Abstract
Because VEGFA has been implicated in follicle development, the objective of this study was to determine the effects of granulosa- and germ cell-specific VEGFA loss on ovarian morphogenesis, function, and female fertility. pDmrt1-Cre mice were mated to floxed VEGFA mice to develop granulosa-/germ cell-specific knockouts (pDmrt1-Cre;Vegfa -/-). The time from mating to first parturition was increased when pDmrt1-Cre;Vegfa -/- females were mated to control males (P = 0.0008) and tended to be longer for heterozygous females (P < 0.07). Litter size was reduced for pDmrt1-Cre;Vegfa -/- females (P < 0.007). The time between the first and second parturitions was also increased for heterozygous females (P < 0.04) and tended to be increased for pDmrt1-Cre;Vegfa -/- females (P < 0.07). pDmrt1-Cre;Vegfa -/- females had smaller ovaries (P < 0.04), reduced plasma estradiol (P < 0.007), fewer developing follicles (P < 0.008) and tended to have fewer corpora lutea (P < 0.08). Expression of Igf1r was reduced (P < 0.05); expression of Foxo3a tended to be increased (P < 0.06); and both Fshr (P < 0.1) and Sirt6 tended to be reduced (P < 0.06) in pDmrt1-Cre;Vegfa -/- ovaries. To compare VEGFA knockouts, we generated Amhr2-Cre;Vegfa -/- mice that required more time from mating to first parturition (P < 0.003) with variable ovarian size. Both lines had more apoptotic granulosa cells, and vascular staining did not appear different. Taken together these data indicate that the loss of all VEGFA isoforms in granulosa/germ cells (proangiogenic and antiangiogenic) causes subfertility by arresting follicular development, resulting in reduced ovulation rate and fewer pups per litter.
Introduction
Vascular endothelial growth factor A (VEGFA) is one of the key factors regulating angiogenesis in the ovary [1]. VEGFA stimulates neovascularization, vascular permeability, acts as a survival factor and can also stimulate proliferation of vascular and nonvascular cells [2–4]. An in situ study showed the in vivo expression of VEGFA in different cell types including theca, cumulus, granulosa and luteal cells in the mouse ovary [5]. Our laboratory has demonstrated that VEGFA and its receptors are localized to pre-granulosa and granulosa cells of all follicle stages and to theca cells and oocytes of advanced-stage follicles in the rat [6]. The Vegfa gene can be alternatively spliced to produce angiogenic or antiangiogenic isoforms which have divergent functions from one another: angiogenic isoforms promote vascular development while antiangiogenic isoforms inhibit vascular development [6,7]. To initiate biological effects, VEGFA binds two related tyrosine kinase receptors: FMS-like tyrosine kinase 1 (FLT1, also known as VEGFR1) and kinase insert domain receptor (KDR, also known as VEGFR2 and FLK1). The primary receptor is KDR [8]. KDR is the major mediator of the mitogenic, angiogenic and permeability enhancing effects of VEGFA. KDR is also involved in mediating endothelial cell proliferation, survival and vascular permeability, whereas FLT1 may be inhibitory by sequestering VEGFA, and preventing its interaction with KDR [9]. Roberts et al. reported ovarian administration of antibodies to KDR resulted in significant depletion of primordial follicle numbers, whereas anti-FLT1 antibodies did not [10]. Thus, it appears that KDR is critical for follicle progression.
Independent of its angiogenic effects, VEGFA may also be important in the regulation of follicle growth with direct effects on granulosa cells. While rat ovaries treated with VEGFA receptor-tyrosine kinase inhibitor had vascular development reduced by 94%, there were more primordial follicles and fewer early primary, transitional, and secondary follicles compared to controls suggesting a block in follicle progression [6]. This study demonstrated a novel role for VEGFA in the recruitment of primordial follicles into the growing follicle pool, as well as being a potential survival factor for primary and later-stage follicles through vascular-dependent and vascular-independent mechanisms [6]. Further studies from our laboratory demonstrated that treatment with proangiogenic isoform VEGFA_164 or neutralization of antiangiogenic VEGFA isoforms enhanced vascular and follicular development in perinatal rat ovaries [7]. These results suggest that VEGFA proangiogenic isoforms stimulate follicle progression and antiangiogenic isoforms inhibit or arrest follicle development [7]. Furthermore, our laboratory has demonstrated that inhibition of antiangiogenic isoforms stimulates progression of follicles to later stages of development providing further support that their normal function is to inhibit folliculogenesis. Antiangiogenic isoforms were localized to granulosa and theca cells of all stages of follicles in rats [7]. KDR-LacZ-positive staining in the oocytes of secondary follicles in mice further suggests a role of VEGFA directly on oocyte development [11].
Thus, in the current study, we hypothesized that production of VEGFA isoforms (both pro and antiangiogenic) by granulosa cells is critical for normal ovarian morphogenesis and function. Therefore, the objective of the present study was to determine the in vivo effects of granulosa (pDmrt1 and Amhr2) and potentially germ cell- (pDmrt1) specific VEGFA loss on ovarian function and morphogenesis through conditional knockout mice in which Vegfa isoforms are eliminated via either the pDmrt1 or Amhr2 promoter.
Materials and Methods
Animals
Granulosa and germ cell VEGFA knockout (KO) mice were generated by mating a Vegfa-floxed mouse line [12] with porcine doublesex and mab-3 related transcription factor 1 (DMRT1) promoter (pDMRT1)-Cre mice [13]. Homozygous Vegfa-floxed mice were mated to mice whose genomic DNA genotyped positively for the pDmrt-1-Cre allele. Mating these parental lines resulted in F1 progeny, and heterozygous mice from the F1 generation were then mated back to the homozygous Vegfa floxed founders to generate the F2 population. Control and homozygous knockout female mice were generated from the F2 generation. We used Cre-negative Vegfa-/- or Vegfa +/- mice as controls and Cre-positive Vegfa-/- mice as knockouts (KOs) (pDmrt1-Cre;Vegfa-/-) or Cre-positive Vegfa +/- as heterozygotes (Het). Dmrt1 is expressed in the indifferent mouse gonad at E10.5 in precursor cells that differentiate into Sertoli and granulosa cells [14]. By E13.5, expression is no longer detectable in granulosa cells but is still present within oocytes until E15.5 [14,15]. The pDmrt1 gene is expressed similarly to mouse Dmrt1, as detected by reverse transcriptase (RT)-PCR in developing ovaries, making it a useful means to eliminate Vegfa in granulosa and germ cells [13]. In our pDmrt1-Cre;Vegfa-/- mouse, Cre recombinase activity is driven by the pDmrt1 promoter which excises exon 3 of the Vegfa gene. Exon 3 is present in both proangiogenic and antiangiogenic isoforms; thus, excision results in a reduction in all Vegfa isoforms where pDmrt1 is expressed (S1 Fig.). Ovaries plus oviducts, uteri, kidneys, and adrenals were collected from 8-month-old female mice and weighed in addition to measuring total body weight (n = 11 controls, n = 10 KO). Females were collected in estrus.
Similarly, we generated a transgenic mouse line by crossing the same floxed Vegfa mice with mice expressing Amhr2-Cre. Expression of the Amhr2-Cre transgene was detected as early as 12.5 dpc and continued through adulthood in pre-granulosa cells, granulosa cells and the female reproductive tract [16]. These mice were harvested at approximately 3-months-of-age, earlier than collection of the Vegfa x pDmrt1-Cre animals (8 months). Reproductive potential of the 8-month-old control animals was not impaired. The mice were harvested at different ages in an attempt to compare reproductive phenotypes resultant from similar cell-specific elimination of VEGFA both early and late in reproductive life.
All animal protocols were approved by the University of Nebraska Institutional Animal Care and Use Committee (IACUC) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal models were procured from collaborating investigators with verbal or written MTA agreements. Thus, the corresponding author does not have rights to freely distribute these mouse lines.
Genotyping
Genomic DNA was extracted from tail samples according to previously reported methods [17]. RNA was extracted from whole ovaries and reverse transcribed to cDNA to be tested for Cre expression at the level of the gonad. Animals were removed from analysis if results differed from genomic DNA genotyping. Primer sequences are supplied in Table 1.
Table 1. Sequences for primers used to conduct conventional and quantitative PCR.
| PCR | Gene | Sequence | Accession Number |
|---|---|---|---|
| Conventional | pDmrt1(forward) | 5’-AGCAGAGGCTTCCTTCGACTT-3’ | AF216651 |
| Cre (reverse) | 5’-AGTGAACGAACCTGGTCGAAA-3’ | ||
| muVEGF419.F | 5’-TCCGTACGACGCATTTCTAG-3’ | ||
| muVEGF567.R | 5’-CCTGGCCCTCAAGTACACCTT-3’ | ||
| qPCR | Foxo3a | 5’-GCAAAGCAGACCCTCAAACTG-3’ | NM_019740 |
| 5’-TGAGAGCAGATTTGGCAAAGG-3’ | |||
| Amh | 5’-TCCTACATCTGGCTGAAGTGATATG-3’ | NM_007445 | |
| 5’-CAGGTGGAGGCTCTTGGAACT-3’ | |||
| Gdf9 | 5’-GCCGGGCAAGTACAGCC-3’ | NM_008110 | |
| 5’-TTTGTAAGCGATGGAGCCG-3’ | |||
| Igf1r | 5’-CAGATGGCTGGAGAGATTGCA-3’ | NM_010513.2 | |
| 5’-GTGGACGAACTTGTTGGCATT-3’ | |||
| Fshr | 5’-TCCGCAGGGACTTCTTCGT | NM_013523.3 | |
| 5’-AATCTGGGCTTGCACCTCAT | |||
| Bmp2 | 5’-TGTCCCCAGTGACGAGTTTCT-3’ | NM_007553.3 | |
| 5’-CCTGTATCTGTTCCCGGAAGAT-3’ | |||
| Sirt1 | 5’-AAACCTCCACGCCCACAA-3’ | NM_019812.2 | |
| 5’-GAAGAGGTGTTGGTGGCAACTC-3’ | |||
| Sirt6 | 5’-AGCCCCCCGTGCATCT-3’ | NM_181586.3 | |
| 5’-TGTTGGGCTTGGACTTATACGA-3’ |
Fertility Trials
Cyclic female mice were paired with control males when they were approximately 2–4 months-of-age and remained together until they were six to eight months-of-age for both pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- mice. Control, Het and KO females were all paired with control males of proven fertility. The time from when males and females were placed together in cages to the time of parturition was measured in days. We also determined the number of pups per litter. Het females were removed from the Vegfa x Amhr2-Cre analysis due to ectopic Cre expression.
Fixation, Embedding, Staining, and Immunohistochemistry (IHC)
Ovaries were fixed at room temperature in Bouin’s solution, embedded in paraffin and sectioned at 5 μm according to standard procedures [6]. The rabbit polyclonal IgG VEGFA primary antibody was raised against a peptide corresponding to amino acids 1–140 of human VEGFA (catalog number: sc-507, Santa Cruz Biotechnology, Santa Cruz, CA). As a pan-antibody, it targeted both proangiogenic and antiangiogenic isoforms to confirm presence of VEGFA isoforms in KO and control mouse ovaries. The antibody was diluted 1:100 in 10% normal goat serum (NGS). As a negative control, serial sections were processed without primary antibody. Biotinylated goat anti-rabbit secondary antibodies were diluted 1:300 in 10% NGS and were used with each primary antibody in this study (catalog number: BA-1000, Vector Laboratories, Burlingame, CA). The secondary antibody was detected using aminoethyl carbazole (AEC) chromagen substrate solution (Invitrogen, Carlsbad, CA). Similar immunohistochemistry protocols were used for additional primary antibodies. The cytochrome P450, family 11, subfamily a, polypeptide 1 (CYP11A1) primary antibody (catalog number: ab78416; Abcam, Cambridge, Mass., USA) was diluted 1:200 in 10% normal goat serum in PBS) and was used to quantify the number of corpora lutea present.
Counts of Follicles and Corpora Lutea
In order to determine the number of follicles per ovary, three images per ovary were counted by two technicians whose counts were averaged, and means were compared between Vegfa x pDmrt1-Cre control (n = 8) and KO (n = 9) ovaries as well as for Vegfa x Amhr2-Cre control (n = 4) and KO (n = 5) ovaries. One image was taken of the same ovary sectioned at representative depths- the most proximal and distal sections as well as the centermost section. Follicles were staged according to previously reported methods [6,7]. Stage 0 or primordial follicles consisted of an oocyte surrounded by a single layer of squamous granulosa cells. Stages 1–5 were grouped together as developing follicles. Stage 1 follicles were characterized by an oocyte surrounded by a single layer of a mixture of squamous and cuboidal granulosa cells. To be classified as Stage 2, all granulosa cells were cuboidal. Two or more layers of squamous granulosa cells had to present to be considered a Stage 3 follicle. Stage 4 follicles had a visible theca cell layer as well as an antrum. Stage 5 follicles had a large antrum and appeared to be ready to ovulate. Corpora lutea (CL) were evidenced by positive staining for the steroidogenic enzyme CYP11A1. The number of CL on one ovary per animal was counted and compared between Vegfa x pDmrt1-Cre control (n = 8) and KO ovaries (n = 9). Follicles were counted at 200x magnification.
Quantitative RT- PCR (qPCR)
One ovary was collected from each animal for RNA extraction, reverse transcription to cDNA and quantitative Real-Time PCR (qPCR) analysis according to previous protocols [6,17]. Primers and probes were designed with Primer Express 3.0 software (Applied Biosystems) and synthesized at Integrated DNA Technologies, Inc. (Coralville, IA). Amplification of genes that required SYBR Green to measure quantification was also plotted on a dissociation curve to ensure primer dimerization did not occur. We designed probes for two genes: BCL2-associated X protein (Bax) and B cell leukemia/lymphoma 2 (Bcl2). Primer and probe sequences for each gene are found in Table 1. Primers were designed for Foxo3a, Igf1r, Fshr, Bmp2, Sirt1, Sirt6, Amh, and Gdf9. Messenger RNA abundance was normalized to expression of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and represented as a fold change relative to the mean control value (set at a value of 1). The expression of Gapdh was determined using a VIC probe and primer kit (Taqman Rodent GapDH Control Reagents; Applied Biosystems; Catalog #4308313). Values were expressed as means±SEM.
Hormone Assays
Trunk blood was collected at the time of euthanasia and prepared for ELISA according to routine methods [17]. Plasma estradiol concentrations were determined by ELISA kit (Catalog #1920, Alpha Diagnostics International Inc., San Antonio, TX). Samples remained undiluted and were run according to the manufacture’s direction. All unknown values were plotted against a standard curve. Assay sensitivity was 10 pg/ml. The progesterone concentrations were determined by ELISA kit (Catalog #1860, Alpha Diagnostics International Inc.). Assay sensitivity was 0.2 ng/ml. Testosterone concentrations were determined by a testosterone ELISA kit (catalog number: 1880; Alpha Diagnostics International Inc., San Antonio, TX) [18]. Assay sensitivity was 0.125 ng/ml.
Immunofluorescence
Ovaries were processed for immunofluorescence as described for IHC and according to previous methods in our laboratory [17] with a rabbit polyclonal primary antibody to cleaved Caspase 3 (CASP3; Asp 175; catalog number: #9661; Cell Signaling, Danvers, MA). An anti-rabbit FITC secondary antibody (Alexa Fluor 488; catalog number: #4412S; Cell Signaling) was added to bind the cleaved CASP3 primary antibody, and it was diluted 1:1000 in 3% bovine serum albumin (BSA) in PBS-Tween. A goat antibody to VE-CADHERIN (catalog number: #sc-6458; Santa Cruz Biotechnology, Inc.) was also used to visualize vasculature. To detect expression of VE-cadherin, we used an anti-goat Texas Red secondary antibody (Alexa Fluor 594; catalog number: #A-21223; Invitrogen/Life Technologies, Grand Island, NY). We also stained ovaries from Vegfa x Amhr2-Cre control and KO (Amhr2-Cre;Vegfa-/-) females to increase the number of representative images since they have a similar mechanism of eliminating VEGFA. Two images were taken at 200x of both control (n = 3) and KO (n = 4) ovaries. Both primary antibodies were diluted 1:50 in 3% BSA (catalog number: #A3311; Sigma-Aldrich Corp., St. Louis, MO) in PBST, and both secondary antibodies were diluted 1:1000 in 3% BSA.
Statistical Analysis
Fertility data were analyzed by one-way ANOVA followed by t-test using SAS software (SAS Institute, Cary, NC). All other data were analyzed using the Dunnett’s procedure in JMP (SAS Institute). Differences in data were considered statistically significant when P < 0.05. Data were considered tending to be different if 0.05 > P < 0.1. All treatments were compared relative to controls. Gene expression data were tested for normality, and data that were not normally distributed were square rooted using SAS. Means reflect the original data, but P-values are representative of the Dunnett’s test following transformation.
Results
pDmrt1-Cre;Vegfa-/- mice were subfertile and had reduced ovarian weight
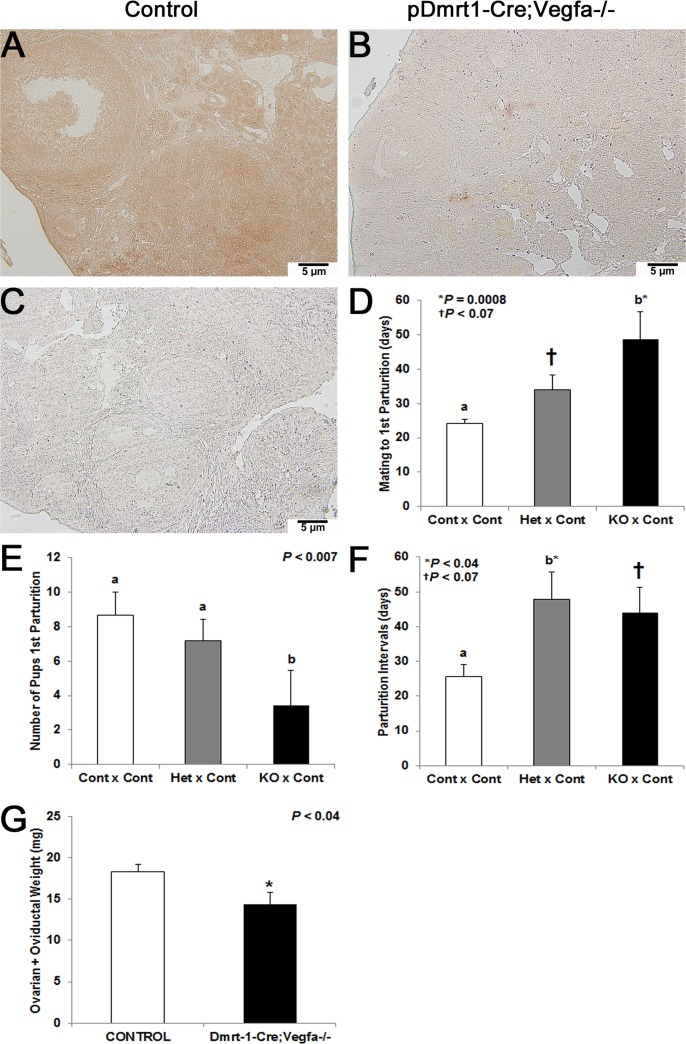
Immunohistochemistry with a pan-VEGFA antibody was performed to confirm the deletion of both pro- and antiangiogenic isoforms of VEGFA in KO ovaries at 8 months-of-age. The intensity of VEGFA-positive staining was dramatically reduced in ovaries taken from pDmrt1-Cre;Vegfa-/- females (Fig. 1B) compared to controls (Fig. 1A). Sections processed without primary antibody did not stain and served as negative controls (Fig. 1C).
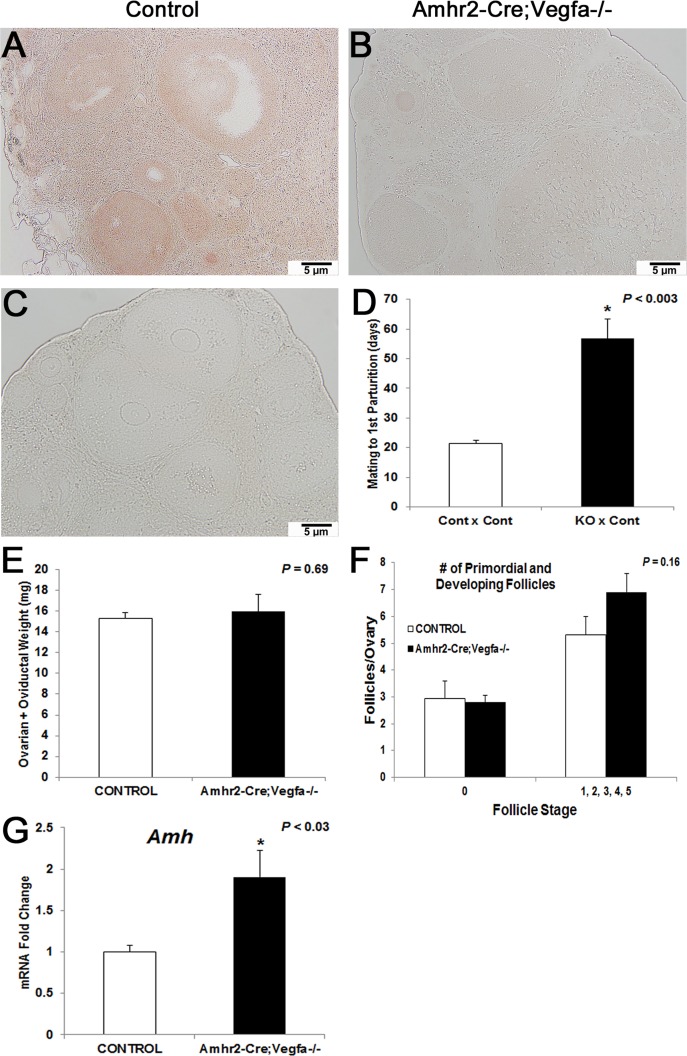
Fig 1. Immunohistochemistry with panVEGFA antibody to confirm VEGFA knockdown via pDmrt1-Cre and female fertility: 200x magnification in a pDmrt1-Cre;Vegfa-/- ovary (B) and a control ovary (A).
Negative control ovaries were processed without primary antibody (C). Females are named first in each fertility trial pairing (Control, Het, KO) for Vegfa x pDmrt1-Cre and were broken into mating to first parturition (D), the number of pups born at the first parturition (E) and the number of days from the first parturition to the second (F). For the Control x Control, Het x Control, and KO x Control pairs in both (D) and (E), n = 12, 10, and 5, respectively. For the Control x Control, Het x Control, and KO x Control pairs in (F), n = 4, 4, and 5, respectively. Ovarian plus oviductal weight (g) is presented in KO vs control females (G), and n = 10 and 11, respectively. Results represented by either different letters or a * were significant when P < 0.05, and a † denoted data that tended to be significant (0.05 < P < 0.1).
Mice were placed in mating pairs as follows: 1) control females with control males (Contl x Contl, n = 12), 2) heterozygous females with control males (Het x Cont, n = 10), and 3) KO females with control males (KO x Contl, n = 5). Het x Cont mating pairs from Vegfa x pDmrt1-Cre mice tended to take 10 days longer to get pregnant during the first mating to parturition interval (P < 0.07), and KO females took significantly longer to get pregnant when mated to control males (P = 0.0008) compared to Cont x Cont pairs (Fig. 1D). While there were no differences in the number of pups born in the first litter for Het x Control (P = 0.32), there were fewer pups born to KO x Cont mating pairs (P < 0.007) compared to Cont x Cont matings (Fig. 1E). Finally as seen in Fig. 1F, the number of days between the first and second parturitions for Het x Control pairs was increased (P < 0.04) and tended to be increased for KO x Cont mating pairs (P < 0.07) compared to the length for Cont x Cont mating pairs. Females were collected at the end of the fertility trial, and the combined weight of ovaries and oviducts from pDmrt1-Cre;Vegfa-/- females was significantly decreased compared to control females (P < 0.04, Fig. 1G). Control mice at the end of the fertility trial were still reproductively viable while the KO mice were less so, as indicated by the preceding data.
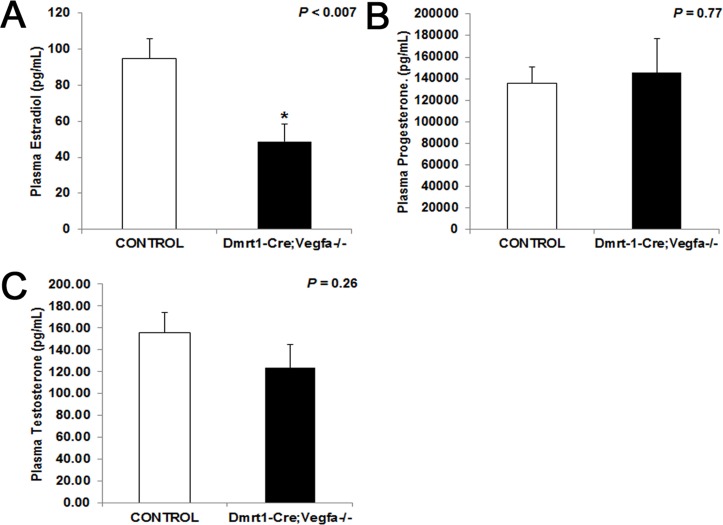
Plasma estradiol concentrations were reduced in pDmrt1-Cre;Vegfa-/- mice
Plasma 17β-estradiol in blood collected at the end of the fertility trial was reduced by almost half in pDmrt1-Cre;Vegfa-/- females (P < 0.007) compared to control females (Fig. 2A). There was no significant difference in plasma progesterone concentrations between the pDmrt1-Cre;Vegfa-/- (P = 0.77) and control females (Fig. 2B). There was also no difference in plasma testosterone concentrations in pDmrt1-Cre;Vegfa-/- females (P = 0.26) compared to control females (Fig. 2C).
Fig 2. Plasma hormone concentrations in Vegfa x pDmrt1-Cre females.
Concentrations of plasma E2 (A), progesterone (B) and testosterone (C) in KO (n = 7–10) compared to controls (n = 9–11). Results were significant when P < 0.05 and were represented with a *.
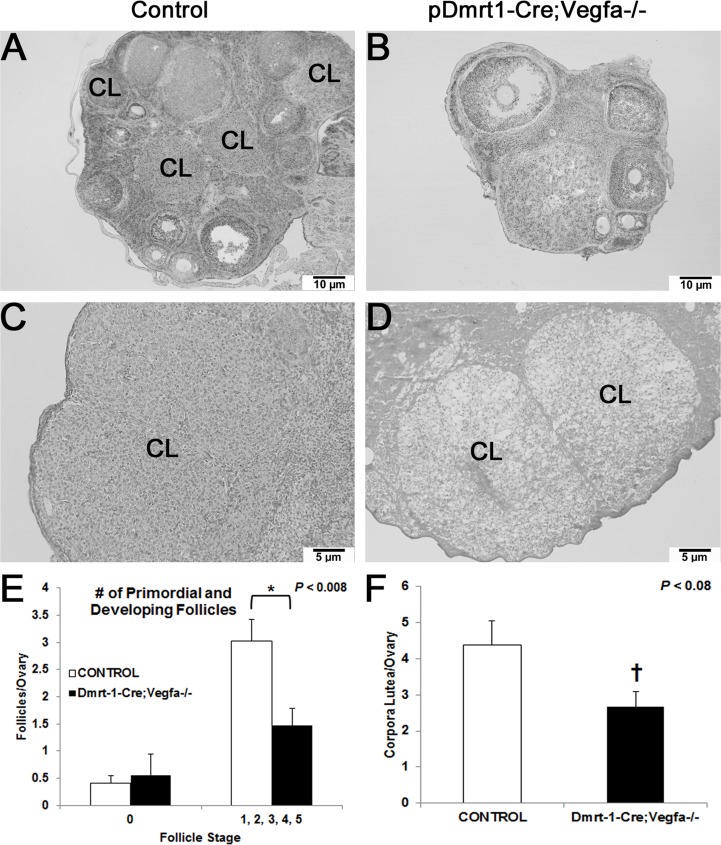
There were fewer developing follicles and a tendency for a reduction in CL number in pDmrt1-Cre;Vegfa-/- ovaries
While the number of primordial follicles (Stage 0) was not different per ovary between pDmrt1-Cre;Vegfa-/- and control females, pDmrt1-Cre;Vegfa-/- females had half as many developing follicles (Stages 1–5) per ovary (P < 0.008) as control females (Fig. 3E). Fewer later stage follicles can be seen on the accompanying 100x images of a pDmrt1-Cre;Vegfa-/- ovary (Fig. 3B) compared to a control ovary (Fig. 3A), and the pDmrt1-Cre;Vegfa-/- ovaries appear smaller than controls. In addition, the number of CL per ovary on pDmrt1-Cre;Vegfa-/- ovaries (P < 0.08) tended to be reduced compared to control mice (Fig. 3F). At 200x, the morphology of two CL in a representative KO ovary appeared to have fewer and less densely compacted luteal cells compared to a control (Fig. 3C, D).
Fig 3. pDmrt1-Cre;Vegfa-/- and control ovary histology.
Hematoxylin-eosin staining of ovarian cross-sections of 200x control (A), 200x control (C), 200x KO (B), and 200x KO (D). The 200x images depict varied CL morphology between a control and KO. Some of the ovarian structures are labeled: corpus luteum (CL) and the stage of follicle (1–4). The number of primordial follicles (stage 0) and developing follicles (stages 1–5) were counted and compared between controls (n = 8) and KO (n = 9) (E). The number of CL per ovarian section was counted based on positive staining for CYP11A1 and compared between controls (n = 8) and KO (n = 6) (F). Results were significant when P < 0.05 and were represented with a *, and a † denoted data that tended to be significant (0.05 < P < 0.1).
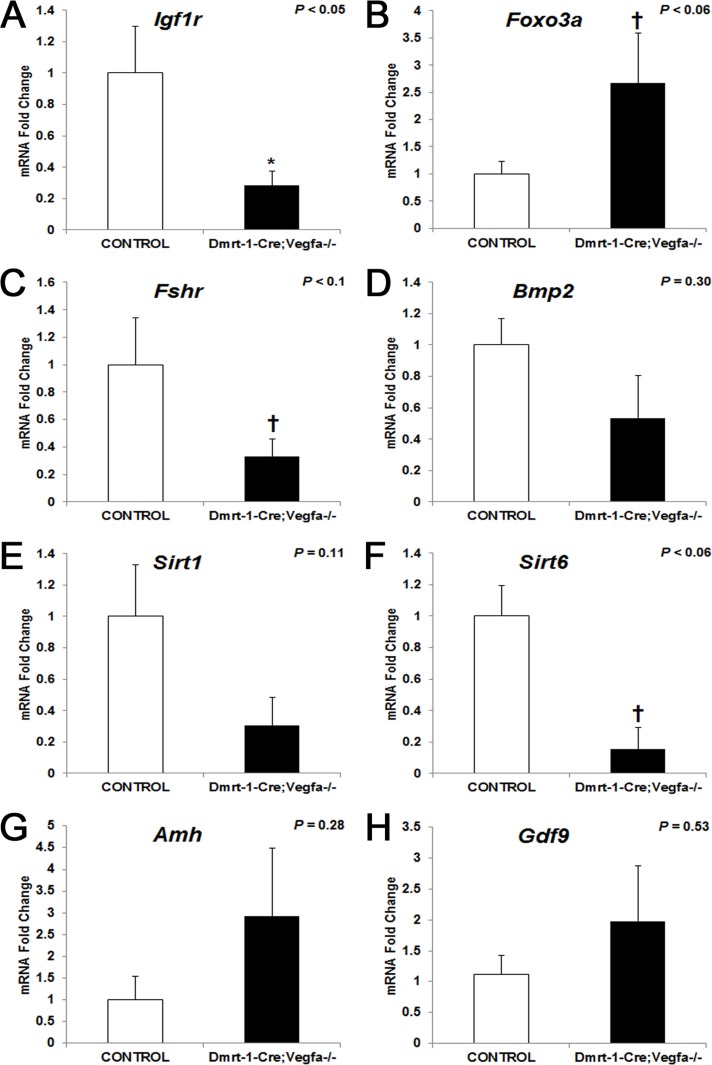
pDmrt1-Cre;Vegfa-/- female ovaries had reduced Igf1r, Fshr, Sirt6 andFoxo3a mRNA at 8 months of age
Quantitative real-time PCR analysis was performed on one whole ovary from each animal to determine the expression of genes known to regulate growth and follicular development. Messenger RNA abundance for insulin-like growth factor 1 receptor (Igfr1) was significantly reduced 3.5-fold in ovaries lacking VEGFA isoforms (P < 0.05) compared to controls (Fig. 4A) at 8 months of age. Its ligand, IGF1 is important for ovarian function and proliferation of granulosa cells [19]. Forkhead boxo3a (FOXO3A) is a transcription factor that inhibits the activation of primordial follicles. Messenger RNA abundance for Foxo3a tended to be increased 4.8-fold in pDmrt1-Cre;Vegfa-/- ovaries (P < 0.06) compared to that of controls (Fig. 4B). Primordial follicles numbers were a maximum of one or two per section; thus, a difference was not detectable statistically. Follicle stimulating hormone receptor (Fshr) mRNA abundance tended to be reduced 3-fold in pDmrt1-Cre;Vegfa-/- ovaries (P < 0.1) compared to controls (Fig. 4C). FSHR is the receptor for the gonadotropin, FSH, and would be indicative of FSH signaling in the ovary. While expression of sirtuin 1 (Sirt1) did not differ between pDmrt1-Cre;Vegfa-/- and control ovaries (Fig. 4E), sirtuin 6 (Sirt6) mRNA abundance tended to be reduced 6.5-fold in pDmrt1-Cre;Vegfa-/- ovaries (P < 0.06) compared to control ovaries (Fig. 4F). Sirtuins are ultimately important in maintenance following caloric restriction. FOXO3A is a substrate for SIRT1, and deletion of SIRT1 results in female infertility [20–22]. There were no differences in mRNA abundance of bone morphogenetic protein 2 (Bmp2), anti-Müllerian hormone (Amh), or growth differentiation factor 9 (Gdf9) between KO and control ovaries (Fig. 4D, G-H) at 8 months of age. BMP family members have been shown to be regulators of the FOXOs [23]. AMH is expressed in granulosa cells prior to their differentiation and until follicles are at the early antral stage [24,25]. GDF9 is expressed by the oocyte, and when knocked out, results in impaired follicular development [26,27].
Fig 4. Whole ovary mRNA abundance for Vegfa x pDmrt1-Cre female mice: Igf1r (A), Foxo3a (B), Fshr (C), Bmp2 (D), Sirt1 (E), Sirt6 (F), Amh (G) and Gdf9 (H).
Mean KO values (n = 2–6) are represented as fold changes ± SEM compared to control (n = 3–9) mean (set to 1). Results were significant when P < 0.05 and were represented with a *, and a † denoted data that tended to be significant (0.05 < P < 0.1).
VEGFA loss via the Amhr2-Cre promoter resulted in an increase in the time from mating to first parturition (three-months-of-age) and increased Amh mRNA abundance
We confirmed the effects on fertility by elimination of VEGFA in another mouse line, Vegfa x Amhr2-Cre. These females were collected at 3-months-of-age, earlier than the Vegfa x Dmrt1-Cre females (8 months). The same pan-VEGFA antibody was used to verify knockdown of VEGFA in ovaries of Amhr2-Cre;Vegfa-/- mice (Fig. 5B) compared to controls (Fig. 5A). Sections that were processed without primary antibody served as negative controls (Fig. 5C).
Fig 5. Immunohistochemistry with panVEGFA antibody to confirm VEGFA knockdown via Amhr2-Cre, female fertility and qPCR analysis: 200x magnification in an Amhr2-Cre;Vegfa control ovary (A) and an Amhr2-Cre;Vegfa-/- ovary (B).
Negative control ovaries were processed without primary antibody (C). For the Control x Control and KO x Control pairs in for mating to first parturition (D), n = 4. Ovarian plus oviductal weight (mg) is presented in KO vs control females (E) while follicle counts are presented in (F) and Amh mRNA is represented similarly (G), and n = 10 controls and 11 KO for each graph, respectively. Results were significant when P < 0.05 and were represented with a *.
On average Amhr2-Cre;Vegfa-/- mice females took 35 days longer to get pregnant during their first mating to control males (P < 0.003; n = 4) than control females mated to control males in the Vegfa x Amhr2-Cre mice (Fig. 5D) with no measurable change in litter size. Additionally, there was no difference in the combined ovarian and oviductal weight from Amhr2-Cre;Vegfa-/- (P = 0.69) mice compared to controls (Fig. 5E) at 3 months-of-age unlike the 8-month-old pDmrt1-Cre;Vegfa-/- females, suggesting that the loss of VEGFA isoforms may affect fertility in a progressive manner.
While there was no difference in follicle numbers on Amhr2-Cre;Vegfa-/- ovaries compared to controls (Fig. 5F) as well as no differences in mRNA abundance of the genes that were statistically significant in pDmrt1-Cre;Vegfa-/- ovaries (S2 Fig.), Amh was increased 2-fold in Amhr2-Cre;Vegfa-/- ovaries (P < 0.03) compared to controls (Fig. 5G).
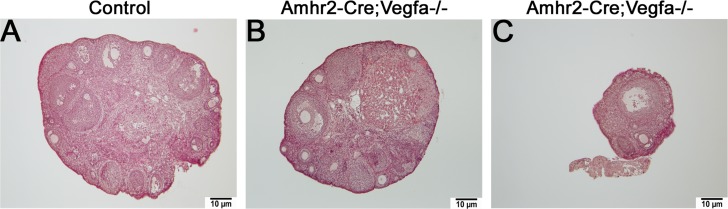
Ovarian size was variable for Amhr2-Cre;Vegfa-/- mice
Ovarian size varied greatly in Amhr2-Cre;Vegfa-/- mice despite no differences in ovarian weight or follicle number. Some of the Amhr2-Cre;Vegfa-/- mice KO ovaries were similar in size to controls (Fig. 6A, B); however, some appeared greatly reduced in size (Fig. 6C). The variability in ovarian size could be due to variability in the levels of Amhr2-Cre [28]. The variability in ovarian size coordinated with estradiol concentrations in the plasma without overall statistical significance: low estradiol concentrations were measured in females with smaller ovaries while the high concentrations were measured in females with larger ovaries.
Fig 6. Amhr2-Cre;Vegfa-/- and control ovary histology.
Hematoxylin-eosin staining of Amhr2-Cre;Vegfa-/- ovaries of variable sizes (B, C) compared to a control (A) at 100x magnification.
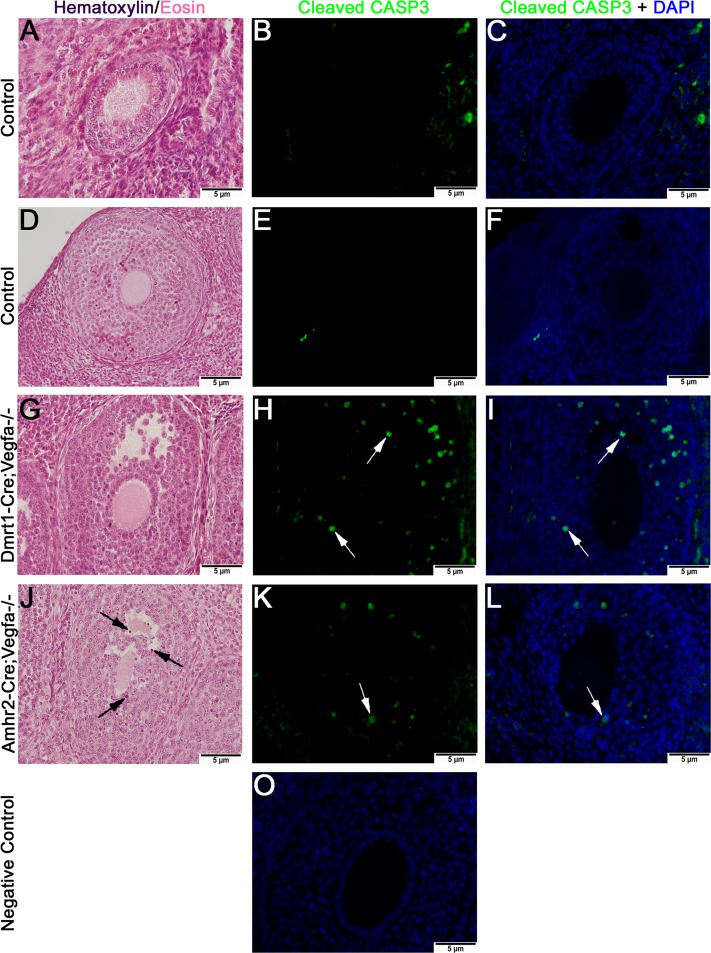
Cleaved Caspase 3-positive staining appeared more abundant in granulosa cells of pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- ovaries. Caspases are involved in apoptosis [29]. Positive staining for cleaved caspase 3 (CASP3) did not appear to be present in control ovaries (Fig. 7B-C, E-F). In both the pDmrt1-Cre;Vegfa-/- and the Amhr2-Cre;Vegfa-/- ovaries, there was cleaved CASP3-positive staining in granulosa cells not seen in controls, suggestive of granulosa cell apoptosis primarily seen in secondary follicles. Hematoxylin-eosin staining depicting the same fluorescently stained follicles is shown at 400x (Fig. 7A, D, G, J). While sections were not serial, the H-E sections were within 4–5 sections of the immunostained sections. Follicles on ovaries from Amhr2-Cre;Vegfa-/- females exhibited granulosa cells with darker H-E staining indicative of pyknosis.
Fig 7. Morphology of stage 4 follicles by hematoxylin-eosin (H-E) staining and cleaved CASP3-positive staining of same follicles.
H-E staining of follicles is shown at 400x for controls (A, D) and KOs (G, J). Green cleaved CASP3-positive staining is shown in follicles at 400x in controls (B, E) and in KOs (H, K). Cleaved CASP3-positive staining is shown in the same follicles but co-localized with DAPI at 400x in controls (C, F) and KOs (I,L). Negative controls were processed without primary antibody (M). Black arrows point to pyknotic granulosa cells on H-E stained follicles while arrows point to cleaved CASP3-positive granulosa cells.
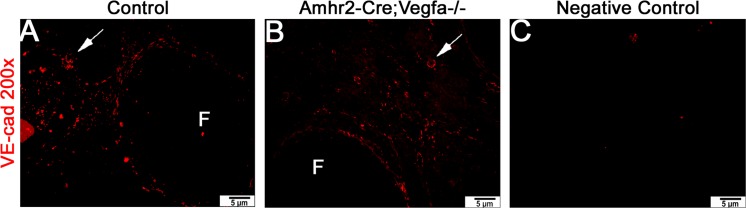
VE-cadherin-positive immunofluorescence did not appear different between control and pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- ovaries
Ovaries from both pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- mice were immunostained with an antibody to VE-cadherin, a known vascular marker. VE-cadherin-positive staining, demonstrating the presence of vasculature was at similar intensities and appeared to stain blood vessels similarly in both control and KO ovaries from both lines of mice Representative sections of Amhr2-Cre;Vegfa-/- and respective control ovaries are depicted (Fig. 8A, B). Similar results were seen in the testes of pDmrt1-Cre;Vegfa-/- males [17]. Negative controls had background reduced and were not treated with primary antibody (Fig. 8C).
Fig 8. VE-cadherin-positive immunostaining in representative ovaries: Amhr2-Cre;Vegfa-/- KO ovaries (B) compared to controls from the same mouse line (A) depicted at 200x magnification.
White arrows denote blood vessels. Negative controls were processed without primary antibody (C).
Discussion
The findings generated from the current study demonstrated that loss of VEGFA in granulosa (pDmrt1- and Amhr2-Cre) and germ cells (pDmrt1-Cre) resulted in subfertile females. While VEGFA has been previously implicated in fertility, this is the first time conditional loss of both pro- and antiangiogenic VEGFA isoforms has been accomplished within the granulosa cells of the ovary. The loss of VEGFA resulted in females taking longer to get pregnant, giving birth to fewer pups per litter, having reduced plasma estradiol, containing more apoptotic granulosa cells, resulting in fewer developing follicles and a tendency to have fewer corpora lutea. Additionally, the subfertility phenotype was supported in Amhr2-Cre;Vegfa-/- ovaries (3 months of age) and appeared more pronounced in the pDmrt1-Cre;Vegfa-/- mice which was presumably due to the later age of these mice at collection (8 months of age); however, we cannot ignore that the differences between the mouse lines could also be due to the different cell types suffering VEGFA isoform loss. This is currently being investigated in our laboratory through a granulosa cell-specific elimination of VEGFA. The phenotypes from both of these mouse lines support earlier findings in our laboratory regarding the importance of VEGFA isoforms in follicle recruitment and development [6,7]. Furthermore, in mice overexpressing antiangiogenic VEGFA165b that were collected between 2- and 4-months-of-age, there is a similar phenotype with half the litter size, fewer mature follicles, and fewer CL [30]. Thus, eliminating all VEGFA isoforms or overexpression of VEGFA165b in granulosa cells appears to reduce female fertility.
Both pDmrt1-Cre;Vegfa-/- (8-months-old) and Amhr2-Cre;Vegfa-/- females (3-months-old) were subfertile. The longer period leading to parturition in female KO mice compared to controls suggested a decreased rate of ovulation in the female KO mice or ovulation of oocytes that are not competent to be fertilized. Ha et al., showed that estrogen administration increases ovarian VEGFA expression and suggested that VEGFA improves the number and quality of oocytes [31]. In our study, the loss of VEGFA in pDmrt1-Cre;Vegfa-/- resulted in reduced estrogen in blood plasma which was presumably due to a reduced number of follicles developing. As mentioned previously, ovarian weight and plasma estradiol concentrations had a positive relationship in Vegfa x Amhr2-Cre females. The reduction in estrogen levels may also contribute to a reduction in the quality of oocytes. The aromatase knockout mouse, the estrogen β receptor knockout mouse, and the estrogen α receptor knockout mouse have demonstrated that estrogen enhances the number of follicles progressing to the early antral stage [32–35]. Furthermore, a positive relationship between VEGFA and E2 concentrations in gilt follicular fluid suggests a possible cause-and-effect relationship [36] with VEGFA stimulating estrogen production by increasing vascular permeability to cholesterol and increasing signal transduction pathways activating enzymes that are critical for estrogen production. One study showed that plasma 17β-estradiol concentration in rats treated with VEGFA120 and 164 were significantly higher than that in the control rats, indicating that VEGFA120 and 164 may indirectly stimulate the production of 17β-estradiol via granulosa cells [37].
In our study, plasma 17β-estradiol levels in the pDmrt1-Cre;Vegfa-/- were significantly lower than that in the controls. Our results indicated that loss of VEGFA in granulosa cells inhibits the production of 17β-estradiol in pDmrt1-Cre;Vegfa-/- and the granulosa cells from both pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- mice had increased staining for cleaved CASP3 as well as an increased number of pyknotic nuclei in granulosa cells. Previous studies have demonstrated that intrabursal injections of VEGFA trap (an inhibitor of VEGFA) resulted in reduced granulosa cell proliferation [38]. Thus, the in vivo phenotype of both of our KO mouse lines supports a role for VEGFA in granulosa cell survival and in enhancing its ability to produce steroid hormones such as estradiol promoting the viability of follicle development and progression.
McFee et al. previously demonstrated a role for VEGFA in primordial follicle activation, maturation and survival [6]. Rat ovaries treated transiently with VEGFA_164 were composed of fewer primordial follicles (stage 0) and more developing follicles (stages 1–4) than controls. Ovaries treated with VEGFAxxxB antibody to neutralize all antiangiogenic isoforms had fewer primordial and early primary follicles and more primary, transitional, and secondary follicles compared to controls (7). These data indicate that VEGFA proangiogenic isoforms promote follicle development, and in contrast, VEGFA antiangiogenic isoforms maintain primordial follicles in an arrested state. Removal of VEGFA antiangiogenic isoforms allows for increased progression of primordial follicles to the developing follicle pool [7]. Given previous findings in our laboratory with transient treatments in culture, we suspect that the number of primordial follicles is increased in the pDmrt1-Cre;Vegfa-/- females’ ovaries given the increase in Foxo3a mRNA even if differences were not detectable. At the same time, the differences may not be statistically relevant given the apparent increase in cleaved CASP3-positive granulosa cells, suggesting that some of the follicles that did advance in the pDmrt1-Cre;Vegfa-/- females regressed prematurely. VEGFA165 also promoted the primary-to-secondary follicle transition in bovine ovaries activated in vitro [39]. Furthermore, in vivo studies showed that inhibition of VEGFA and KDR can inhibit follicular development or prevent ovulation [40,41]. Thus, in addition to follicular development VEGFA also appears to affect ovulation in later stage preovulatory follicles.
In our study, ovarian weight in KO mice was reduced compared to that of control females. This suggests that loss of VEGFA in granulosa and germ cells within mouse ovaries affects ovarian size through reductions of follicle development and/or maturation. Another reason for the reduced ovarian size in the KO females is due to the reduced number of CL despite no change in progesterone. An in vivo study showed that the VEGFA angiogenic isoform promoted ovarian follicular angiogenesis leading to stimulatory effects on subsequent follicular development and numbers of oocytes ovulated in mature cycling rats [37]. Furthermore, inhibition of VEGFA and KDR suppressed follicular development and ovulation [40,41]. Presumably, the fewer CL in ovaries of pDmrt1-Cre;Vegfa-/- females may be caused by fewer antral follicles and subsequently fewer ovulations in those mice, ultimately resulting in smaller ovaries and a marked reduction in litter size compared to controls.
Another plausible theory for the reduction in ovarian weight in the KO females would be that loss of VEGFA in granulosa cells may reduce angiogenesis, the blood supply for oxygen, nutrients and influence the growth of the ovary and follicle maturation. However, this model utilizes the Cre-lox approach to eliminate VEGFA from granulosa cells and oocytes, cells that are avascular. Still, we investigated ovarian vasculature via immunostaining. Ovarian vasculature in two similar KO mouse models (pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/-) did not appear different from controls based on positive staining of VE-cadherin. Similarly, we did not see effects in pDmrt1-Cre;Vegfa-/- male mice where VEGFA was eliminated in Sertoli and germ cells [17].
To further characterize the reproductive phenotypes caused by VEGFA loss, we performed qPCR analysis of genes that are known regulators of ovarian function. FOXO3A is a key oocyte factor critical for suppressing primordial follicle activation [22]. Liu et al. reported that loss of Foxo3a expression in mice results in premature follicle activation [42]. Foxo3a mRNA was 4.8-fold higher in KO ovaries than controls, suggestive of potential suppression of follicle progression in these ovaries. Moreover, members of the FOXO family have been shown to regulate many vital cellular processes including metabolism [43,44]. Interestingly, it has been suggested that insulin and IGF-1 signaling inactivate the FOXOs in other tissues [45]. FOXO transcription factors are further implicated in this study by the 3.5-fold decrease in Igf1r mRNA in the ovaries of the females following VEGFA loss. Additionally, IGF1R expression has been demonstrated to be highest in large luteal cells of the bovine CL; thus, the disruption in follicle progression in our mice and subsequent reduction in CL number could be a result of the reduction in Igf1r [46] since whole ovaries were analyzed. It has also been demonstrated that FSH and IGF1 work synergistically in human, mouse and rat granulosa cells to stimulate AKT-dependent granulosa cell proliferation [47]. These findings, together with our observed reductions in mRNA abundance for Igf1r and Fshr, further support the apoptotic granulosa cells seen in ovaries of both pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- mice as well as the reduction in litter size of pDmrt1-Cre;Vegfa-/- females. Also striking is the 6.5-fold reduction in Sirt6 because of the direct relationship of sirtuins with FOXO3A and infertility [48]. It has been posited that AMH increases VEGFA; thus, the increase in Amh mRNA abundance in Amhr2-Cre;Vegfa-/- ovaries could be to atone for the elimination of VEGFA in follicular cells [49]. While we did not detect differences in follicle counts, ovarian weight or plasma estradiol concentrations when Vegfa was lost via the Amhr2-Cre promoter, we did see prolonged first mating period and variability in ovarian size as well as the presence of apoptotic granulosa cells. Thus, the increase in Amh mRNA could also be explained to be a block against the follicle’s sensitivity to FSH [49]. Again, these mice were harvested at 3-months-of-age, and the phenotype did not appear as progressed as in the pDmrt1-Cre;Vegfa-/- mice at 8 months of age.
In conclusion, we demonstrated that removal of VEGFA angiogenic and antiangiogenic isoforms in granulosa cells via the pDmrt1 promoter resulted in reduced ovarian weight, fewer CL and reduced estrogen concentrations (by 50%). Furthermore, the time period between mating and parturition was longer in both pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- mice than in controls, and pDmrt1-Cre;Vegfa-/- females gave birth to fewer pups. Additionally, pDmrt1-Cre;Vegfa-/- females had increased Foxo3a, reduced Igf1r, Fshr, and Sirt6 mRNA levels. These data are suggestive of primordial follicle arrest and reduced proliferation, further implicated by increased apoptotic granulosa cells as indicated by immunofluorescence that was observed in both pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- mice. The present data suggest that VEGFA is necessary for normal follicular development, estrogen synthesis, and ovulation. These reductions in ovarian function likely contributed to the increased interval from mating to parturition in both pDmrt1-Cre;Vegfa-/- and Amhr2-Cre;Vegfa-/- mice. Ultimately, these data support the concept that the loss of VEGFA results in subfertility by limiting follicle progression and subsequent ovulation and that this phenotype appears to be more dramatic as the female progress in age as demonstrated by the 3-month Amhr2-Cre;Vegfa-/- and 8-month pDmrt1-Cre;Vegfa-/- phenotypes or due to differences in cell-specific VEGFA loss.
Supporting Information
(PPTX)
Mean KO values (n = 3–5) are represented as fold changes ± SEM compared to control (n = 4) mean (set to 1).
(TIF)
Acknowledgments
We thank Renata Spuri Gomes for her efforts in refining the Cre-Lox system schematic and Dr. John Davis for his careful reading of this manuscript. We also thank John Essink for his assistance in staining and imaging ovarian sections.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by NIH/NICHD HD051979 and a contribution from the University of Nebraska Agricultural Research Division, Lincoln, Nebraska.
References
- 1. McFee RM, Cupp AS (2013) Vascular contributions to early ovarian development: potential roles of VEGFA isoforms. Reprod Fertil Dev 25: 333–342. 10.1071/RD12134 [DOI] [PubMed] [Google Scholar]
- 2. Byrne AM, Bouchier-Hayes DJ, Harmey JH (2005) Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9: 777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K (2006) The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci 63: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caires KC, de Avila JM, Cupp AS, McLean DJ (2012) VEGFA family isoforms regulate spermatogonial stem cell homeostasis in vivo. Endocrinology 153: 887–900. 10.1210/en.2011-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E (1993) Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest 91: 2235–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McFee RM, Artac RA, McFee RM, Clopton DT, Longfellow Smith RA, et al. (2009) Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biol Reprod 81: 966–977. 10.1095/biolreprod.109.078071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Artac RA, McFee RM, Smith RA, Baltes-Breitwisch MM, Clopton DT, et al. (2009) Neutralization of vascular endothelial growth factor antiangiogenic isoforms is more effective than treatment with proangiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary. Biol Reprod 81: 978–988. 10.1095/biolreprod.109.078097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9: 669–676. [DOI] [PubMed] [Google Scholar]
- 9. Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25: 581–611. [DOI] [PubMed] [Google Scholar]
- 10. Roberts AE, Arbogast LK, Friedman CI, Cohn DE, Kaumaya PT, et al. (2007) Neutralization of endogenous vascular endothelial growth factor depletes primordial follicles in the mouse ovary. Biol Reprod 76: 218–223. [DOI] [PubMed] [Google Scholar]
- 11. Bott RC, Clopton DT, Fuller AM, McFee RM, Lu N, et al. (2010) KDR-LacZ-expressing cells are involved in ovarian and testis-specific vascular development, suggesting a role for VEGFA in the regulation of this vasculature. Cell Tissue Res 342: 117–130. 10.1007/s00441-010-1038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerber HP, Hilan KJ, Ryan AM, Kowalski J, Keller GA, et al. (1999) VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159. [DOI] [PubMed] [Google Scholar]
- 13. Boyer A, Dornan S, Daneau I, Lussier J, Silversides DW (2002) Conservation of the function of DMRT1 regulatory sequences in mammalian sex differentiation. Genesis 34: 236–243. [DOI] [PubMed] [Google Scholar]
- 14. Lei N, Hornbaker KI, Rice DA, Karpova T, Agbor VA, et al. (2007) Sex-specific differences in mouse DMRT1 expression are both cell type- and stage-dependent during gonad development. Biol Reprod 77: 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krentz AD, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, et al. (2011) DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol 356: 63–70. 10.1016/j.ydbio.2011.05.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, et al. (2008) A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev 75: 1154–1162. 10.1002/mrd.20858 [DOI] [PubMed] [Google Scholar]
- 17. Lu N, Sargent KM, Clopton DT, Pohlmeier WE, Brauer VM, et al. (2013) Loss of Vascular Endothelial Growth Factor A (VEGFA) Isoforms in the Testes of Male Mice Causes Subfertility, Reduces Sperm Numbers, and Alters Expression of Genes That Regulate Undifferentiated Spermatogonia. Endocrinology 154: 4790–4802. 10.1210/en.2013-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhat GK, Sea TL, Olatinwo MO, Simorangkir D, Ford GD, et al. (2006) Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis-related genes in the mouse. J Androl 27: 302–310. [DOI] [PubMed] [Google Scholar]
- 19. Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, et al. (1996) Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 10: 903–918. [DOI] [PubMed] [Google Scholar]
- 20. Li X, Kazgan N (2011) Mammalian sirtuins and energy metabolism. Int J Biol Sci 7: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, et al. (2003) Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A 100: 10794–10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA (2003) Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301: 215–218. [DOI] [PubMed] [Google Scholar]
- 23. Richards JS, Sharma SC, Falender AE, Lo YH (2002) Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol 16: 580–599. [DOI] [PubMed] [Google Scholar]
- 24. Sadeu JC, Adriaenssens T, Smitz J (2008) Expression of growth differentiation factor 9, bone morphogenetic protein 15, and anti-Mullerian hormone in cultured mouse primary follicles. Reproduction 136: 195–203. 10.1530/REP-08-0065 [DOI] [PubMed] [Google Scholar]
- 25. Durlinger AL, Visser JA, Themmen AP (2002) Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction 124: 601–609. [DOI] [PubMed] [Google Scholar]
- 26. McGrath SA, Esquela AF, Lee SJ (1995) Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol 9: 131–136. [DOI] [PubMed] [Google Scholar]
- 27. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, et al. (1996) Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383: 531–535. [DOI] [PubMed] [Google Scholar]
- 28. Hernandez Gifford JA, Hunzicker-Dunn ME, Nilson JH (2009) Conditional deletion of beta-catenin mediated by Amhr2cre in mice causes female infertility. Biol Reprod 80: 1282–1292. 10.1095/biolreprod.108.072280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, et al. (1998) Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med 187: 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu Y, Seager M, Osman A, Castle-Miller JS, Bevan HS, et al. (2012) Ovarian VEGF165b expression regulates follicular development, corpus luteum function and fertility. Reproduction Epub. [DOI] [PMC free article] [PubMed]
- 31. Ha CS, Joo BS, Kim SC, Joo JK, Kim HG, et al. (2010) Estrogen administration during superovulation increases oocyte quality and expressions of vascular endothelial growth factor and nitric oxide synthase in the ovary. J Obstet Gynaecol Res 36: 789–795. 10.1111/j.1447-0756.2010.01212.x [DOI] [PubMed] [Google Scholar]
- 32. Fisher CR, Graves KH, Parlow AF, Simpson ER (1998) Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A 95: 6965–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, et al. (1998) Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A 95: 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, et al. (1999) Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology 140: 2733–2744. [DOI] [PubMed] [Google Scholar]
- 35. Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, et al. (2003) Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod 68: 1736–1741. [DOI] [PubMed] [Google Scholar]
- 36. Mattioli M, Barboni B, Turriani M, Galeati G, Zannoni A, et al. (2001) Follicle activation involves vascular endothelial growth factor production and increased blood vessel extension. Biol Reprod 65: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 37. Iijima K, Jiang JY, Shimizu T, Sasada H, Sato E (2005) Acceleration of follicular development by administration of vascular endothelial growth factor in cycling female rats. J Reprod Dev 51: 161–168. [DOI] [PubMed] [Google Scholar]
- 38. Abramovich D, Irusta G, Parborell F, Tesone M (2010) Intrabursal injection of vascular endothelial growth factor trap in eCG-treated prepubertal rats inhibits proliferation and increases apoptosis of follicular cells involving the PI3K/AKT signaling pathway. Fertil Steril 93: 1369–1377. 10.1016/j.fertnstert.2009.01.127 [DOI] [PubMed] [Google Scholar]
- 39. Yang MY, Fortune JE (2007) Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol Reprod Dev 74: 1095–1104. [DOI] [PubMed] [Google Scholar]
- 40. Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, et al. (2001) Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J Clin Endocrinol Metab 86: 768–772. [DOI] [PubMed] [Google Scholar]
- 41. Zimmermann RC, Xiao E, Bohlen P, Ferin M (2002) Administration of antivascular endothelial growth factor receptor 2 antibody in the early follicular phase delays follicular selection and development in the rhesus monkey. Endocrinology 143: 2496–2502. [DOI] [PubMed] [Google Scholar]
- 42. Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, et al. (2007) Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 134: 199–209. [DOI] [PubMed] [Google Scholar]
- 43. van der Vos KE, Coffer PJ (2008) FOXO-binding partners: it takes two to tango. Oncogene 27: 2289–2299. 10.1038/onc.2008.22 [DOI] [PubMed] [Google Scholar]
- 44. Rached MT, Kode A, Silva BC, Jung DY, Gray S, et al. (2010) FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest 120: 357–368. 10.1172/JCI39901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakae J, Oki M, Cao Y (2008) The FoxO transcription factors and metabolic regulation. FEBS Lett 582: 54–67. [DOI] [PubMed] [Google Scholar]
- 46. Berisha B, Meyer HH, Schams D (2010) Effect of prostaglandin F2 alpha on local luteotropic and angiogenic factors during induced functional luteolysis in the bovine corpus luteum. Biol Reprod 82: 940–947. 10.1095/biolreprod.109.076752 [DOI] [PubMed] [Google Scholar]
- 47. Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, et al. (2013) IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol 27: 511–523. 10.1210/me.2012-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tao R, Xiong X, DePinho RA, Deng CX, Dong XC (2013) FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J Biol Chem 288: 29252–29259. 10.1074/jbc.M113.481473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, et al. (2011) Anti-Mullerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril 96: 1246–1251 e1241. 10.1016/j.fertnstert.2011.08.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX)
Mean KO values (n = 3–5) are represented as fold changes ± SEM compared to control (n = 4) mean (set to 1).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.