Abstract
Objective
There is little information about vaccine schedule compliance in very-low-birth-weight infants in developing countries. The aim of the study was to describe the compliance with the vaccine schedule among this population in Lima, Peru.
Patients and Methods
We conducted a prospective cohort study in four hospitals in Lima in infants with a birth-weight of less than 1500g, followed from birth up to 12 months of age every 2 weeks. The date and age at administration of each vacccine was recorded.
Results
222 infants were enrolled. The median birth-weight was 1,250g (range 550-1,499g) and the median gestational age was 30.0 weeks (range 23-37 weeks). The mean age for the first pentavalent (DPT, Hib, HepB) and oral polio vaccine administration was 4.3 ± 1.4 months in infants with a birth-weight of <1000g vs. 3.1 ± 1.0 in infants with a birth-weight 1000- 1500g (p<0.001); 4.1 ± 0.9 vs. 3.3 ± 1.1 for rotavirus (p<0.05); and 5.1 ± 2.1 vs. 4.3 ± 1.8 for the 7-valent pneumococcal conjugated vaccine. Only 35% had received the three doses of oral polio and pentavalent vaccine by seven months, although by nine months 81% had received these vaccines.
Conclusions
Vaccination of very-low-birth-weight infants in Peru is significantly delayed, especially in infants with a birth-weight of <1000g. Urgent educational interventions targeting physicians and nurses should be implemented in order to improve vaccination rates and timing in these high risk populations.
Keywords: Vaccines, Very Low Birth Weight Infant, Developing Countries
INTRODUCTION
Premature infants are at increased risk of infectious diseases, including vaccine-preventable diseases (1-3), due to multiple factors like decreased IgG concentrations and impaired innate immunity (4-8). The number of reported cases of pertussis and pertussis-related hospitalizations are higher in low birth-weight infants (9). Pneumococcal diseases also are more common and severe in premature infants (1). Timely and complete immunization is extremely important in these infants.
Current recommendations state that premature infants should be immunized at the same chronological age as other infants, as long as they are clinically stable. The only exceptions for this rule are the dose of the hepatitis B vaccine given at birth and the BCG, which is only used in selected countries. No corrections should be made regarding gestational age or birth-weight when planning the immunization of premature infants (3,10).
Multiple studies in developed countries have shown that premature infants are immunized with a significant delay and it is more pronounced in infants with lower birth-weight (11-14). The most important reasons for this delay are the concerns by parents and providers, including pediatricians, about the immunogenicity and safety of vaccines in preterm infants (2).
Information on this topic in developing countries is lacking. Their immunization programs are typically less developed and access to medical care is more difficult. Also, the education level of the population is lower which could lead to a greater refusal of vaccination in premature infants. Accurate information about the magnitude of these problems is necessary to justify focused educational intervention for both families and medical personnel. To answer this question we conducted a prospective cohort study to describe the compliance with the vaccine schedule among very low birth-weight (VLBW) infants in Lima, Peru.
METHODS
We conducted a prospective cohort study in four hospitals in the city of Lima: Hospital Nacional Edgardo Rebagliati Martins, Instituto Nacional Marteno Perinatal, Hospital Nacional Guillermo Almenara Irigoyen and Hospital Nacional Madre Nino San Bartolome. All infants with a birth-weight <1500g and gestational age <37 weeks that were born or transferred to one of these hospitals were invited to participate. Infants who could not complete the one-year follow up due to social factors (e.g. no home phone or cell phone, or infants returning to a hometown outside of the city of Lima), had severe congenital malformations or were older than 6 months at hospital discharge were excluded. The patients were followed from the time of discharge until 12 months of age by clinic visits or phone calls to the parents every 2 weeks. This study was part of a larger cohort study aimed at determining the incidence of respiratory syncytial virus in premature infants during their first year of life (15).
We recorded the age at administration of each immunization during the first year of life. According to the Peruvian vaccination calendar the diphtheria-pertussis-tetanus (DPT), Haemophilus influenza type b (Hib),Hepatitis B (HepB), and oral polio vaccine (OPV) is administered at 2, 4 and 6 months with two boosters of the DPT vaccine at 18 and 48 months. The 7-valent conjugated pneumococcal vaccine (7vCPV) is given in a 2 + 1 schedule, the first two doses at 2 and 4 months (in some hospitals at 3 and 5 months) and a booster at 12 months. The monovalent rotavirus vaccine is given at 2 and 4 months. We evaluated the compliance for the first three doses of the DPT-Hib-HepB (pentavalent vaccine) and OPV and the first two doses of the 7vCPV and the rotavirus vaccine. We selected these vaccines because they are scheduled to be administered before 7 months of age; this allowed us to have a follow up of 5 months to evaluate the delay in the immunizations. BCG vaccine was excluded because in premature infants it is given based on weight, rather than age, and this was not recorded in all cases. The measles, mumps and rubella vaccine and the third dose of the 7vCPV was excluded from the analysis because they are administrated at 12 months and the delay of administration could not be measured accurately since the follow up ended at this age.
Differences in proportions (vaccination rates) were calculated using Fisher’s exact test. We tested differences in the age of vaccine administration between groups of birth weight (<1000g vs. 1000-1500g) and gestational age (<32 weeks vs 32 or more weeks) using Two-sample Wilcoxon rank-sum (Mann-Whitney) test. A p < 0.05 was considered significant. Statistical analysis was performed using STATA 11 (StataCorp LP, College Station, Texas, United States). The study was reviewed and approved by the Institutional Ethics Review Boards of Universidad Peruana Cayetano Heredia and of each of the participating hospitals.
RESULTS
Characteristics of study population
From March 2009 to March 2010, 335 infants were assessed for eligibility. Of these, 222 (66.3%) were enrolled; 21 (6.3%) parents did not consent to participate, 57(17%) could not complete the follow up and 35 (10.4%) had some other exclusion criteria (4 congenital malformation, 11 transferred to other hospital before discharge, 1 older than 6 months of age at hospital discharge and 15 with other reasons). On-hundred and ninety-eight children completed the study, 14 were lost during follow up, and 10 children died. We only included the infants that completed the follow up in the analysis.
Forty-eight infants had a birth-weight less than 1,000g and 157 had a birth-weight between 1,000g and 1,500g. Male infants represented 44.6% of the study population. The median birth-weight was 1,250g (range 550-1,499g), the median gestational age was 30.0 weeks (range 23-37 weeks) and 36.5% of infants were small for gestational age. The mean duration of hospitalization was 82.1 days (range 36-153) for infants with a birth-weight less than 1 000g and 42.7 days (range 8-126) for infants with a birth-weight of 1000-1500g.
Vaccination rate
The first doses of the DPT-Hib-HepB and OPV were the only immunizations that were administered to all infants. The second dose of the DPT-Hib-HepB and OPV and the first dose of the 7vCPV were administered to 97.5% of infants. The rotavirus vaccine rate was the lowest among the analyzed immunizations with only 74.2% and 62.1% of infants receiving the first and second dose, respectively. Infants with a birth-weight of less than 1000g were less likely to be immunized compared to infants with a birth-weight of 1000g-1500g. This difference was statistically significant for all immunizations except for the second dose of the rotavirus vaccine (p=0.11) (Table 1). Also, infants with a gestational age of less than 32 weeks were less likely to be immunized than those born at 32 weeks or more.
Table 1.
Vaccination rates by birth-weight group in premature infants
| Total (N=198) | <1000g (N=41) | 1000-1500g (N=157) |
P** | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| DPT-Hib-HepB* 1 | 198 | 100.0 | 41 | 100.0 | 157 | 100.0 | - |
| DPT-Hib-HepB* 2 | 193 | 97.5 | 38 | 92.7 | 155 | 98.7 | 0.028 |
| DPT-Hib-HepB* 3 | 185 | 93.4 | 33 | 80.5 | 152 | 96.8 | <0.001 |
| Oral Polio 1 | 198 | 100.0 | 41 | 100.0 | 157 | 100.0 | - |
| Oral Polio 2 | 193 | 97.5 | 38 | 92.7 | 155 | 98.7 | 0.28 |
| Oral Polio 3 | 185 | 93.4 | 33 | 80.5 | 152 | 96.8 | <0.001 |
|
7-valent
Pneumococcal 1 |
193 | 97.5 | 37 | 90.2 | 156 | 99.4 | 0.001 |
|
7-valent
Pneumococcal 2 |
184 | 92.9 | 33 | 80.5 | 151 | 96.4 | 0.001 |
|
Monovalent
Rotavirus 1 |
147 | 74.2 | 23 | 56.1 | 124 | 79.0 | 0.003 |
|
Monovalent
Rotavirus 2 |
123 | 62.1 | 21 | 51.2 | 102 | 65.0 | 0.106 |
Diphtheria-pertussis-tetanus, Haemophilus influenza type b and Hepatitis B vaccine
For the comparison between birth weight groups
Vaccination age
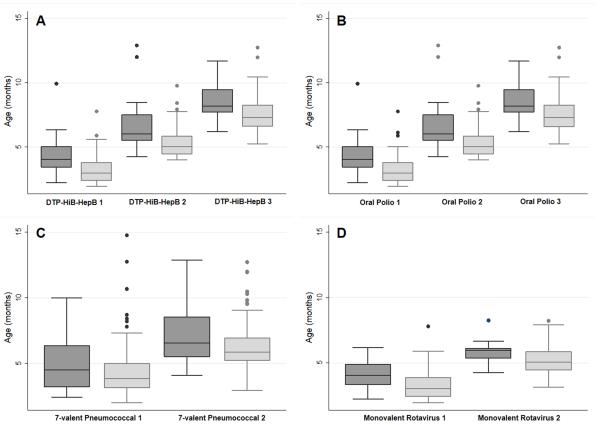
The mean age for the first DPT-Hib-HepB and OPV administration was 3.4±1.2 months; 5.5±1.3 for the second and 7.7±1.3 for the third administration. The first 7vCPV was administered at a mean age of 4.4 ± 1.9 months and the second at 6.5 ± 1.8 months. Mean age at the first rotavirus vaccination was 3.4±1.1 months and 5.3±1 months for the second dose. Figure 1 show the ages at vaccination according to birth-weight. Infants with a birth-weight less than 1000g were immunized at significantly higher ages (p<0.05), except in the case of the first 7vCPV (p=0.57). Infants with a gestational age of less than 32 weeks were immunized at latter ages than those born at 32 weeks or more. None of the newborns received any of the evaluated vaccination prior to discharge.
Figure 1.
Age of administration of (A) the three doses of the pentavalent vaccine (DTP-HiB-HepB); (B) the three doses of the oral polio vaccine; (C) the two doses of the 7-valent conjugated pneumococcal vaccine; (D) the two doses of the monovalent rotavirus vaccine. Dark grey boxes: <1000g; Light grey boxes: 1000-1500g.
Completion of programed vaccine doses
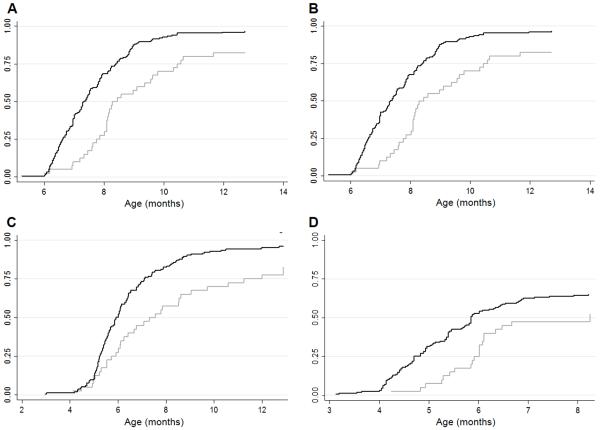
At 7 months of age only 34.3% and 35.4% of infants had received three doses of the DPT-Hib-HepB and OPV, respectively. By 9 months, 80.8% had received the three doses of the DPT-Hib-HepB and OPV. The 7vCPV coverage was slightly higher with 84.3% of infants vaccinated with two doses by 9 months of age. The percentage of infants vaccinated against rotavirus was the lowest; only 61.6% had received two doses by 9 months. Figure 2 shows the cumulative incidences for the administration of the primary course of the DPT-Hib-HepB, OPV, 7vCPV and rotavirus vaccine. Vaccine coverage of infants with a birth-weight less than 1000g was lower than infants with a birth-weight of 1000-1500g throughout the entire follow up. The coverage in infants with a gestational age less than 32 weeks was lower than infants of 32 weeks or more.
Figure 2.
Cumulative administration of (A) third dose of the pentavalent vaccine (DTP-HiB-HepB); (B) third dose of the oral polio vaccine; (C) second dose of the 7-valent conjugated pneumococcal vaccine; (D) second dose of the monovalent rotavirus vaccine. Light grey line: <1000g; Dark grey line: 1000-1500g.
DISCUSION
We found that very-low-birth-weight infants are immunized with a delay. The mean age for vaccination was more than one month after the recommended age; this is considered the grace period to define the upper limit for age-appropriate vaccination (11,14,16). By 7 months of age all of the evaluated vaccines should have been administered; nevertheless vaccination rates at this age were less than 70%. Most infants received the third dose of the DPT-Hib-HepB and OPV between 7 and 10 months of age, and the second dose of the 7vCPV between 5 and 9 month of age. The delay in vaccination was more pronounced in infants with lower birth-weight or lower gestational age.
Studies conducted in developed countries have obtained similar results (11-14). Langkamp et al found that low birth-weight infants received the first two doses of polio and the first three doses of DPT at significantly older ages than normal birth-weight infants (12). The same was observed in Switzerland and France (13,17). More recently Batra et al. found that infants with a birth-weight of less than 1500g were less likely to be immunized at an appropriate age compared to normal birth-weight infants. Additionally, extremely-low-birth-weight infants (<1000g) had lower age appropriate immunization rates than larger premature infants (1000-1500g) (11). On the other hand Davis et al concluded than premature infants were vaccinated at levels approaching that of the general population. However, when considering only VLBW infants the immunization levels were significantly lower than normal birth-weight infants (14). This delay is more pronounced during the first year of life and reduced after this (11,12,14). Extremely-low-birth-weight infants are the exception since they continue to receive immunizations at significantly higher ages after 12 months (14).
There are concerns regarding the effectiveness and safety of immunizations in VLBW (2), despite multiple studies showing that immunizations are both safe and effective in VLBW infants (2,8,18). VLBW infants may have lower antibody responses initially, but most of them achieve protective antibody titers after vaccination (19-26). Even in extremely premature infants, where an initial appropriate response is not achieved by all, protective antibody concentrations are obtained by 9-12 months (2,27-29). Regarding safety, the severity and frequency of most vaccine-related adverse events in VLBW infants does not differ from that observed in normal birth-weight infants (2,3,8).
Only apnea associated with the DPT vaccine has been reported consistently, yet this is still widely debated and seems to only affect patients with the most severe illnesses (2,30-33). Most vaccination centers in Lima are not managed by neonatologist but instead by nurses that are not familiarized with VLBW vaccination guidelines. Also most parents are not informed of VLBW vaccination schedule and may have safety concerns. These two factors lead to unjustified immunization postponement.
It is recommended that vaccination schedule should be started during hospitalization to improve long term coverage of preterm infants. (10,34) However this is not always followed, as showed in a recent study. (35) In the evaluated NICUs the standard practice is to start immunizations after the patient is discharged which could be after 2 months for VLBW infants. This practice also contribute of the delay in immunizations.
More premature newborns are surviving the neonatal period in developing countries; this brings a new challenge to pediatricians who are not used to managing this population. Also, infectious diseases are more common and health services less available. In these settings vaccination is an essential tool against transmissible diseases in these countries. A previous study from South America concluded that premature infants were immunized with a significant delay and this delay was considered unjustifiable 76% of the time. (36).
Most studies in this topic were made before the implementation of the pneumococcal and rotavirus vaccine (11-14), so the compliance for these vaccines has not been well studied. Because of their recent inclusion in the immunization calendar we expected to find lower immunization rates and a more pronounced delay in administration. This was true for the rotavirus vaccine but not for the 7vCPV which achieved immunizations levels near those of older vaccines like the DPT-Hib-HepB. The reasons for this are unclear but it may be due to a high population awareness of the severity of the diseases prevented by the vaccine. When the pneumococcal vaccine was introduced it was accompanied by an aggressive advertising campaign, this caused parents to demand the vaccination of their children. Advertising campaigns focused on improving public awareness may be an effective method to increase vaccination rate in VLBW infants.
The main limitation of this study is its low number of subjects. Unlike previous studies (11,12,14) we didn’t try to obtain national representative samples. These studies were made using national registries, which are not available or are incomplete in Peru, and used retrospective cohorts. The prospective design allowed us to get accurate and reliable information. Unfortunately this approach dictated that a limited the number of patients could be followed. Another limitation is the lack of a comparison group of term neonates. Because our cohort is part of a larger cohort focusing on RSV infection in VLBW infants we couldn’t compare it to normal birth-weight infants. However, because of the reasons exposed above, we believe our results suggests a delay in immunizations in VLBW infants. Unfortunately, these limitations are difficult to assess in a country like Peru due to the lack of systematic registries of vaccinations. Future studies should address these limitations.
CONCLUSION
VLBW infants are vaccinated with a delay despite current guidelines which advise following the same vaccination schedule used in normal birth-weight infants. This is true for all evaluated vaccines but the delay is more prominent in the rotavirus vaccine. Extreme low birth-weight infants are vaccinated at even later ages than patients with a birth-weight from 1000-1500g. Urgent educational interventions targeting physicians and nurses, as well as the general population, should be implemented in order to improve vaccination rates and timing in these high risk populations.
ACKNLOWDEGMENTS
This study was funded by a Research Grant from Abbott Laboratories to Dr. Ochoa. We will like to thank the research nurses Consuelo Benites (Rebagliati), Gregoria Torvisco and Saida Ocrospoma (Maternidad), Virginia Loo and Martha Torres (Almenara), Patricia Segovia and Socorro Torres (San Bartolome); and the study monitors Karen Bazalar and Erika Bazalar.
The authors thank the patients and their families for participation in this study.
Funding source: This study was funded by a Research Grant from Abbott Laboratories.
Footnotes
Conflict of interests: There are no competing interests for any of the authors.
REFERENCES
- 1.Bonhoeffer J, Siegrist CA, Heath PT. Immunisation of premature infants. Arch. Dis. Child. 2006;91(11):929–35. doi: 10.1136/adc.2005.086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito S, Serra D, Gualtieri L, et al. Vaccines and preterm neonates: why, when, and with what. Early Hum. Dev. 2009;85(10 Suppl):S43–45. doi: 10.1016/j.earlhumdev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Saari TN, American Academy of Pediatrics Committee on Infectious Diseases. American Academy of Pediatrics Committee on Infectious Diseases Immunization of preterm and low birth weight infants. Pediatrics. 2003;112(1 Pt 1):193–8. doi: 10.1542/peds.112.1.193. [DOI] [PubMed] [Google Scholar]
- 4.Landor M. Maternal-fetal transfer of immunoglobulins. Ann. Allergy Asthma Immunol. 1995;74(4):279–283. [PubMed] [Google Scholar]
- 5.Chiou YB, Blume-Peytavi U. Stratum corneum maturation. A review of neonatal skin function. Skin Pharmacol. Physiol. 2004;17(2):57–66. doi: 10.1159/000076015. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez KM, Moss RL. Necrotizing enterocolitis. Clin. Perinatol. 2012;39(2):387–401. doi: 10.1016/j.clp.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Chang AB, Widdicombe JG. Cough throughout life: children, adults and the senile. Pulm. Pharmacol. Ther. 2007;20(4):371–82. doi: 10.1016/j.pupt.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Esposito S, Fumagalli M, Principi N. Immunogenicity, safety and tolerability of vaccinations in premature infants. Expert Rev. Vaccines. 2012;11(10):1199–209. doi: 10.1586/erv.12.93. [DOI] [PubMed] [Google Scholar]
- 9.Langkamp DL, Davis JP. Increased risk of reported pertussis and hospitalization associated with pertussis in low birth weight children. J. Pediatr. 1996;128(5 Pt 1):654–9. doi: 10.1016/s0022-3476(96)80131-4. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics, Committee on Infectious Diseases . Red Book. 29th Edition 2012. Immunization in special circumstances. Preterm and low birth weight infants. [Google Scholar]
- 11.Batra JS, Eriksen EM, Zangwill KM, et al. Evaluation of vaccine coverage for low birth weight infants during the first year of life in a large managed care population. Pediatrics. 2009;123(3):951–8. doi: 10.1542/peds.2008-0231. [DOI] [PubMed] [Google Scholar]
- 12.Langkamp DL, Hoshaw-Woodard S, Boye ME, et al. Delays in receipt of immunizations in low-birth-weight children: a nationally representative sample. Arch. Pediatr. Adolesc. Med. 2001;155(2):167–72. doi: 10.1001/archpedi.155.2.167. [DOI] [PubMed] [Google Scholar]
- 13.Tillmann BU, Tillmann HC, Nars PW, et al. Vaccination rate and age of premature infants weighing <1500 g: a pilot study in north-western Switzerland. Acta Paediatr. 2001;90(12):1421–6. doi: 10.1080/08035250152708842. [DOI] [PubMed] [Google Scholar]
- 14.Davis RL, Rubanowice D, Shinefield HR, et al. Centers for Disease Control and Prevention Vaccine Safety Datalink Group Immunization levels among premature and low-birth-weight infants and risk factors for delayed up-to-date immunization status. JAMA. 1999;282(6):547–53. doi: 10.1001/jama.282.6.547. [DOI] [PubMed] [Google Scholar]
- 15.Ochoa T, Bautista R, Dávila C, et al. Respiratory syncytial virus-associated hospitalizations in premature infants in Lima, Peru. doi: 10.4269/ajtmh.13-0648. Submited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez SR, Parrott JS, Lauderdale DS, et al. On-time immunization rates among children who enter Chicago public schools. Pediatrics. 2004;114(6):e741–747. doi: 10.1542/peds.2004-1053. [DOI] [PubMed] [Google Scholar]
- 17.Pinquier D, Adde-Michela C, Ploin D, et al. Vaccination rate of premature infants at 6 and 24 months of age: a pilot study. Arch. Pediatr. 2009;16(12):1533–9. doi: 10.1016/j.arcped.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Baxter D. Vaccine responsiveness in premature infants. Hum. Vaccin. 2010;6(6):506–11. doi: 10.4161/hv.6.6.12083. [DOI] [PubMed] [Google Scholar]
- 19.Faldella G, Alessandroni R, Magini GM, et al. The preterm infant’s antibody response to a combined diphtheria, tetanus, acellular pertussis and hepatitis B vaccine. Vaccine. 1998;16(17):1646–9. doi: 10.1016/s0264-410x(98)00060-7. [DOI] [PubMed] [Google Scholar]
- 20.Esposito S, Faldella G, Giammanco A, et al. Long-term pertussis-specific immune responses to a combined diphtheria, tetanus, tricomponent acellular pertussis and hepatitis B vaccine in pre-term infants. Vaccine. 2002;20(23-24):2928–32. doi: 10.1016/s0264-410x(02)00230-x. [DOI] [PubMed] [Google Scholar]
- 21.Slack MH, Cade S, Schapira D, et al. DT5aP-Hib-IPV and MCC vaccines: preterm infants’ response to accelerated immunisation. Arch. Dis. Child. 2005;90(4):338–41. doi: 10.1136/adc.2004.052720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adenyi-Jones SC, Faden H, Ferdon MB, et al. Systemic and local immune responses to enhanced-potency inactivated poliovirus vaccine in premature and term infants. J. Pediatr. 1992;120(5):686–9. doi: 10.1016/s0022-3476(05)80228-8. [DOI] [PubMed] [Google Scholar]
- 23.D’Angio CT, Maniscalco WM, Pichichero ME. Immunologic response of extremely premature infants to tetanus, Haemophilus influenzae, and polio immunizations. Pediatrics. 1995;96(1 Pt 1):18–22. [PubMed] [Google Scholar]
- 24.Lumbiganon P, Kowsuwan P, Lumbiganon P, et al. Comparison of immunogenicity of hepatitis B vaccine between low and normal birth weight infants. Asian Pac. J. Allergy Immunol. 1992;10(1):61–3. [PubMed] [Google Scholar]
- 25.Esposito S, Pugni L, Bosis S, et al. Immunogenicity, safety and tolerability of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months post-natally to pre- and full-term infants. Vaccine. 2005;23(14):1703–8. doi: 10.1016/j.vaccine.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Omenaca F, Sarlangue J, Szenborn L, et al. Safety, reactogenicity and immunogenicity of the human rotavirus vaccine in preterm European Infants: a randomized phase IIIb study. Pediatr. Infect. Dis. J. 2012;31(5):487–93. doi: 10.1097/INF.0b013e3182490a2c. [DOI] [PubMed] [Google Scholar]
- 27.D’Angio CT, Heyne RJ, O’Shea TM, et al. Heptavalent pneumococcal conjugate vaccine immunogenicity in very-low-birth-weight, premature infants. Pediatr. Infect. Dis. J. 2010;29(7):600–6. doi: 10.1097/INF.0b013e3181d264a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruggeberg JU, Collins C, Clarke P, et al. Immunogenicity and induction of immunological memory of the heptavalent pneumococcal conjugate vaccine in preterm UK infants. Vaccine. 2007;25(2):264–71. doi: 10.1016/j.vaccine.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Kristensen K, Gyhrs A, Lausen B, et al. Antibody response to Haemophilus influenzae type b capsular polysaccharide conjugated to tetanus toxoid in preterm infants. Pediatr. Infect. Dis. J. 1996;15(6):525–9. doi: 10.1097/00006454-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Carbone T, McEntire B, Kissin D, et al. Absence of an increase in cardiorespiratory events after diphtheria-tetanus-acellular pertussis immunization in preterm infants: a randomized, multicenter study. Pediatrics. 2008;121(5):e1085–1090. doi: 10.1542/peds.2007-2059. [DOI] [PubMed] [Google Scholar]
- 31.Botham SJ, Isaacs D, Henderson-Smart DJ. Incidence of apnoea and bradycardia in preterm infants following DTPw and Hib immunization: a prospective study. J. Paediatr. Child Health. 1997;33(5):418–21. doi: 10.1111/j.1440-1754.1997.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 32.Slack MH, Schapira D. Severe apnoeas following immunisation in premature infants. Arch. Dis. Child. Fetal Neonatal Ed. 1999;81(1):F67–68. doi: 10.1136/fn.81.1.f67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein NP, Massolo ML, Greene J, et al. Risk factors for developing apnea after immunization in the neonatal intensive care unit. Pediatrics. 2008;121(3):463–9. doi: 10.1542/peds.2007-1462. [DOI] [PubMed] [Google Scholar]
- 34.Denizot S, Fleury J, Caillaux G, et al. Hospital initiation of a vaccinal schedule improves the long-term vaccinal coverage of ex-preterm children. Vaccine. 2011;29(3):382–6. doi: 10.1016/j.vaccine.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Wilson K, Hawken S, Holdt Henningsen K, et al. On-time Vaccination Coverage in Premature Infants in Ontario, 2002-2009. Can. J. Public Health. 2012;103(5):e359–362. doi: 10.1007/BF03404441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderón CG, Moore VR, Pittaluga PE, et al. Adherence to immunizations in newborns less than 1500 gr at birth and/or younger than 32 weeks, in two chilean centers. Rev. Chil. Infectol. 2011;28(2):166–73. [PubMed] [Google Scholar]