all four serotypes.
Conclusions
Dengue infection is highly prevalent among pregnant women in this dengue-endemic area. Most of the cord blood had transferred dengue antibodies, which may have an impact on the disease burden in this population.
Author Summary
Dengue is the fastest spreading mosquito-borne viral infection. Infections cause mild to severe diseases, including dengue hemorrhagic fever (DHF), a severe form that may kill infants and young children. Dengue virus antibody transfer from mother to fetus in pregnancy confers protection at birth, thereafter subsiding to a lower level that may cause DHF in infants. Infant dengue antibodies levels also influence the optimal age for dengue vaccination because of neutralization of the proposed live virus vaccine by the protective antibody levels in the newborn. To establish the optimal age, we identified mother-child pairs in which maternal dengue antibodies were transferred from mother to fetus in this study. Then a follow-up study would measure the infant antibody levels. Our study found that 97.2% of pregnant women giving birth in a dengue-endemic area had evidence of previous dengue infection. All umbilical cord blood from fetuses had the same proportion of positive tests for the presence of dengue antibodies, but had a higher dengue antibody levels compared to their mothers. The period of protection provided by maternally transferred dengue antibodies might affect the disease burden among infants and offer a better understanding of the optimal age for dengue vaccination.
Introduction
Dengue is the most rapidly disseminating mosquito-borne viral infection [1]. Any of the 4 antigenically-related serotypes DEN-1, DEN-2, DEN-3 or DEN-4 may cause an infection with a wide variety of manifestations from mild to severe such as asymptomatic infection, undifferentiated febrile illness, dengue and severe dengue infection [2]. The pathological processes of the severe forms of infection, including dengue hemorrhagic fever (DHF), remain unclear. Because cases of dengue virus infected infants <1 year old with maternal dengue virus antibodies at a subneutralizing level have shown a greater probability of contracting DHF, antibody-dependent enhancement (ADE) has been suggested as a possible process [3–6].
Having been first detected in hospitalized Thai patients in Bangkok in 1958 [7], dengue virus infections have occurred in other regions of the country [8, 9]. The majority of dengue infections in Asia are in children, and it is one of the 10 most common causes of morbidity and mortality for children in the region [9]. An initial estimate of up to 3.97 billion people might be at risk of infection [10]. Of an estimated 390 million dengue infections, 96 million have clinical manifestations. This is just over four times that of the dengue burden estimated by the World Health Organization (WHO) [11]. Globally, Asia, the Americas and some Pacific islands have had dengue epidemics. The majority of epidemics (75%) occur in the WHO defined regions of Southeast Asia (SEA) and the Western Pacific [2]. With the exception of the Maldives, Nepal and Thailand, other SEA countries reported increasing numbers of cases between 2011 and 2012 [12].
The only immunological substance recognized to be transferred from mother to fetus are antibodies, of which most are in the IgG subclass [13–16]. Measurements of high levels of transferred neutralizing dengue antibodies have been found in neonates at delivery [17, 18]. Proven by serum sampling, the presence of these antibodies in SEA region infants prevents clinical dengue before around 6–9 months of age [9, 17–19].
To address the increasing need for dengue treatment, the WHO’s ‘Global Strategy for Dengue Prevention and Control, 2012–2020’ set targets to reduce dengue morbidity by 25% and mortality by 50% (calculated from a baseline of the 2010 estimate) by 2020 [1, 20]. To reach these targets, the use of potential vaccines and sustainable vector control measures are the main prevention strategies, as well as appropriate clinical management. At the time of writing, no licensed vaccine exists. Recombinant live-attenuated, CYD tetravalent dengue vaccine was reported to provide around 30% effective protection in a trial of Thai school children [21]. In Asia and Latin America, a multicentered phase III trial has been ongoing since 2011. In Thailand, this trial involves Ban Pong and Photharam Districts in Ratchaburi Province and Kamphaeng Phet Province. One of the top 10 provinces in incidence of dengue in Thailand, Ratchaburi Province is situated 100 km west of Bangkok. The incidence of clinical dengue rose from 123.45 per 100,000 population in 2003 to 394.25 in 2008 [22].
One of two key Phase III efficacy studies of a potential dengue vaccine has successfully attained its primary clinical endpoint. From a preliminary press release, this study reported a 56% decrease in dengue disease cases, and preliminary safety data suggests a good safety profile similar to previous studies [23]. To decide future vaccination strategies, accurate data of measurements of disease impact and of the kinetics of maternal dengue antibodies are crucial.
We designed this study to evaluate the seroprevalence of neutralizing dengue antibody of pregnant women living in an area of high endemicity of dengue—Ban Pong, Ratchaburi Province—and to ascertain the proportion of transference of maternal neutralizing dengue antibody to the cord blood.
Materials and Methods
Setting
Ban Pong Hospital, a 350-bed state-operated hospital, services a district of 169,900 people and an average of 160 babies are delivered there every month.
Study design
At Bang Pong Hospital, we conducted a prospective observational cross-sectional study and enrolled 151 mother-infant pairs between December, 2011 and January, 2013. We excluded pregnant women who had a history of immunodeficiency, had taken immunosuppressive medications within a month before the study (inclusive of corticosteroid treatment >2 weeks), and had been transfused blood or blood components within 3 months before the study.
Sample size calculation
We determined the sample size to evaluate the seroprevalence of dengue infection in pregnant women by Epi Info software (2002). The basis of our calculation was a survey of pregnant women in Bangkok, Thailand from 2000 to 2001. In a sample size of 124, that survey used a 50% plaque reduction neutralization test (PRNT50), and reported a prevalence of dengue antibody at 97% [20] with a confidence interval of 95% and a precision of +/- 3%. From this basis, we derived a total sample size of 150 pregnant women.
Data collection
We noted the demographic data of the mothers and neonates within 24 hours after delivery. We collected a 5-ml blood sample from each mother and a 5-ml sample of cord blood at delivery for dengue serological testing.
Serology
Every sample was centrifuged within several hours after collection and stored at -20°C. The Armed Forces Research Institute of Medical Sciences (AFRIMS), Bangkok, Thailand performed the antibody assays.
Dengue antibody levels against all 4 dengue reference strains were performed by PRNT50 technique previously described by Russell et al [24] in 141 dengue hemagglutination assay seropositive maternal sera. The dengue strains used were DEN-1(16007) (isolated from a child with DHF in Thailand in 1964), DEN-2 (16681) (isolated from a child with DHF in Thailand in 1964), DEN-3 (16562) (from a child with DHF in the Philippines in 1964) and DEN-4 (C0036/06) (from a child with DF in Thailand in 2006). PRNT titer ≥1:10 to one dengue serotype at least was considered seropositive.
Data analysis
We calculated serotype-specific seroprevalence rates to be the percentage of samples with neutralizing (NT) antibody levels ≥1:10 against at least one dengue serotype. We calculated the geometric mean titers (GMTs) at a 95% confidence interval (CI) for each of the 4 serotypes. The comparisons of seroprevalence and GMTs between mother and paired cord blood were performed by symmetry test and paired t-test, respectively. Comparisons between seroprevalences among different age specific groups were based on chi-square test. Correlations between maternal and cord blood of DEN-1, DEN-2, DEN-3 and DEN-4 antibody titers were computed by Spearman’s rho. The paired titers of mothers and cord bloods were also plotted using lowess smoothing curve. All statistical analyses were performed using STATA software (version 10). The level of significance was set at 0.05.
Ethics
The Ethics Committee of Ban Pong Hospital approved the study protocol. All the pregnant women gave written informed consent prior to enrollment.
Results
We enrolled 141 mothers with a mean (± SD) maternal age of 23.6 years (± 5.8 years; range 15–41 years). All participants resided in Bang Pong District, Ratchaburi Province.
Just over one-third of the mothers were <20 years of age. Slightly less than 44% of them were primigravida. Table 1 displays the demographic data of the mothers including age, level of education, occupation, number of parity, gestational age and mode of delivery. All neonates were healthy at birth. Two had low birth weights (<2,500 grams) at 2,190 grams and 2,460 grams. A proportion of 99.3% were full term. Mean birthweight (± SD) was 3,196 (± 399) grams (range 2,190–4,430 grams). The male to female ratio was 1.1:1.
Table 1. Demographic characteristics of 141 pregnant women.
| Variable | Number (%) |
|---|---|
| Age | |
| - ≤20 y | 54 (38.3) |
| - 21–25 y | 40 (28.4) |
| - 26–30 y | 20 (14.2) |
| - 31–35 y | 24 (17.0) |
| - ≥36 y | 3 (2.1) |
| Education | |
| - primary school | 58 (41.1) |
| - secondary school | 66 (46.8) |
| - vocational school | 11 (7.8) |
| - bachelor degree | 6 (4.3) |
| Occupation | |
| - housewives | 67 (47.5) |
| - employee | 54 (38.3) |
| - other | 20 (13.2) |
| Parity | |
| - 0 | 61 (43.3) |
| - 1 | 51 (36.2) |
| - >2 | 29 (20.5) |
| Gestational age (wks) | |
| - 37–41 | 140 (99.3) |
| - ≥42 | 1 (0.7) |
| Mode of delivery | |
| - spontaneous delivery | 134 (95.0) |
| - vacuum extraction | 2 (1.4) |
| - cesarean section | 5 (3.6) |
Some factors suspected to influence the prevalence of dengue infection such as age, level of education, occupation and the household income of the mothers were analyzed by logistic regression. These analyses produced no statistically significant differences.
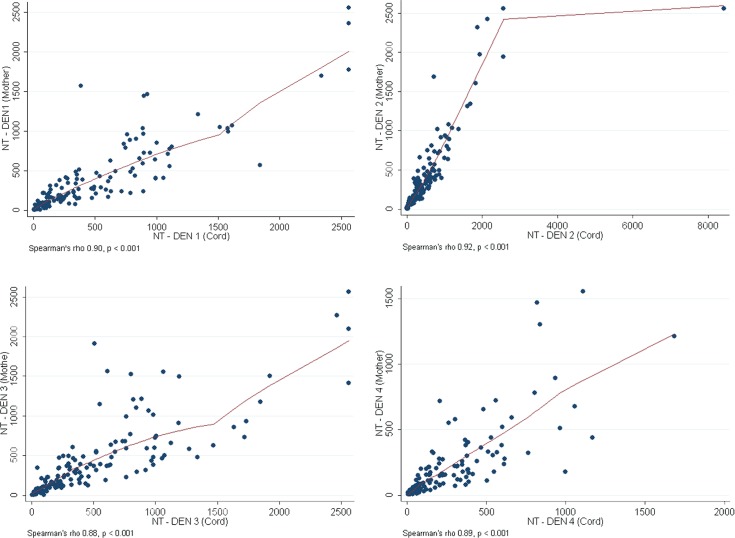
Most mothers (137/141, 97.2%) had NT antibody to at least 1 dengue serotype in their sera. Table 2 displays the proportions of seroprevalence of all 4 dengue serotypes, and GMT levels of the pregnant women and their cord sera. At birth, the proportion of cord sera with NT antibodies to DEN-1, DEN-2, DEN-3 and DEN-4 were high and similar to the sera of their mothers at 93.6, 97.2, 97.9 and 92.2%, respectively. The GMT levels of dengue NT titers in cord blood were significantly higher than the maternal antibody titers (p<0.001): the highest in DEN-2, followed by DEN-3, and DEN-1. The GMT of DEN-4 was the lowest of all the 4 serotypes. Maternal and cord NT of DEN-1, DEN-2, DEN-3, and DEN-4 antibody titers correlated well (Spearman correlation <0.001), as shown in Fig. 1.
Table 2. The proportion and geometric mean titer of NT dengue antibodies of 141 maternal and cord paired sera.
| Positive (N) | % | GMTs | (95%CI) | ||
|---|---|---|---|---|---|
| DEN-1 | mother | 132 | 93.6 | 180.3 | 141.5–229.7 |
| Cord | 132 | 93.6 | 219.0 | 169.6–282.9 | |
| p-value | 1.00 | <0.001 | |||
| DEN-2 | mother | 137 | 97.2 | 246.0 | 201.0–301.1 |
| Cord | 137 | 97.2 | 308.1 | 249.7–380.1 | |
| p-value | 1.00 | <0.001 | |||
| DEN-3 | mother | 136 | 96.5 | 233.9 | 187.1–292.4 |
| Cord | 138 | 97.9 | 288.1 | 232.0–357.6 | |
| p-value | 0.157 | <0.001 | |||
| DEN-4 | mother | 127 | 90.1 | 90.3 | 71.0–114.8 |
| Cord | 130 | 92.2 | 120.7 | 95.2–152.9 | |
| p-value | 0.180 | <0.001 | |||
Note: p-values for comparisons of seroprevalence are based on symmetry test; p-values for comparisons of GMTs between mother and cord are based on paired test of log (NT)
Fig 1. Correlation between maternal and cord blood of DEN-1 to DEN-4 neutralizing antibodies titers.
X-axis shows GMTs of cord blood dengue antibody and Y-axis shows GMTs of maternal dengue antibody of DEN-1, DEN-2, DEN-3 and DEN-4. The Spearman’s rho of DEN1, DEN2, DEN3 and DEN4 are 0.90, 0.92, 0.88 and 0.89 respectively.
All mothers aged >35 years and their cord sera showed 100% seropositive to all 4 dengue serotypes compared with the younger age groups. However, dengue seroprevalence by maternal age grouping was not statistically significantly different.
Discussion
In our study, we found that 97.2% of pregnant women giving birth at Ban Pong Hospital showed serological evidence of previous dengue infection. Most transferred dengue antibodies to cord blood. The high levels of antibody transference at delivery in our study are similar to previous results from Bangkok, Thailand, from 1998 to 2001 [17,18, 25,26]. The high proportion of mothers with antibodies against multiple dengue virus serotypes reflects the high rate of transmission of dengue viruses in an endemic area, Thailand.
Seronegative sera occurred in 2.8% of pregnant women possibly susceptible to primary dengue infection, and another 2.8% (4/141) would be at risk to secondary dengue infection because they had only one dengue serotype positivity.
A detectable dengue IgG of 53.9% in the pregnant women using dengue IgG indirect enzyme-link immunosorbent assay (ELISA) with a high degree of agreement of IgG seropositivity between the pairs of pregnant women and their neonates (99.3%) was reported in a cross-sectional study in central Brazil during a large outbreak (2009–2010) [27]. Approximately half of the adult population screened was still immunologically naïve to dengue virus exposure. This finding appears compatible with the more recent reintroduction of the virus to central Brazil.
Table 3 shows a comparison of maternal dengue seroprevalence, maternal and cord sera antibody titers in various published articles [17, 18, 25–28]. However, the dengue seroprevalence of IgG in pregnant women in previous reports detected by ELISA IgG was 93.6–95.2% [17, 18]. Our finding that maternally transferred neutralizing dengue antibody in cord blood in all 4 dengue serotypes had a higher GMT than maternal blood with statistically significant differences (p <0.001) is consistent with previous reports [13, 25]. Higher cord blood GMTs compared to maternal blood in all 4 dengue serotypes using HAI assay were reported [26] although only 52.9% of mean dengue antibody was higher in cord sera than maternal sera [25]. Only higher GMTs of DEN-2 in cord sera compared to maternal sera were found while GMTs of DEN-1, DEN-3 and DEN-4 were higher in maternal sera compared to cord sera [18]. This could be explained by the preferential movement of the antibody across the placenta, which promotes both greater avidity to antigens and greater ease of transplacental passage, and reflects the dynamic of IgG placental transfer [13, 28]. Most infants experience a decline in maternal antibody level during the first 12 months [17, 18, 25, 26], and some infants are at risk of developing various spectra of dengue infection [18]. Undifferentiated fever and asymptomatic dengue infection have been diagnosed in infants after a decline of maternal-transferred antibody [18, 19]. Because of this, refinement and reconsideration of the current ADE pathogenesis should be encouraged [19].
Table 3. Comparison of maternal dengue seroprevalence, maternal and cord sera dengue antibody titers in various articles.
| Methods | Seroprevalence (%) | Maternal GMTs | Cord GMTs | p* | |
|---|---|---|---|---|---|
| Pengsaa K, et al[17] | PRNT50 | 95.2 | - | 53.5–258.2 a | n/a |
| Watanaveeradej V, et al[25] | HAI | 96.8 | - | - | n/a |
| IgG | - | 62.7±85.8 b | 99.8±448 b | n.d. | |
| Perret C, et al[26] | HAI | 94.7 | 41.3–104 a | 50.8–128.2 a | <0.01 d |
| Pengsaa K, et al[18] | PRNT50 | 97.3 | 82.1–731 a | 78.6–692 a | n.d. |
| Chau TNB, et al[28] | PRNT50, | 98 | 26–53 a 913 (582–1,297) c | 30–80 a 1,025 (600–1,429) c | <0.001 e |
| IgG | 913 (582–1,297) c | 1,025 (600–1,429) c | 0.002 | ||
| Argolo AF, et al[27] | IgG | 53.9 | - | - | n/a |
| This study | PRNT50 | 97.2 | 90.3–246 a | 120.7–308.1 a | <0.001 |
* p-values are based on paired sample t-test
a range
b mean±SD
c mg/dL, GMT (range)
d p-value 0.002 for DEN-1, 0.003 for DEN-3 and <0.001 for DEN-2 and DEN-4
e p-value <0.001 for DEN-2 and DEN-3 only, p-value for DEN-1 and DEN-4 ≥ 0.5
n/a = not applicable, n.d. = no data available
Epidemiological, clinical, and virological studies indicate that the disease severity could be due to viral virulence, as well as the presence of passively acquired heterotypic sub-neutralizing maternally-transferred dengue antibodies which cause ADE [3–6].
In our study, we demonstrated that the seroprevalence of dengue infection in pregnant women to all 4 dengue serotypes was the highest in DEN-2, followed by DEN-3, DEN-1 and DEN-4. These finding agree with the high incidence of dengue infection with all 4 dengue serotypes in primary school children in the Namuang Subdistrict of Muang District, Ratchaburi Province from 2006 through 2009 [29]. However, the rank order of seroprevalence in that study was dissimilar to that of the pregnant women in our study. DEN-1 was the most common infecting serotype reported in those school children (43%), followed by DEN-2 (29%), DEN-3 (20%) and DEN-4 (8%). These findings indicate that dengue transmission is continuous in Ratchaburi Province.
The high dengue seroprevalence in older mothers in our study is similar to a previous study in Bangkok in which all mothers aged >35 years transferred antibodies to their infants [25]. The higher prevalence of dengue infection among older mothers compared to younger mothers reflects the high transmission rate of dengue viruses in Thailand, and confirms the findings of previous studies [18, 25, 26].
Conclusion
A high neutralizing dengue seroprevalence of 97.2% was found in pregnant women at Ban Pong Hospital, Ratchaburi Province. Nearly all those pregnant women had been infected with the 4 dengue serotypes. All cord blood had the same proportion of seropositivity, but had a higher dengue antibody titer to each dengue serotype compared to their mothers. Most infants appeared to be protected from dengue infection in early life by maternal transferred dengue antibodies. In this dengue-endemic area, the period of protection provided by maternally transferred dengue antibodies might have an impact on the disease burden among infants, and offer a better understanding of the optimal age for dengue vaccination.
Disclaimer: The views expressed in this article are those of the author (s) and do not reflect the official policy of the Department of the Army, Department of Defense, or the U.S. Government.
Supporting Information
(DOC)
Acknowledgments
We would like to thank Napaporn Lattiwongsakorn, Yongyuth Poolpanichaupatam, Taweewan Hunsawong, Winai Kaneechit, and Suwannee Hunsawong from the Armed Forces Research Institute of Medical Sciences (AFRIMS) for providing serological analysis. We would also like to thank participating mothers, and all other personnel involved in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.WHO (2012). Global strategy for dengue prevention control: 2012–2020. Available at: http://reliefweb.int/sites/reliefweb.int/files/resources/9789241504034_eng.pdf. Accessed on May 16, 2014
- 2. WHO (2009) Dengue haemorrhagic fever: diagnosis, treatment and control 3rd ed. Geneva: WHO; [Google Scholar]
- 3. Halstead SB (1998) Pathogenesis of dengue: challenges to molecular biology. Science 239:476–481. [DOI] [PubMed] [Google Scholar]
- 4. Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS (1989) Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40:444–451. [DOI] [PubMed] [Google Scholar]
- 5. Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, et al. (1998) Dengue and dengue haemorrhagic fever. Lancet 352:971–977. [DOI] [PubMed] [Google Scholar]
- 6. Sullivan NJ (2001) Antibody–mediated enhancement of viral disease. Curr Trop Microbiol Immunol 260:145–169. [DOI] [PubMed] [Google Scholar]
- 7. Hammon WM, Rudnick A, Sather GE (1960) Viruses associated with epidemic hemorrhagic fevers of the Philippines and Thailand. Science 131:1102–1103. [DOI] [PubMed] [Google Scholar]
- 8. Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR (1969) Dengue and Chikungunya virus infection in man in Thailand, 1962–1964. Am J Trop Med Hyg 18:954–971. [DOI] [PubMed] [Google Scholar]
- 9. Halstead SC, Lan NT, Myint TT, Shwe TN, Nisalak A, et al. (2002) Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis 8:1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, et al. (2012) Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6:e1760 10.1371/journal.pntd.0001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496:504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO-Regional Office for South East Asia (2012) SEARO Dengue situation update, 24 September 2012. Available at: http://www.searo.who.int/entity/vector_borne_tropical_diseases/data/seardengueupdate.pdf. Accessed on May 16, 2014.
- 13. Ventura AK, Ehrenkranz NJ, Rosenthal D (1975) Placenta passage of antibodies to dengue virus in persons living in a region of hyperendemic dengue virus infection. J Infect Dis 131:S62–68. [DOI] [PubMed] [Google Scholar]
- 14. Gasparoni A, Avanzini A, Probizer FR, Chirico G, Rondini G (1992) IgG subclasses compared in maternal and cord serum and breast milk. Arch Dis Child 67:41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simister NE (2003). Placental transport of immunoglobulin G. Vaccine 21:3365–3369. [DOI] [PubMed] [Google Scholar]
- 16.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M (2012) IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol [DOI] [PMC free article] [PubMed]
- 17. Pengsaa K, Yoksan S, Limkittikul K, Wisetsing P, Sirivichayakul C, et al. (2003) Maternally transferred neutralising dengue antibodies in Thai infants: a pilot study. Ann Trop Paediatr 23:159–165. [DOI] [PubMed] [Google Scholar]
- 18. Pengsaa K, Luxemburger C, Sabchareon A, Limkittikul K, Yoksan S, et al. (2006) Dengue virus infections in the first 2 years of life and the kinetics of transplacentally transferred dengue neutralizing antibodies in Thai children. J Infect Dis 194:1570–1576. [DOI] [PubMed] [Google Scholar]
- 19. Libraty DH, Acosta LP, Tallo V, Segubre-Mercado E, Bautista A, et al. (2009) A prospective nested case-control study of Dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med 6:e1000171 10.1371/journal.pmed.1000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO (2012) Report of a WHO technical working group meeting on dengue prevention and control. WHO Headquarters, Geneva, Switzerland. 10–12 December, 2012.
- 21. Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, et al. (2012) Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567. 10.1016/S0140-6736(12)61428-7 [DOI] [PubMed] [Google Scholar]
- 22. Tanayapong S, Pengsaa K, Thisyakorn U (2013) Changing epidemiology of dengue patients in Ratchaburi, Thailand. Asian Biomed 7:561–566. 10.1016/S1995-7645(13)60097-8 [DOI] [PubMed] [Google Scholar]
- 23. http://www.dengue.info/#overlay=content/world%25E2%2580%2599s-first-large-scale-dengue-vaccine-efficacy-study-successfully-achieved-its-primary-cl. Accessed May 16, 2014.
- 24. Russell PK, Nisalak A, Sukhavachana P, Vivona S (1967) A plaque reduction test for dengue virus neutralizing antibodies. J Immunol 99:285–290. [PubMed] [Google Scholar]
- 25. Watanaveeradej V, Endy TP, Samakoses R, Kerdpanich A, Simasathien S, et al. (2003) Transplacentally transferred maternal-infant antibodies to dengue virus. Am J Trop Med Hyg 69:123–128. [PubMed] [Google Scholar]
- 26. Perret C, Chanthavanich P, Pengsaa K, Limkittikul K, Hutajaroen P, et al. (2005) Dengue infection during pregnancy and transplacental antibody transfer in Thai mothers. J Infect 51:287–293. [DOI] [PubMed] [Google Scholar]
- 27. Argolo AF, Féres VC, Silveira LA, Oliveira AC, Pereira LA, et al. (2013) Prevalence and incidence of dengue virus and antibody placental transfer during late pregnancy in central Brazil. BMC Infect Dis 3:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chau TNB, Hieu NT, Anders KL, Wolbers M, Lien LB, et al. (2009) Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J Infect Dis 200:1893–1900. 10.1086/648407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sabchareon A, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. (2012) Dengue infection in children in Ratchburi, Thailand: a cohort study I. Epidemiology of symptomatic acute dengue infection in children, 2006–2009. PLoS Negl Trop Dis 6:e1732 10.1371/journal.pntd.0001732 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.