Abstract
We describe the neuropathologic procedure utilized in the Stillbirth Collaborative Research Network (SCRN), focusing on the examination of central nervous system (CNS) in stillbirth (SB). The SCRN was organized to perform a case-control study to determine the scope and causes of SB. Pathologists at all the participating centers agreed on and used the same standardized neuropathologic techniques. Standardized sections were taken and detailed data were collected. Fresh brain tissue was saved for investigative purposes. A total of 663 women with SB were enrolled into the case-control study: 620 delivered a single stillborn, 42 delivered twins, and 1 delivered triplets. Of the 560 (84.5%) who consented to postmortem examination, 465 (70.1%) also gave consent to the examination of the CNS. In the 440 stillborn infants in whom CNS examination was possible, 248 (56.4%) of the brains were intact, 72 were fragmented (16.4%), and 120 (27.3%) were liquefied. In summary, this is the largest prospective study dedicated to investigate the causes of SB and collect essential information and biological samples in the United States. A protocol for neuropathologic examination was instituted, and a brain tissue repository was created to provide samples and related data for future investigations.
Keywords: SCRN, neuropathology, stillbirth, central nervous system
Despite a 35% decline in infant mortality in the United States over the last decade, the number of stillbirths declined by only 17%.1–3 As a result, fetal deaths have now become the leading contributor to perinatal mortality in the United States. In 2007, The American College of Obstetricians and Gynecologists (ACOG) Committee on Genetics recommended that macroscopic and microscopic examination of the placenta and detailed postmortem examination should be performed in all cases of stillbirth to be able to explain the cause of death.4 After the ACOG’s recommendations, the number of postmortem examinations performed on the stillborn have not significantly increased.5 Even today, these procedures are not standardized.
In 2003, the Eunice Kennedy Shriver National Institute of Child Health and Human Development established the Stillbirth Collaborative Research Network (SCRN) to study the extent and causes of stillbirth in the United States.6 The scientists responsible from the SCRN developed a prospective, multicenter, population-based, case-control study that would include all stillbirths and a representative sample of live births occurring to residents in five geographically diverse regions. The study enrolled at 59 hospitals, as a whole performing >80,000 deliveries per year, from March 2006 to August 2008. Participants underwent a standardized protocol including maternal interview, medical record abstraction, biospecimen collection, placental pathology, and, for cases, postmortem examination. Further details on the study design are reported in the companion article on placenta. General information regarding the overall SCRN study design, the development of the SCRN placental and postmortem pathology protocols and associated data collection procedures, and the technical standards for digital photographs have been previously published in the companion article on placenta, and are not repeated here. In this article, we discuss the neuropathologic elements of the SCRN postmortem procedures.
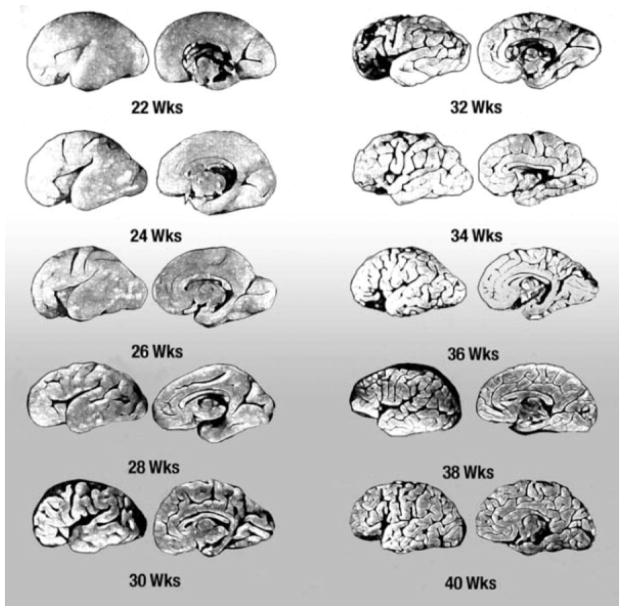
Because the examination of the central nervous system is a major and specialized component of the postmortem examination, we developed the neuropathologic examination protocol as a separate document. There is a rich body of information about the developmental landmarks and cellular processes of the human fetal brain that have been developed over the last century by many neuropathologists, neuroanatomists, and other neuroscientists. The landmark study examining the fetal brain is the National Collaborative Perinatal Project, overseen by the pediatric neuropathologist Dr. Floyd Gilles.7,8 This study provided a database of fetal brain development, growth, and formation, including gyri, ventricular and ependymal formation, and myelination.7–13 It provided important standards for the developmental assessment of the fetal brain. These standards relate, for example, to specific gyri and sulci of the cerebral cortex and the times they appear and brain weight at each gestational age, as well as the onset and timing of myelination, which is rapid in the fetal brain stem and spinal cord over the last half of gestation.14–16 In addition, Marín-Padilla and Armstrong and Hawkes, to name a few major investigators, delineated the dendritic geometry and the speed of dendritic and axonal growth in the fetal cerebral cortex. Also, the cycles of central myelination and neuronal migration patterns were elucidated.17–20 Others have helped to identify the stages of cerebral vascularization, neurotransmitter maturation, oligodendrocyte and astrocyte development, and antioxidant enzyme maturation.21,22 In addition, there are published atlases on fetal brain anatomy, neuropathology, and neuroradiology, with some combining these three fields.23–26 In an area where there was a significant accumulation of knowledge, our task was to devise a relatively user-friendly and rather simple but complete procedure to be used as the standard in the Network. The “basic” technique of examination of an organ in a postmortem examination varies according to the purpose of the investigator. This is true for brains, too.27–30 The brains are examined for different purposes in forensic sciences versus neuropathology research, so details of the examinations differ widely. Because our objective was to devise a simpler research protocol, we modified some aspects of the detailed investigative neuropathologic examination previously described by various authors by decreasing the number of steps involved and preparing easy-to-understand instructions and complementary questionnaires so that data and the biospecimens could be collected correctly and effectively. It is important to underline that this is a research protocol and is not intended to be a standardized and simplified procedure for the use of nonperinatal neuropathologists. Some of the procedures not frequently used, such as removal of the eyes or the spinal column in toto, are briefly described in the text.
METHODS
The initial assessment of the stillborn involved the evaluation of autolysis and fragmentation. They were graded using the standardized criteria previously described.31,32 At times, the grade of external maceration did not reflect the physical condition of the brain. In general, brain tissue is softer and autolyzes faster compared with other internal organs. This is true especially for the brains from early gestational ages, when the myelination process is just starting.7,8,11,12,14 The degree of integrity of the brain was determined after opening the cranial cavity (Table 1).
Table 1.
Initial Evaluation of the Condition of the Brain
| Condition | Description |
|---|---|
| Intact | In one piece |
| Fragmented | Most fragments large enough to be able to determine their origin; they measure an average of 5 cm in their largest diameter |
| Liquefied | When the specimen pours from the cranial cavity during removal |
| Other | Neither intact nor liquefied; these were obtained by various dilatation and extraction methods; they are considered fragmented and after describing and weighing, best available fragments were sampled |
Consent
A full neuropathologic examination included procurement of the brain and, if indicated, the spinal cord and eyes. Due to regional differences, some centers had to obtain special consent to examine the spinal cord and the eyes. This topic is discussed in detail in the companion article about the postmortem examination procedure.
Stages of Neuropathologic Examination
The neuropathologic examination was completed in three stages. The first stage involved the removal, preliminary external examination, and weighing of the brain. The second stage involved fixing the specimen for 1 to 2 weeks. The third stage was repeating the external examination and then slicing the brain in the coronal plane to examine the internal structures.27–30
STAGE 1
Removal of the Brain
There are several descriptions in the literature claiming to be the easiest and simplest method to remove the brain in the perinatal age group.27–30 Removing the brain intact from an early gestational-age stillborn requires a certain level of skill. The soft and pliable cranium due to incomplete ossification and the open fontanelles allowed the prosector to cut through the cranium with a pair of scissors with ease. But special bone cutters were used to sample the base of the cranium such as the sella turcica. The main disadvantage was the incompleteness of myelination causing these brains to be so soft they easily fell apart during the removal process. In addition, hypoxic and ischemic changes that usually affect the perinatal brain made them even softer. Although these usually resulted in fragmented brain tissue, they did not preclude the examination and gaining significant insights about the pathology in this organ. A description of removal of the brain from a stillborn or a neonate is described in Fig. 1.
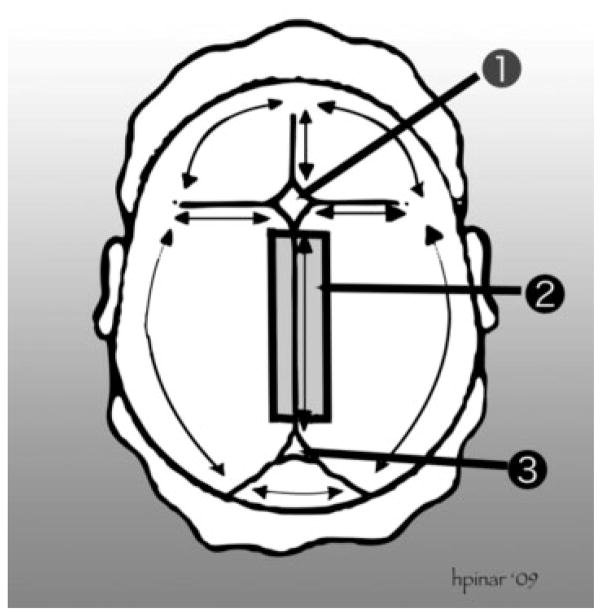
Figure 1.
Removal of the brain. This technique is suitable for fetuses of early gestations and involves cutting through the sutures and the connecting part of the bone flaps to remove the entire calvarium. (1) Anterior fontanelle. (2) The thick black lines represent the cutting lines to remove the sagittal sinus. (3) Posterior fontanelle. The scalp is incised starting behind one ear, extending to the back of the opposite ear. The first incision is made as far posterior as possible, in the area of the posterior fontanelle. The fetal scalp is soft and can easily be stretched without tearing. The scalp is then reflected downward to the level of the brows ventrally and the neck muscles dorsally. The cranial vault is opened by cutting through the membranous sutures. Cuts lateral to the sagittal suture are made to leave a strip of calvarial bone with the underlying sagittal sinus. Freeing the attachments of the brain is accomplished by turning the head on the occipital bone (or faceup) with the vertex directed to the prosector and both lateral flaps deflected. As the cranium is tilted gently to one side and then the other, all the cranial nerves and large vessels are identified and transected as close to the bone as possible, proceeding from front to back, and the anterior portion of the falx cerebri cut loose. As the posterior fossa is approached, the tentorium is cut away from the interior of the skull, around its entire perimeter, and then the falx cerebri is transected just above the tentorium. The cerebrum is then allowed to fall backward, and the pituitary stalk, anterior border of the tentorium, and the cervical spinal cord are cut as far distally as possible. With that, the entire brain slides out easily into a container and the specimen is weighed fresh. The external appearance of the brain is examined and any abnormalities are noted and photographed.28–30
Weighing the Brain
The next step after the removal of the brain was to weigh it fresh using an electronic scale. No other structures were included in the weighing process. If the spinal cord, sagittal sinus, and other structures were obtained, they were fixed in the same container.
Research Sample
The next step was to obtain a fresh brain sample by slicing the tip of the left frontal pole to include cortex and white matter. This sample was chosen because of its simple identification from the usually fragmented brain samples and also the fact that it contained gray and white matters, which constitute the greatest percentage of the fetal brain. Multiple research samples were not collected because of problems with standardization and cost. In fragmented specimens, any tissue fragment containing gray and white matters was frozen. The size of the sample depended on the gestational age and weight of the brain. The sample was immediately placed in a 15-mL cryovial and placed in −80°C freezer (Table 2). These were initially stored in the local freezer and subsequently batched and shipped on dry ice by airmail to the central tissue repository. This sample could be used for DNA extraction, protein analysis, or immunohistochemistry using antibodies that only react in unfixed tissue.
Table 2.
Sections from Intact Brains
| Block | Location and Type of Tissue |
|---|---|
| 11A | Hippocampus, choroid plexus |
| 11B | Basal ganglia (includes germinal matrix and internal capsule) |
| 11C | Orbital frontal cortex and white matter |
| 11D | Cerebrum (parietal) (no particular gyrus) |
| 11E | Cerebrum (occipital) (includes calcarine cortex and periventricular white matter) and meninges |
| 11F | Cerebellum, pons (one section, depends on the size of the specimen—if it fits into one cassette, it is submitted as such) |
| 11G | Medulla, midbrain |
| 11H | Cervical spinal cord |
| 11+ | Additional sections—specify |
Removal of the Pituitary Gland
The pituitary gland was removed with the cartilaginous sella.
Fragmented Brain Tissue
When the brain tissue was completely fragmented, the prosectors collected all the available pieces and weighed them in toto. In most cases, determination of the exact origin of the fragments was not possible, so random sections were taken. In fragmented and liquefied brains, submitting less than the standard number of blocks was acceptable (Tables 2 and 3).
Table 3.
Sections from Fragmented Brains
| Block | Location and Type of Tissue |
|---|---|
| 12A | Cortical tissue—anatomic site if identified |
| 12B | White matter—anatomic site if identified |
| 12C | Choroid plexus and meninges |
| 12D | Cerebellum and brain stem—if identified |
| 12+ | Additional sections—specify |
Identifiable cortical and white matter, choroid plexus, meninges, and cerebellar tissue should be sampled.
Very Soft (Liquefied) Brain Tissue
When the brain tissue was very soft and almost liquid in consistency, prosectors again tried to collect as much brain tissue as possible to obtain an approximate weight. If the tissue was so soft that it was impossible to use the standard tissue cassettes, no samples were submitted.
Examination of the Eyes
In certain conditions, it was desirable to remove the globes for further detailed measurements and examination. The main indications for removing the eyes included developmental abnormalities in prosencephalon (forebrain) such as cyclopia, synophthalmus, anophthalmos, and congenital cystic eye. Conditions such as aniridia, congenital ectropion of iris, all types of colobomas, failure of formation of the lid fold, cryptophthalmos, all types of embryotoxon, some aneuploidies such as trisomy 13, and all skeletal dysplasias with connective tissue gene mutations were additional indications for detailed examination of the eyes. Examination of the eyes included a careful review of the periorbital structures. During the external examination of the eyes, presence of periorbital edema or pigmentation was noted. The presence of deep set or prominent eyes and deep creases under the eyes were evaluated. Hypotelorism, hypertelorism, and colobomas were also checked. Sclera was examined, particularly if it was consistent with osteogenesis imperfecta. The eyelids and eyelashes were also examined. In some of the centers, the need of an additional consent above and beyond the federal and the local consent forms made the utilization of this procedure cumbersome.
In our protocol, we recommended removing the eyes using an anterior approach: the eyelids were held apart with the aid of retractors. Using curved scissors, the conjunctival attachments to the limbus were severed, being careful not to cut the eyelids. Tenon’s capsule was left intact to avoid leakage. The four rectus muscles were cut so that ~5.0 mm (shorter in preterm babies) of muscle was left attached to the globe; this allowed orientation of the globe at a later time. The inferior oblique muscle was then cut. Rotation of the eye temporally by traction on the stump of the inferior oblique muscle allowed access to the optic nerve and ensured that a long piece of the intraorbital portion of the optic nerve was obtained. Before examination, the eyes were fixed in a fixative of choice such as 10% buffered formalin.26–30
Examination of the Spinal Cord
A standard, single section that included the lumbar spinal cord and vertebral body at the level of L3 to L5 was considered as part of the routine postmortem procedure. The rationale for obtaining this section was to have at least one sample in cases where the spinal cord was not examined in its entirety. The spinal cord was sampled at the lumbar level because of its easy access and its large cross section that would provide a nice sample from the distal spinal cord. When the brain was removed, it was recommended to reach as deeply as possible into the narrowing spinal canal and make that cut as far down as possible. This way, both ends of the cord would have been sampled.
The removal of the entire spinal cord was recommended when there was an obvious spinal cord lesion. Because in some centers obtaining a special consent was necessary for this procedure, it was not frequently done. It is recommended to remove the entire spinal cord in suspected skeletal, muscular, or neuromuscular diseases that usually present with oligohydramnios, decreased fetal movements, and arthrogryposis, Arnold-Chiari malformation, iniencephaly, neural tube closure defects, spinal cord duplication malformations such as diastematomyelia, diplomyelia, and filum terminale malformations.33–35
After the removal of the organ block, the thoracic and lumbar portions of the spinal column could be viewed in their entirety. Next, one of the lowermost lumbar intervertebral discs was transected using a scalpel blade. Then the end of a rounded pair of scissors was inserted into this opening, and the pedicles of the vertebrae on both sides along the entire spine were cut. The dura was left intact. Once all of the pedicles were cut, the freed vertebral column was lifted, exposing the spinal cord. Next, the cord was transected caudally at the lumbar end. The dura was gently lifted. Then the dura and the cord were dissected from the spinal canal along its entire length, without exerting tension on the cord.
In the cervical region, the dissection was blind. By keeping the scissors close to the bone, damage to the cord was prevented. The cervical region could also be approached from the base of the skull through the foramen magnum.
The spinal cord also needed to be fixed for a minimum of 1 to 2 weeks and examined and sampled with the brain after this time.
STAGE 2
Fixing the Brain Tissue
After the research sample was obtained, the brain was transferred to a container filled with fixative. The fixative had to be at least 10 times the specimen’s estimated volume. Because different neuropathologists were responsible from different centers, it was decided for each center to use their fixative of choice. At the end of the fixation period, and before cutting the specimens (stage 3), the samples were gently washed with tap water overnight. If there were indications, the specimens were imaged again before starting cutting.
STAGE 3
Examination of the Brain in Fixed State
This was the stage where the fixed brain was sliced according to a strict protocol and the entire specimen was again evaluated. Before the brain was sliced, a thorough external examination was performed to evaluate the gestational age of the fetus according to external anatomic landmarks (Fig. 2). Then for the same purpose transcerebellar diameter was measured (Fig. 3). The details of the rest of the brain-cutting procedure are described in Fig. 4.
Figure 2.
Figure 3.
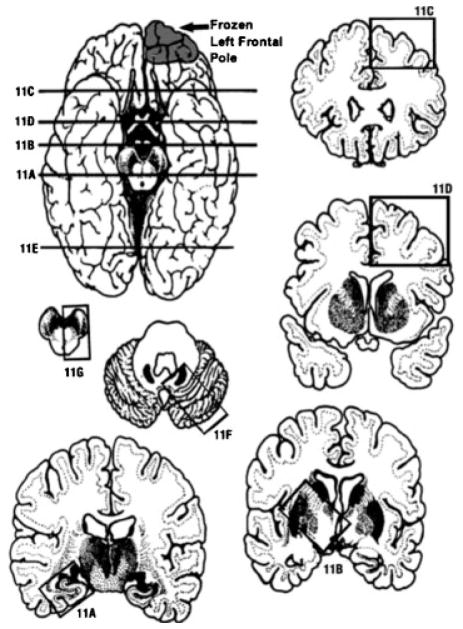
Location of the brain slices and blocks obtained. Part of the left frontal pole was submitted fresh for research purposes. (11A) The first slice was at the level of hippocampus. (11B) Basal ganglia level. (11C) Frontal cortex and white matter. (11D) Parietal cortex and white matter. (11E) Occipital cortex and white matter. (11F) Cerebellum and pons. (11G) Medulla and midbrain. (11H) Cervical spinal cord.27–30
Figure 4.
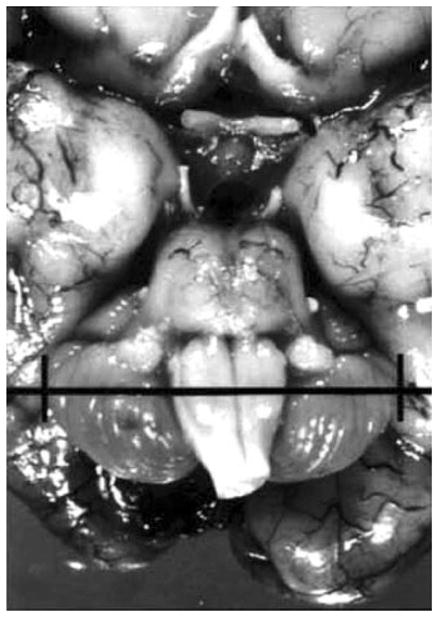
Measurement of the transcerebellar diameter (TCD) after fixation of the brain. The TCD is measured at the widest transverse diameter.39
Processing the Fragmented or Liquefied Brain after Fixation
These specimens were also reviewed after fixation. Any fragment 2 to 3 cm in its largest diameter was sliced. Any lesions that may have become more visible after fixation were then photographed.
Labeling and Submitting of Standard Tissue Blocks
In intact samples without any unilateral lesions, the SCRN research samples were obtained from one half of the organ. The clinical samples were obtained from the other symmetrical half. Information regarding the exact location of the section was entered into the database. The clinical blocks were labeled according to local procedures and stored in the originating hospital. These were used to generate the clinical report and to comply with legal and regulatory requirements. The duplicate SCRN blocks were sent to the tissue repository according to the SCRN protocol.
The block numbers were standardized to facilitate future retrieval. Numbers 11 through 13 were reserved for tissue blocks for the neuropathologic examination (Table 3). They were subclassified using an alphabetical notation. The anatomic locations of the submitted sections were recorded on the trim sheet/SCRN data collection form. If the spinal cord was taken or lesions were present, these samples were labeled starting with block “11i.” The main reason for numbering the blocks as described was to keep the large number of blocks in a sequence and make retrieval easier.
Sections were processed in a routine manner and embedded in paraffin. Then 5-μm sections placed on glass slides were stained with hematoxylin and eosin and in some instances hematoxylin and eosin and Luxol fast blue. The slides were examined and the appropriate data forms were completed. When indicated, additional slides were prepared using special histochemical or immunohistochemical stains. As needed, digital photomicrographs were taken.
In the small specimens from earlier gestational-age fetuses, it was not always possible to obtain identical blocks for the tissue repository. In these situations, five unstained slides and one 25-μm-thick paraffin curl were prepared from the clinical blocks and sent to the tissue repository.
In fragmented samples, identifiable cortical and white matter, choroid plexus, meninges, and cerebellar tissue were sampled (Table 2). Lesions were sampled separately. The exact locations of the sections, if known, were then recorded.
Data Collection
Figure 5 shows all the data points that were collected during the brain-cutting (macroscopic) session. Figure 6 depicts all the data elements that were collected during the microscopic examination of the sections.
Figure 5.
Data elements collected in the macroscopic examination of the perinatal brain.
Figure 6.

Data elements collected in the microscopic examination of the perinatal brain.
RESULTS
A total of 663 women with stillbirth were enrolled into the case-control study, and 620 delivered a single stillborn infant, 42 delivered twins (13 sets with 2 stillborn infants and 29 sets with 1 stillborn and 1 live-born infant), and 1 delivered triplets (1 stillborn and 2 live-born infants), for a total of 707 infants. Of these women, 560 (84.5%) consented to postmortem examination for a total of 572 stillborn infants. Of the latter, consent for neuropathologic examination was available for 465 (70.1%). Of 440 stillborn infants with assessment of maceration and brain intactness, the brain was reported as intact in 248 (56.4%), fragmented in 72 (16.4%), and liquefied in 120 (27.3%); fragmentation and liquefaction were less commonly observed at later gestational ages, with only 8.8% of the brains reported as fragmented and 8.8% reported as liquefied among term stillborns. Among 100 (22.7%), 287 (65.2%), and 53 (12.0%) stillborn infants with no, mild, or marked maceration, the brain was intact in 78.0%, 37.5%, and 9.4%, fragmented in 18.0%, 15.7%, and 17.0%, and liquefied in 4.0%, 26.8%, and 73.6%, respectively.
DISCUSSION
Over its lifetime, the human organism develops at its fastest rate during embryonic and fetal development. After their formation, all the organs undergo major changes and maturation to prepare for extra-uterine existence. This is not true for the fetal central nervous system, where the maturation and differentiation of many of its structures are still not complete by the time of birth. The fetal brain continues its development after birth adding another layer of difficulty when studying the developing nervous system.
Study of the fetal nervous system has been hampered by the difficulty of distinguishing “normal” from “abnormal” in mostly autolyzed brains. Over the last decades, many investigators have successfully studied the developing fetal brain and, while describing its normal development, also identified distinct pathological condition. Another reason for this slow pace was due to delayed recognition of intrauterine conditions that over time compromise the fetus and result in either impaired newborns or stillbirths.
During the SCRN preparatory stages, this issue was particularly discussed.6 According to the consensus reached at the end of the symposium, it was decided that if all the studies including the postmortem and placental examinations fail to reveal a cause, examination of the brain and its injury patterns may provide insight into the pathophysiology of the stillbirth.6,34–39 This led to the development of a standardized neuropathologic examination as one of the tools used to test the main hypotheses.
Although the basic technique of the neuropathologic examination in the fetus had been published, they all are relatively dated.27–30 In addition, most of the recent publications focusing on different lesions do not include the details of this procedure.38 Thus, it was decided to devise a research protocol that all the contributing centers would use, one simple enough that a nonperinatal neuropathologist would feel comfortable using it. We hope that this publication will give interested parties a useful and up-to-date tool to perform, collect data, and complete a very significant part of the postmortem examination in a stillborn.
One of the realities of our times is that the time allocated to learning perinatal pathology and especially perinatal neuropathology in residency training has been either nonexistent or rapidly decreasing. Most trainees are therefore unfamiliar with neuropathologic examination of the stillbirth. A major advance in this area is the increase in the number of stillbirth brains examined by nonneuropathologists during their training. We hope that this article will help anybody who is looking for a standardized and concise procedure to review.
We think that case-control studies comparing the pathological findings of the placental and postmortem examination, which includes a detailed neuropathologic component, bolstered by the addition of detailed maternal information, have the potential to explain some of the real causes of fetal death and the impact of the responsible pathogenetic mechanisms on the fetal brain.
Acknowledgments
FUNDING
Supported in part by grant funding from the Stillbirth Collaborative Research Network sites: U10-HD045953 (Brown University, Rhode Island); U10-HD045925 (Emory University, Georgia); U10-HD045952 (University of Texas Medical Branch at Galveston, Texas); U10-HD045955 (University of Texas Health Science Center at San Antonio, Texas); U10-HD045944 (University of Utah Health Sciences Center, Utah); and U01-HD45954 (RTI International, North Carolina); and by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
- 1.Goldenberg RL, Kirby R, Culhane JF. Stillbirth: a review. J Matern Fetal Neonatal Med. 2004;16:79–94. doi: 10.1080/14767050400003801. [DOI] [PubMed] [Google Scholar]
- 2.Fretts RC, Boyd ME, Usher RH, Usher HA. The changing pattern of fetal death, 1961–1988. Obstet Gynecol. 1992;79:35–39. [PubMed] [Google Scholar]
- 3.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 4.Committee on Genetics. ACOG Committee Opinion No 383: Evaluation of stillbirths and neonatal deaths. Obstet Gynecol. 2007;110(4):963–966. doi: 10.1097/01.AOG.0000263934.51252.e0. [DOI] [PubMed] [Google Scholar]
- 5.Silver RM, Varner MW, Reddy U, et al. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196:433–444. doi: 10.1016/j.ajog.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grafe MR, Kinney HC. Neuropathology associated with stillbirth. Semin Perinatol. 2002;26:83–88. doi: 10.1053/sper.2002.29862. [DOI] [PubMed] [Google Scholar]
- 7.Gilles FH, Dooling E, Fulchiero A. Sequence of myelination in the human fetus. Trans Am Neurol Assoc. 1976A;101:244–246. [PubMed] [Google Scholar]
- 8.Gilles FH. Myelination in the neonatal brain. Hum Pathol. 1976B;7:244–248. doi: 10.1016/s0046-8177(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 9.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 10.Dooling EC, Chi JG, Gilles FH. Ependymal changes in the human fetal brain. Ann Neurol. 1977;1:535–541. doi: 10.1002/ana.410010605. [DOI] [PubMed] [Google Scholar]
- 11.Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 13.McLennan JE, Gilles FH. A growth model for the total weight of the prenatal human brain. Trans Am Neurol Assoc. 1976;101:271–272. [PubMed] [Google Scholar]
- 14.Kinney HC. Human myelination and perinatal white matter disorders. J Neurol Sci. 2005;228:190–192. doi: 10.1016/j.jns.2004.10.006. (Review) [DOI] [PubMed] [Google Scholar]
- 15.Billiards SS, Haynes RL, Folkerth RD, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- 16.Dorovini-Zis K, Dolman CL. Gestational development of brain. Arch Pathol Lab Med. 1977;101:192–195. [PubMed] [Google Scholar]
- 17.Marín-Padilla M. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory. J Comp Neurol. 1992;321:223–240. doi: 10.1002/cne.903210205. [DOI] [PubMed] [Google Scholar]
- 18.Marín-Padilla M. Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: a Golgi study. J Comp Neurol. 1995;357:554–572. doi: 10.1002/cne.903570407. [DOI] [PubMed] [Google Scholar]
- 19.Marín-Padilla M. Three-dimensional structural organization of layer I of the human cerebral cortex: a Golgi study. J Comp Neurol. 1990;299:89–105. doi: 10.1002/cne.902990107. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong CL, Hawkes R. Pattern formation in the cerebellar cortex. Biochem Cell Biol. 2000;78:551–562. [PubMed] [Google Scholar]
- 21.Haynes RL, Borenstein NS, Desilva TM, et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- 22.Kinney HC, Belliveau RA, Trachtenberg FL, Rava LA, Paterson DS. The development of the medullary serotonergic system in early human life. Auton Neurosci. 2007;132:81–102. doi: 10.1016/j.autneu.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Feess-Higgins A, Laroche J-C. Development of the Human Foetal Brain: An Anatomical Atlas. Paris: INSERM; 1987. [Google Scholar]
- 24.Huang H, Xue R, Zhang J, et al. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci. 2009;29:4263–4273. doi: 10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habas PA, Kim K, Rousseau F, Glenn OA, Barkovich AJ, Studholme C. A spatio-temporal atlas of the human fetal brain with application to tissue segmentation. Med Image Comput Comput Assist Interv. 2009;12(Pt 1):289–296. doi: 10.1007/978-3-642-04268-3_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habas PA, Kim K, Yang P, et al. MICCAI 2009 Lecture Notes in Computer Science. Berlin/Heidelberg: Springer; 2009. A Spatio-temporal Atlas of the Human Fetal Brain with Application to Tissue Segmentation: Medical Image Computing and Computer-Assisted Intervention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bove KE Autopsy Committee of the College of American Pathologists. Practice guidelines for autopsy pathology: the perinatal and pediatric autopsy. Arch Pathol Lab Med. 1997;121:368–376. [PubMed] [Google Scholar]
- 28.Barnes EG, editor. Handbook of Pediatric Autopsy Pathology. New York, NY: Humana Press; 2004. [Google Scholar]
- 29.McPherson TA, Valdes-Dapena M. The perinatal autopsy. In: Wigglesworth JS, Singer DB, editors. Textbook of Fetal and Perinatal Pathology. 2. Boston: Blackwell Scientific Publications; 1998. pp. 87–110. [Google Scholar]
- 30.Keeling J. The perinatal necropsy. In: Keeling J, editor. Fetal and Neonatal Pathology. 3. London: Springer-Verlag; 2001. pp. 1–45. [Google Scholar]
- 31.Genest DR, Williams MA, Greene MF. Estimating the time of death in stillborn fetuses: I. Histologic evaluation of fetal organs; an autopsy study of 150 stillborns. Obstet Gynecol. 1992A;80:575–584. [PubMed] [Google Scholar]
- 32.Genest DR, Singer DB. Estimating the time of death in stillborn fetuses: III. External fetal examination; a study of 86 stillborns. Obstet Gynecol. 1992B;80:593–600. [PubMed] [Google Scholar]
- 33.Hori A, Fischer G, Dietrich-Schott B, Ikeda K. Dimyelia, diplomyelia, and diastematomyelia. Clin Neuropathol. 1982;1:23–30. [PubMed] [Google Scholar]
- 34.Hutchins GM, Meuli M, Meuli-Simmen C, Jordan MA, Heffez DS, Blakemore KJ. Acquired spinal cord injury in human fetuses with myelomeningocele. Pediatr Pathol Lab Med. 1996;16:701–712. [PubMed] [Google Scholar]
- 35.Griffiths PD, Variend D, Evans M, et al. Postmortem MR imaging of the fetal and stillborn central nervous system. AJNR Am J Neuroradiol. 2003;24:22–27. [PMC free article] [PubMed] [Google Scholar]
- 36.Sims ME, Turkel SB, Halterman G, Paul RH. Brain injury and intrauterine death. Am J Obstet Gynecol. 1985;151:721–723. doi: 10.1016/0002-9378(85)90503-4. [DOI] [PubMed] [Google Scholar]
- 37.Grafe MR. The correlation of prenatal brain damage with placental pathology. J Neuropathol Exp Neurol. 1994;53:407–415. doi: 10.1097/00005072-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Becher JC, Bell JE, Keeling JW, Liston WA, McIntosh N, Wyatt B. The Scottish Perinatal Neuropathology Study—clinicopathological correlation in stillbirths. BJOG. 2006;113:310–317. doi: 10.1111/j.1471-0528.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 39.Pinar H, Burke SH, Huang CW, Singer DB, Sung CJ. Reference values for transverse cerebellar diameter throughout gestation. Pediatr Dev Pathol. 2002;5:489–494. doi: 10.1007/s10024-001-0262-4. [DOI] [PubMed] [Google Scholar]