Abstract
After reviewing the state of knowledge about the scope and causes of stillbirth (SB) in a special workshop sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the participants determined that there is little guidance regarding the best use of postmortem examination (PM) to address the pathogenesis of stillbirth. In this report, we describe the PM procedure designed and used in the NICHD-supported Stillbirth Cooperative Research Network (SCRN). Perinatal pathologists, clinicians, epidemiologists, and biostatisticians at four tertiary care centers, a data coordinating center, and NICHD developed a standardized approach to perinatal PM, which was applied to a population-based study of stillbirth as part of the SCRN. The SCRN PM protocol was successfully instituted and used at the four medical centers. A total of 663 women with stillbirth were included: 620 delivered a single stillborn infant, 42 delivered twins, and one delivered triplets for a total of 676 stillborn infants. Of these women, 560 (84.5%) consented to PM (572 stillborn infants) that was conducted according to the SCRN protocol. A standardized PM protocol was developed to evaluate stillbirth consistently across centers in the United States. Novel testing and approaches that increase the yield of the PM can be developed using this model.
Keywords: SCRN, postmortem examination, stillbirth, perinatal pathology
There are ~26,000 stillbirths, defined as fetal death at 20 weeks of gestation or greater, every year in the United States. The cause of half of all stillbirths is undetermined.1–3 Although considered an integral part of the investigation of fetal death, postmortem examination is underutilized, being performed in less than half of stillbirths.3–6 Some of the reasons for this underutilization include insufficient knowledge about the usefulness of the postmortem examination not only among the lay public but also in the medical community. Other reasons include lack of reimbursement, discomfort of the caregivers when asking for consent, and some parents’ reluctance due to cultural or religious reasons.3 Perinatal pathology can be more complex than regular pathology because of developmental changes throughout gestation as well as the effect of often prolonged postmortem latency in utero complicating the interpretation of postmortem examination findings. Although some guidelines exist, they are dated, do not incorporate newer diagnostic testing now available, and do not use a standard procedure in conjunction with uniform objective data collection.7–13 The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) held a workshop on March 26, 2001, “Setting a Research Agenda for Stillbirth” that reviewed the state of knowledge about the scope and causes of stillbirth.14,15 Experts concluded that there is little guidance regarding the best use of postmortem examination to address the pathogenesis of stillbirth, noting that present practice lacked consensus and that standardization of anatomic, histological, and laboratory protocols was absent. The purpose of this report is to describe the development of standardized protocol for postmortem examinations that was used in a case-control study of stillbirths.
METHODS
Research Design
In 2003, the NICHD established the Stillbirth Collaborative Research Network (SCRN) to study the extent and causes of stillbirth in the United States.14 The SCRN encompasses five clinical sites, a data coordinating and analysis center, and NICHD. Stillbirth was defined as a fetal death at 20 weeks’ gestation or greater. The SCRN further defined fetal death as Apgar scores of 0 and 0 at 1 and 5 minutes with no other signs of life by direct observation. The SCRN investigators developed a prospective, multicenter, population-based case-control study of all stillbirths and a representative sample of live births occurring to residents in five geographically diverse regions. The study enrolled patients at 59 hospitals, averaging >80,000 deliveries per year, from March 2006 to August 2008. Participants underwent a standardized protocol including maternal interview, medical record abstraction, biospecimen collection, placental pathology, and, for cases, postmortem examination. General information regarding the overall SCRN study design, the development of the SCRN pathology protocols and associated data collection procedures, and the technical standards for digital photographs were previously published. In this article, we review the specific procedures for the SCRN postmortem examinations. Neuropathologic elements of the postmortem procedures are reported separately.
Consent and Initial Handling
Prior to the postmortem examination, informed consent was obtained from eligible participants.16 The SCRN consent provided the details of the examination procedures, as well as information about the potential value gained from the results of the examination.3,5,6,17 Consenting participants agreed to either a complete evaluation with no limitations or to an opt-out consent that included specified restrictions to the procedure. Almost every hospital had a different consent form for postmortem examination. Some included all possible scenarios on how the examination could be performed and some were very general and did not give any details. So participants were asked to sign both the consents specific to the hospital as well as the SCRN consent forms. During this process, participants also received detailed information about the study in general and the procedure itself. If discrepancies existed between these two forms, the pathologist performed the examination according to the more restricted consent. In addition to the different local consent forms, some hospitals had varying requirements for additional procedures such as removing the eyes, brain, or the entire spinal cord when indicated. In these centers, the SCRN research coordinators obtained special consents to perform these particular procedures.
Per standard clinical practice, the SCRN pathologist prepared a clinical report for each case in addition to completing the study forms. The research coordinators provided the obstetric provider a copy of the clinical report. The provider or the clinical site principal investigator shared these results with the family.
Pathologists attempted to complete the SCRN external and internal macroscopic examination within 2 working days of receipt of the fetus and the microscopic examination within 30 days. During transport, medical records and other documents were kept in opaque and sealed envelopes. Study pathologists received a copy of the clinical and SCRN informed consents and other available clinical documents along with the fetus. The family determined the disposition of the fetus upon completion of the postmortem examination.
Initial Assessment
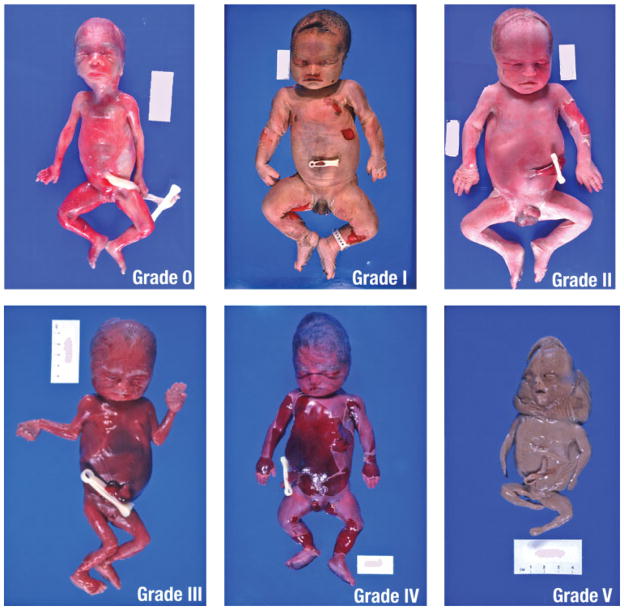
First the pathologists assessed the intactness and degree of maceration of the fetus. These were evaluated using criteria defined a priori. If the fetus was received fragmented, the fetal fragments were separated from the placental tissue and blood clots and then weighed separately. Fragmentation was classified as none, all small (<5 cm) fragments, some large (≥5 cm) fragments, or mostly large fragments. Large fragments were measured and described separately. Maceration was graded on a scale of 0 to 5 based on observable changes in the skin.18 The categories for maceration started with changes that can be seen as soon as 4 to 6 hours after fetal demise (Fig. 1).
Figure 1.
Grades of maceration. Grade 0: No maceration. Tissue appears normal. Grade I: Desquamation involving ≤1% of total body surface and brown-red discoloration of umbilical cord stump. Tissue appears red/pink and fresh with focal discoloration. Grade II: Desquamation of face, abdomen, or back involving ≥1% and ≤5% total body surface. Tissue appears red/pink and fresh with focal discoloration and serous fluid collection. Grade III: Desquamation involving >5% of body surface. Tissue appears red/pink and mixed with brown. Grade IV: Total brown skin discoloration. Tissue appears brown/gray. Grade V: Mummification. Tissue appears gray.
Macroscopic Examination
FETAL MEASUREMENTS
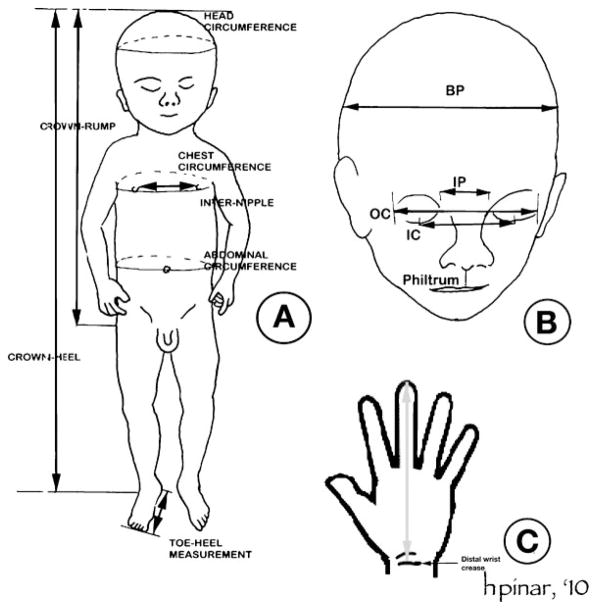
An external examination included a thorough inspection of the body followed by anthropometric measurements. Fetal dimensions were measured using steel calipers, flexible steel tape measures, and steel rulers to the nearest millimeter, and weights were recorded to the nearest one-tenth gram (Fig. 2).19–21 When only unilateral dimensions were recorded, the measurements were obtained on the right side. Because in fetuses with marked maceration (grades 4 to 5), the integrity of the tissues is frequently compromised, only intact body parts were measured.
Figure 2.
(A) External measurements of the body and descriptions of correct techniques. Body weight: the stillborn is weighed without any wrappings or other paraphernalia, except plastic cord clamp or plastic identification bands. Toe-heel length: the distance from the posterior prominence of the heel to the tip of the longest toe. Crown-heel length: the distance from the vertex of the calvarium to the soles of the feet. Crown-rump length: the distance from the vertex of the calvarium to the lowest part of the trunk, which corresponds to either the perineum or the most distant surface of the buttocks. Chest circumference: measured in the transverse plane, over the nipples. Abdominal circumference: measured in the transverse plane, at the level of umbilicus. Internipple distance: The distance between the centers of the nipples. When the nipples were not clearly identified in very early or macerated stillborns, no measurement was obtained. (A) External measurements of the body. (B) External measurements of the head. Biparietal diameter (BP): the distance between the two parietal bones. It is measured by using a caliper. Occipital-frontal circumference (not shown): the circumference of the head just above the eyebrows anteriorly and at the most distant point of the occiput posteriorly. Inner canthal distance (IC): the distance between the inner canthi of both eyes. Outer canthal distance (OC): the distance between the outer canthi of both eyes. Interpupillary distance (IP): the distance between the centers of the pupils with the eyes looking straight. If pupils are not visible, the distance between the midpoints of upper eyelids is measured. Philtrum length: the distance from the base of the columella to the midline depression of the vermilion border. Position of the ears: there are different methods to determine this. When an imaginary line is drawn between the outer canthus of the eye and the most distant part of the occiput, the superior attachment of the pinna should be on or above this line. If this criterion is not met, the ears are determined to be low-set. (C) Hand length: the distance between the distal wrist crease and the tip of the middle finger. This is useful especially in fragmented specimens where feet cannot be identified.
FETAL IMAGING
Technical standards for digital photography are described elsewhere. Standard photographs included (1) anterior and posterior views of the whole body; (2) anterior, right and left side views of the head; and (3) close-up views of any notable findings. We performed whole-body radiographs after positioning the legs and arms flat against the film cassette. Placement of a standard radio-opaque ruler adjacent to one side of the body allowed the measurement of diaphyseal lengths of the femur and humerus in the radiographic images.22,23 The presence of ossification in the distal epiphysis of the femur, humeral diaphysis, and talus were recorded. When a diagnosis of skeletal dysplasia was suspected, additional radiographs were obtained. Fetal fragments were photographed and radiographed after spreading them in a single layer.
Data Elements
EXTERNAL MACROSCOPIC EXAMINATION
The external examination was performed in a systematic manner starting with anthropometric measurements (Fig. 2). After all the measurements were completed, detailed examination was performed starting with the head and followed by the chest, abdomen, and all extremities. All the orifices were probed and external genitalia were evaluated. Any abnormality irrespective of its severity was noted. Severe abnormalities were separately described and photographed. The data elements collected in the external macroscopic examination are provided in Fig. 3.
Figure 3.
Data elements in the postmortem external macroscopic examination.
INTERNAL MACROSCOPIC EXAMINATION
The postmortem examination was performed using the classical method requiring the removal of the organ block in toto. The proximal end of the organ block was at the superior border of the thyroid cartilage. The distal end was at the anorectal junction just above the skin. Before starting the dissection of individual organs, bacterial cultures from the heart blood and lung were obtained. Blood for bacterial culture was obtained by lifting the cardiac apex ventrally with a small toothed forceps, exposing the still sterile inferior vena cava, from which up to 2 mL of blood were aspirated using a sterile 22-gauge needle. The aspirated sample was divided equally into aerobic and anaerobic blood culture bottles and processed. The lung culture was obtained using a hemostat to clamp on the lateral aspect of the base of the right lung. By pulling on the clamps, the lung was then exteriorized out of the thoracic cavity, carefully avoiding contamination of the surfaces to be cultured. A scalpel was sterilized in an alcohol flame and the red-hot blade pressed against the ventral aspect of the right lower lobe. The blade was reheated and, when cool, was used to open the sterilized area. A sterile Dacron® swab (Becton-Dickinson, Franklin Lakes, NJ) was inserted into the lung tissue and then placed in a culture tube with transport medium for aerobic and anaerobic cultures.
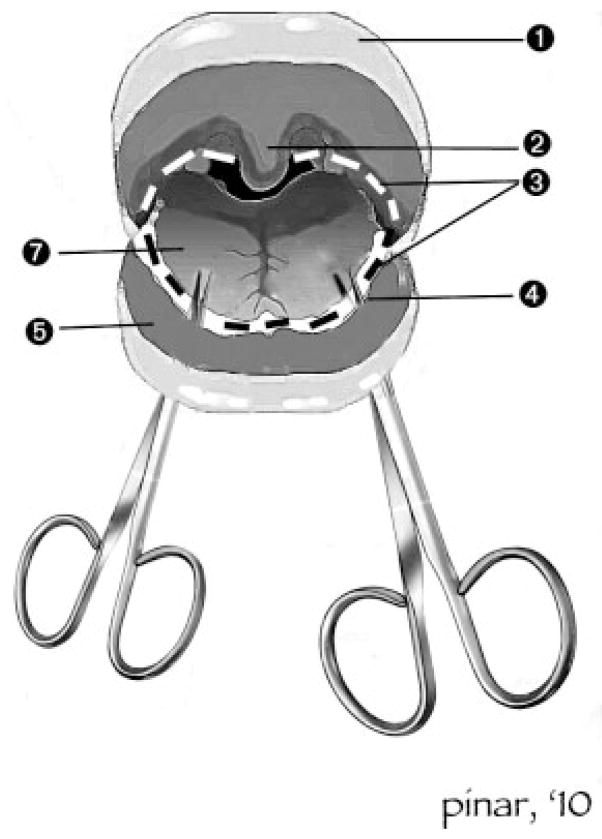
Before removing the organ block, all the internal organs and their anatomic relationships were inspected. When a malformation was identified in any organ system, these structures were carefully dissected. Removal of the tongue in continuity with the neck organs was reserved for fetuses with head and neck malformations (Fig. 4).
Figure 4.
Removal of the tongue. (1) Upper lip; (2) uvula; (3) incision line; (4) perforation site with the sharp scissors; (5) lower lip; (6) lower gum line; (7) tongue. After the skin overlying the ventral and lateral aspects of the neck is dissected free, the tongue is approached ventrally. The muscular base of the tongue is cut from its attachment to the inner aspect of the mandible. Next the incision is extended encircling the tongue laterally and dorsally so that both tonsils, the posterior wall of the pharynx, and the soft palate with the uvula remain attached to the tongue. Once the tongue is freed from its attachments, it is pulled down from the mouth in the caudal direction.
The data elements collected in the internal macroscopic examination are provided in Fig. 5.
Figure 5.

Data elements collected in the postmortem internal macroscopic examination.
DISSECTION AND EXAMINATION OF THE ORGAN BLOCK
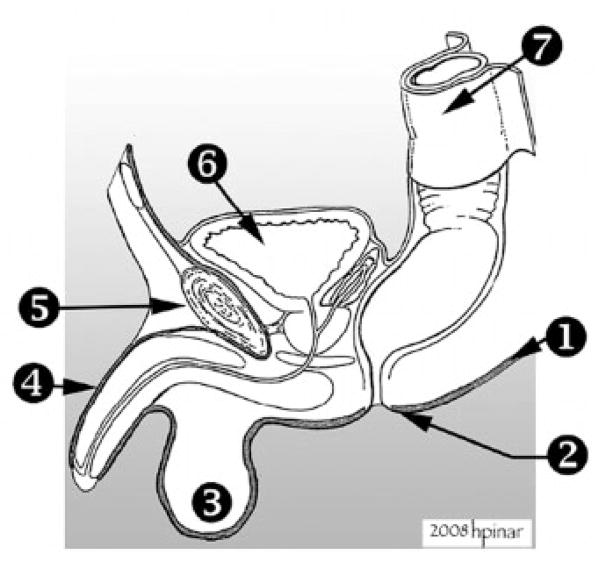
The heart and great vessels were initially examined in situ, and a more detailed examination was performed later. Once the organ block was removed from the body, costochondral samples and a section of psoas muscle were obtained and fixed in 10% buffered formalin. Next, the chest and abdominal organs were separated after dissecting the esophagus away from the trachea and severing the inferior vena cava and aorta. In cases with congenital heart disease, the lungs and heart were left together. When there were any developmental abnormalities of the external genitalia, such as cloacal abnormalities or evidence of urinary tract outflow obstruction, the entire genitourinary system was dissected as one continuous piece, which in the males included the penile structure (Fig. 6).
Figure 6.
Removal of the genitourinary system in toto. (1) Plane of dissection. This dissection is performed close to the skin so extra attention should be give not to damage the overlying skin. The plane of dissection is indicated by the dark thick line. (2) Anal opening. (3) Testes. (4) Penis. The skin over the penis is dissected and cut around the glans. (5) Symphysis pubis. (6) Bladder. (7) Rectum. In cases with suspected genitourinary abnormalities (cloacal dysgenesis, ambiguous genitalia, posterior urethral valves, anal atresia, or fistulas), dissection was performed at the caudal end of the organ block before it was separated from its pelvic attachments. After dissection of pelvic viscera, the pubic rami and the symphysis were then incised, so the ilia could be pushed laterally to open the bony pelvis. The entire length of the urethra and colon/rectum were thus freed from the pelvis and surrounding tissues. The organs connected to the base of the pelvic cavity were dissected as close as possible to the perineal skin. In males, the urethra was removed without disrupting the appearance of the external genitalia by using blunt dissection to free the cavernosa and glans penis from the penile skin. This skin segment was left intact and reexpanded with a small piece of gauze to return it to its normal outward appearance. The testes were removed with the organ block. Posteriorly, the rectum was dissected, as close to anal opening as possible, and resected with an ellipse of skin around it. This site should be sutured at the conclusion of the postmortem examination.
After all the structures were dissected and examined, the heart was reexamined using a simpler method devised by C.E. Oyer, M.D. (personal communication, 2008).24 In this protocol, the ventricular walls were incised in their ventral aspects close to the septum. The aorta and pulmonary valves were incised in continuity with the ventricular incisions unless abnormalities of the atrioventricular valves were noted. Openings of the vena cava and pulmonary veins were kept intact, and the aorta was cut at the level of the diaphragm. This dissection preserved anatomic relations for later inspection (Fig. 7). Most of the various other techniques for the dissection of the fetal/neonatal cardiovascular system can be accessed through these references.8–13,24,25 The indications and procedures for the removal of the brain, spinal cord, and eyes are described in detail in the companion article on the neuropathologic examination of the stillborn.
Figure 7.
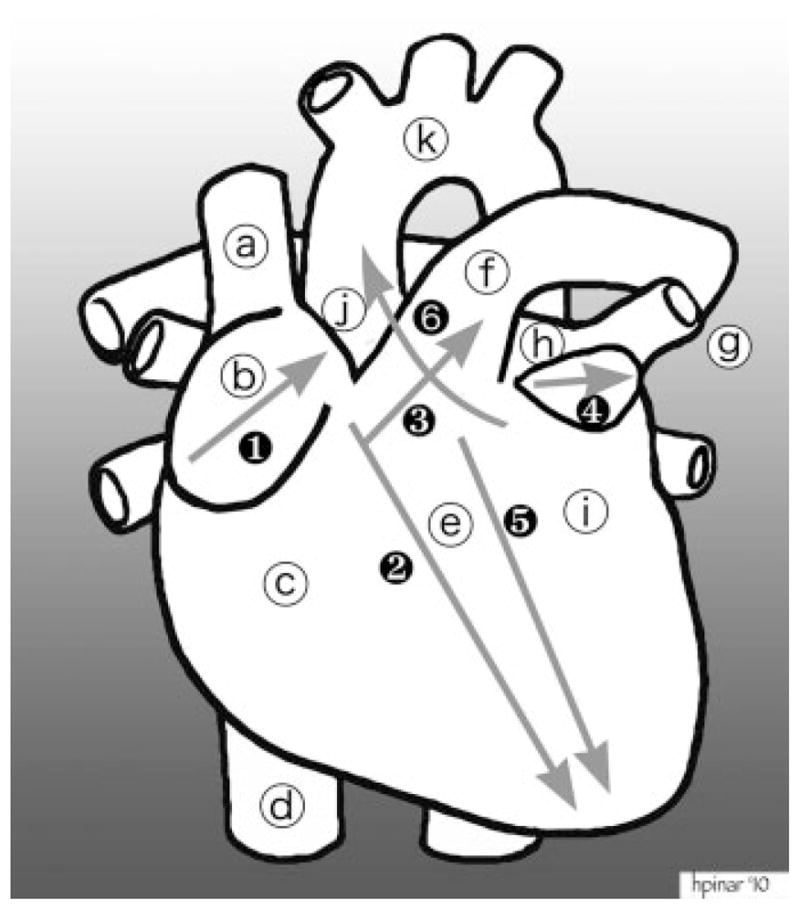
Dissection of the heart. Structures: (a) superior vena cava; (b) right atrial appendage; (c) right ventricle; (d) inferior vena cava; (e) septum; (f) pulmonary artery; (g) pulmonary veins; (h) left atrium; (i) left ventricle; (j) ascending aorta; (k) aortic arch. The cuts during dissection: (1) first cut opens the right atrial appendage; (2) second cut follows close to the interventricular septum all the way to the tip of the heart; (3) third cut goes through the pulmonary artery all the way to the splitting off the right and left arteries (pig’s snout appearance); (4) fourth cut opens the left ventricular appendage. After the initial small incision, this incision can be expanded so that the interior of the left atrium can be examined; (5) fifth cut opens the left ventricle adjacent to the interventricular septum; (6) sixth cut goes through the mitral valve, cuts the pulmonary artery above the valves, and enters into the ascending aorta.
SAMPLE COLLECTION
Samples collected during the postmortem examination included tissue sections for histology, samples snap frozen and stored at −80°C, and samples collected fresh for local analyses. Table 1 summarizes the tissue samples collected from the fetus. In fragmented and macerated cases, the pathologists were instructed to obtain any sample possible, and these were recorded in the database. The samples for histology were fixed in 10% buffered formalin, processed, and embedding in paraffin. The cassettes were labeled with the postmortem and the SCRN IDs along with standard block numbers used for specific organs. Using this method facilitates organ-specific searches and retrieval of tissue blocks. Table 2 is a list of the clinical samples obtained from the fetuses. The samples procured for investigative purposes are listed in Table 3.
Table 1.
Standard Block Numbers and Types of Tissues
| Block Number | Type of Tissue |
|---|---|
| 1A | Right atrium, tricuspid valve, and right ventricle |
| 1B | Left atrium, mitral valve, left ventricle, and aorta |
| 2A | Right upper lobe and trachea |
| 2B | Right middle and lower lobes |
| 2C | Left upper and lower lobes |
| 3A | Upper gastrointestinal tract (gastroesophageal junction, stomach, small bowel) |
| 3B | Lower gastrointestinal tract (terminal ileum, colon) |
| 4 | Liver |
| 5A | Right kidney |
| 5B | Left kidney |
| 5C | Bladder, prostate/uterus |
| 6A | Right adrenal, right testis/adnexa |
| 6B | Left adrenal, testis/adnexa |
| 6C | Trachea with thyroid and larynx, pancreas, pituitary gland |
| 7 | Spleen, mesentery, and lymph nodes |
| 8 | Thymus |
| 9A | Diaphragm and psoas muscle (longitudinal and cross section) |
| 9B | Skin |
| 10A | Ribs with costochondral section |
| 10B | Vertebra with spinal cord |
| 11 + | Samples from intact brain* |
| 12 + | Samples from fragmented brain* |
| 13 + | Samples from liquefied brain* |
| 14 + | Samples of lesions identified during the postmortem examination |
As outlined in the neuropathology protocol.
Table 2.
Checklist of Clinical Samples Obtained from Fetuses*
| Bacteriologic Cultures | Metabolic (When Indicated) |
|---|---|
| Lung culture | Bile |
| Blood culture | Tissue for fibroblast culture |
| Toxicology | Tissue—frozen |
| Meconium† | Tissue—electron microscopy |
| Tissue samples | Neuromuscular (when indicated) |
| Formalin fixed, paraffin blocks | Peripheral nerve—frozen— electron microscopy |
| Tissue for karyotype analysis | Skeletal muscle—frozen— electron microscopy |
| Skeletal dysplasia (when indicated) | |
| Femur or humerus | |
| Costochondral junction | |
| Tissue for fibroblast culture | |
| Cartilage/bone—frozen | |
| Tissue—electron microscopy |
The pathologists attempted to obtain these samples in all fetuses whether they were intact, fragmented, nonmacerated, or macerated. When the degree of maceration was ≥4, samples that required tissue to grow in a culture medium were obtained from the placenta instead of the fetus. When a sample was not obtained for various reasons, the information was entered into the database.
In some centers, meconium was collected for clinical purposes and frozen. It was processed when there was an indication.
Table 3.
Samples Obtained for Research Purposes
| Type | Tissue Origin | Processing |
|---|---|---|
| Frozen samples (fresh) | Approximately 0.5 to 3 g of liver and psoas muscle were placed in 4-mL cryovials | Kept at −80°C and shipped on dry ice to SCRN tissue repository at 2- to 4-mo intervals |
| Brain from the frontal lobe (see neuropathology procedure for details) obtained. | Similar to other frozen samples | |
| Umbilical cord segment (see placenta procedure for details) obtained. | Sent for toxicology analysis | |
| Fixed in 10% formalin | A second set of tissue sections identical to the clinical set (see Table 1) were obtained and processed for the network. | Kept at room temperature and sent to SCRN tissue repository at 2- to 8-mo intervals |
| When the amount of tissue was insufficient to yield two blocks, thick paraffin sections (paraffin curls) and five unstained slides from the single block were prepared. | Kept at room temperature and sent to SCRN tissue repository along with the paraffin blocks | |
| Tissue for karyotype analysis (fresh) | Skin, tendon, and pericardium were obtained from all fetal specimens. The sizes of the samples varied, but they were as large as 1 cm3. In macerated fetuses, fresh samples were obtained from the placenta. | Processed locally |
| Heart blood | Whenever possible, the amount also depended on the size of the fetus and degree of maceration. | Refrigerated and shipped to the repository in batches |
SCRN, Stillbirth Cooperative Research Network.
SAMPLES FOR SPECIFIC INDICATIONS
Certain conditions such as skeletal dysplasias and inborn errors of metabolism require additional samples to reach a diagnosis. For suspected skeletal dysplasia cases, tissue for fibroblast culture to be used for molecular diagnosis was the first specimen collected. These cases required additional radiographs of all the available skeletal structures.26 If possible, a complete long bone such as a femur or humerus was dissected, fixed in formalin, and processed after decalcification. Samples usually required to diagnose cases with suspected inborn errors of metabolism include bile, frozen fresh tissue, and tissue for fibroblast culture. Tissue samples from the liver, heart, or skeletal muscle were obtained and fixed in glutaraldehyde to be used for ultrastructural examination using electron microscopy.27–30 Investigation of hereditary neuromuscular disease requires frozen nerve and skeletal muscle.
Microscopic Examination
The data elements collected in the postmortem microscopic examination are provided in Fig. 8.31–35 Nearly all the organs were sampled, and routine hematoxylin and eosin–stained sections were prepared. The tissue blocks were submitted in a predetermined and standardized order (Table 1). This facilitates the future retrieval of blocks specific to certain organs or tissue types. No histochemical or immunohistochemical stains were routinely used. If there was a peculiar or rare morphological pattern that would be of interest to the future investigators, or if any additional diagnostic methods were used, the pathologists were encouraged to obtain microscopic images. When immunohistochemical stains were required, the most frequently used antibodies were those to detect viral infections such as cytomegalovirus, parvovirus B19, and herpes simplex. When it was impossible to obtain enough tissue for two blocks from an organ, paraffin curls and unstained slides were prepared and these were sent to the tissue repository.
Figure 8.
Data elements collected in the postmortem microscopic examination.
RESULTS
The standardized postmortem examination protocol was instituted and followed by seven pathologists at four medical centers. A total of 663 women with stillbirth were enrolled into the case-control study: 620 delivered a single stillborn infant, 42 delivered twins (13 sets with two stillborn infants and 29 sets with1stillborn and 1 live-born infant), and 1 delivered triplets (1 stillborn and 2 live-born infants), for a total of 707 infants. Of these women, 560 (84.5%) consented to postmortem examination (572 stillborn infants), and in 500 (75.4%), the examination was considered to have been adequate and included internal examination with the exception of the brain and an adequate placental examination (512 stillborn infants). Five of the 572 stillborn infants had some degree of fragmentation. Mild maceration was reported for 378 (66.9%) infants and marked maceration for 75 (13.3%).
DISCUSSION
Postmortem examination is one of the most important diagnostic tools employed by physicians. It began as an anatomic dissection where the purpose was to define normal structures; later, the focus changed to identifying the abnormal conditions (pathology) involving the human body. However, with the development of newer diagnostic and therapeutic tools and techniques, its use declined. Despite numerous studies proving the important contribution of postmortem examination to our understanding of perinatal diseases, it is still considered by some as a waste of time and resources unless performed for forensic reasons.17,31–35
Stillborns represent a unique group of patients who are inaccessible to most diagnostic tools in utero. Postmortem examination in the case of stillbirth is crucial as it is the procedure most likely to yield a cause of death.3,31–39 A major aim of the SCRN was to develop standardized research protocols for postmortem and placental examination to provide uniform data collection and to best identify stillborns where a fetal or placental condition caused or significantly contributed to the fetal death. The SCRN postmortem examination protocol was designed by consensus by the participating pathologists. Although the basic techniques of performing the postmortem examination have been described before,7–13 several aspects of our examination and data collection procedures were unique.
One of the main objectives of the developed protocol was to standardize the various pathologists’ approach to a diverse group of cases and improve on the current practice of using different techniques ranging from no examination to the implementation of complete postmortem examination protocol.
SCRN pathologists recorded the presence or absence of lesions and exact measurements instead of recording diagnostic categories, allowing the correlation of specific postmortem findings with causes of fetal demise. Furthermore, the pathologists obtained anthropomorphic and other measurements but did not compare them with historic reference values produced without benefit of modern methods for gestational dating.36–39 The plan is to compare them with more reliable reference values produced at the completion of the SCRN study and after accounting for the presence of maceration.
Determination of what is “normal” and “abnormal” is a significant step when examining samples from a fetus still actively growing. As the size, weight, and appearance of the organs changes progressively over gestation, reliable information regarding the temporal change in morphology of these organs is required. To accomplish this, the SCRN postmortem protocol specified the collection of maximum number of samples and measurements from as many fetuses as was practicable, taking into consideration the extent of the various conditions included.
The basic challenge in diagnostic perinatal pathology is to acquire the maximum information, often from minimal lesions, and with limited knowledge of preceding events. The difficulties are compounded in the antepartum stillborn by the delay between fetal death and delivery. This is not an easy task because there may be limited information regarding the timing of death. The fetal tissues may have lost weight following fetal death, and the maceration process may obscure structural details of the tissue. It can also be difficult to distinguish between pathological changes, which preceded fetal death, and the autolytic changes, which followed death. Within the SCRN cases, a subset of stillbirths with detailed information about the course of the pregnancy and timing of fetal death within 24 hours is available and can be matched with the grade of maceration defined a priori as part of the standardized postmortem examination. Analysis of this subset of stillbirths will allow correlation of anthropometric measurements and degree of maceration with gestational age at fetal death and the interval between death and delivery, thereby potentially improving the ability to predict gestational age at demise among those for which the timing is uncertain. In addition to deriving the reference values, this approach will allow us to examine the yield of various measurements under various maceration conditions.
One of the most novel aspects of this protocol is the creation of a computerized database covering all aspects of the protocol including anatomic pathology findings. This database is supplemented with the biological samples obtained from the fetuses and placentas. This unique database will allow the correlation of various pathological conditions with various biological markers and pathogenic pathways. The extensive collection and banking of postmortem biospecimens will provide a valuable resource for additional research.
Finally, from an organizational perspective, the basic structure of SCRN involved numerous medical facilities and personnel from different specialties and expertise. Execution of all the standard protocols covering a gamut of techniques from maternal interview to postmortem examination required the creation of efficient and extensive collaboration between the clinicians, their support staff, and the corresponding colleagues in perinatal pathology. Although the structure of this collaboration was not uniform between the participating institutions, the collaboration was nevertheless successfully accomplished. As a consequence, all the participants gained invaluable experience in collaboration. Sharing this and other experiences will improve patient care in an area that has been neglected for a long period of time.
Acknowledgments
FUNDING
Supported in part by grant funding from the Stillbirth Collaborative Research Network sites: U10-HD045953 (Brown University, Rhode Island); U10-HD045925 (Emory University, Georgia); U10-HD045952 (University of Texas Medical Branch at Galveston, Texas); U10-HD045955 (University of Texas Health Science Center at San Antonio, Texas); U10-HD045944 (University of Utah Health Sciences Center, Utah); and U01-HD-45954 (RTI International, North Carolina); and by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
- 1.Frøen JF, Arnestad M, Frey K, Vege A, Saugstad OD, Stray-Pedersen B. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986–1995. Am J Obstet Gynecol. 2001;184:694–702. doi: 10.1067/mob.2001.110697. [DOI] [PubMed] [Google Scholar]
- 2.Barfield WD, Tomashek KM, Flowers LM, Iyasu S. Contribution of late fetal deaths to US perinatal mortality rates, 1995–1998. Semin Perinatol. 2002;26:17–24. doi: 10.1053/sper.2002.29850. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Kirby R, Culhane JF. Stillbirth: a review. J Matern Fetal Neonatal Med. 2004;16:79–94. doi: 10.1080/14767050400003801. [DOI] [PubMed] [Google Scholar]
- 4.MacDorman MF, Minino AM, Strobino DM, Guyer B. Annual summary of vital statistics—2001. Pediatrics. 2002;110:1037–1052. doi: 10.1542/peds.110.6.1037. [DOI] [PubMed] [Google Scholar]
- 5.MacDorman MF, Hoyert DL, Martin JA, Munson ML, Hamilton BE. Fetal and perinatal mortality, United States, 2003. Natl Vital Stat Rep. 2007;55:1–17. [PubMed] [Google Scholar]
- 6.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 7.Bove KE Autopsy Committee of the College of American Pathologists. Practice guidelines for autopsy pathology: the perinatal and pediatric autopsy. Arch Pathol Lab Med. 1997;121:368–376. [PubMed] [Google Scholar]
- 8.Valdes-Dapena M, Huff D. Perinatal Autopsy Manual. Washington, DC: Armed Forces Institute of Pathology; 1983. [Google Scholar]
- 9.McPherson TA, Valdes-Dapena M. The perinatal autopsy. In: Wigglesworth JS, Singer DB, editors. Textbook of Fetal and Perinatal Pathology. 2. Boston: Blackwell Scientific Publications; 1998. pp. 87–110. [Google Scholar]
- 10.Gilbert-Barness E, editor. Handbook of Pediatric Autopsy Pathology. New York, NY: Humana Press; 2004. [Google Scholar]
- 11.Gilbert-Barness E, Debich-Spicer D. Embryo and Fetal Pathology. New York: Cambridge University Press; 2004. [Google Scholar]
- 12.Gilbert-Barness E, editor. Potter’s Pathology of the Fetus, Infant and Child. Philadelphia, PA: Mosby; 2007. [Google Scholar]
- 13.Keeling J. The perinatal necropsy. In: Keeling J, editor. Fetal and Neonatal Pathology. 3. London: Springer-Verlag; 2001. pp. 1–45. [Google Scholar]
- 14.Hankins G, Willinger M, Spong CY. Stillbirth—introduction. Semin Perinatol. 2002;26:1–2. [Google Scholar]
- 15.Spong CY, Erickson K, Willinger M, Hankins GD, Schulkin J. Stillbirth in obstetric practice: report of survey findings. J Matern Fetal Neonatal Med. 2003;14:39–44. doi: 10.1080/jmf.14.1.39.44. [DOI] [PubMed] [Google Scholar]
- 16.Chichester M. Requesting perinatal autopsy: multicultural considerations. MCN Am J Matern Child Nurs. 2007;32:81–86. doi: 10.1097/01.NMC.0000264286.03609.bd. quiz 87–88. [DOI] [PubMed] [Google Scholar]
- 17.Saller DN, Jr, Lesser KB, Harrel U, Rogers BB, Oyer CE. The clinical utility of the perinatal autopsy. JAMA. 1995;273:663–665. doi: 10.1001/jama.273.8.663. [DOI] [PubMed] [Google Scholar]
- 18.Genest DR, Singer DB. Estimating the time of death in stillborn fetuses: III. External fetal examination; a study of 86 stillborns. Obstet Gynecol. 1992;80:593–600. [PubMed] [Google Scholar]
- 19.Aase JM. Diagnostic Dysmorphology. New York and London: Plenum Medical Book Company; 1990. The physical examination in dysmorphology. [Google Scholar]
- 20.Merlob P, Sivan Y, Reisner SH. Anthropometric measurements of the newborn infant (27 to 41 gestational weeks) Birth Defects Orig Artic Ser. 1984;20:1–52. [PubMed] [Google Scholar]
- 21.Hansen K, Sung CJ, Huang C, Pinar H, Singer DB, Oyer CE. Reference values for second trimester fetal and neonatal organ weights and measurements. Pediatr Dev Pathol. 2003;6:160–167. doi: 10.1007/s10024-002-1117-3. [DOI] [PubMed] [Google Scholar]
- 22.Caffey J. Pediatric X-Ray Diagnosis. 6. Chicago: Year Book Medical; 1972. [Google Scholar]
- 23.Olsen EØE, Espeland A, Maartmann-Moe H, Lachman RS, Rosendahl K. Diagnostic value of radiography in cases of perinatal death: a population based study. Arch Dis Child Fetal Neonatal Ed. 2003;88:F521–F524. doi: 10.1136/fn.88.6.F521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly WH, Hawkins H. Optimal examination of the normally formed perinatal heart. Hum Pathol. 1987;18:55–60. doi: 10.1016/s0046-8177(87)80194-6. [DOI] [PubMed] [Google Scholar]
- 25.Devine WA, Debich DE, Anderson RH. Dissection of congenitally malformed hearts, with comments on the value of sequential segmental analysis. Pediatr Pathol. 1991;11:235–259. doi: 10.3109/15513819109064762. [DOI] [PubMed] [Google Scholar]
- 26.Rimoin DL, Cohn D, Krakow D, Wilcox W, Lachman RS, Alanay Y. The skeletal dysplasias: clinical-molecular correlations. Ann N Y Acad Sci. 2007;1117:302–309. doi: 10.1196/annals.1402.072. [DOI] [PubMed] [Google Scholar]
- 27.Rinaldo P, Yoon H-R, Yu C, Raymond K, Tiozzo C, Giordano G. Sudden and unexpected neonatal death: a protocol for the postmortem diagnosis of fatty acid oxidation disorders. Semin Perinatol. 1999;23:204–210. doi: 10.1016/s0146-0005(99)80052-4. [DOI] [PubMed] [Google Scholar]
- 28.Wapner RJ, Lewis D. Genetics and metabolic causes of stillbirth. Semin Perinatol. 2002;26:70–74. doi: 10.1053/sper.2002.29853. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox RL, Nelson CC, Stenzel P, Steiner RD. Postmortem screening for fatty acid oxidation disorders by analysis of Guthrie cards with tandem mass spectrometry in sudden unexpected death in infancy. J Pediatr. 2002;141:833–836. doi: 10.1067/mpd.2002.130259. [DOI] [PubMed] [Google Scholar]
- 30.Christodoulou J, Wilcken B. Perimortem laboratory investigation of genetic metabolic disorders. Semin Neonatol. 2004;9:275–280. doi: 10.1016/j.siny.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Faye-Petersen OM, Guinn DA, Wenstrom KD. Value of perinatal autopsy. Obstet Gynecol. 1999;94:915–920. doi: 10.1016/s0029-7844(99)00468-8. [DOI] [PubMed] [Google Scholar]
- 32.Bendon RW. Review of some causes of stillbirth. Pediatr Dev Pathol. 2001;4:517–531. doi: 10.1007/s10024001-0084-4. [DOI] [PubMed] [Google Scholar]
- 33.Magee JF. Investigation of stillbirth. Pediatr Dev Pathol. 2001;4:1–22. doi: 10.1007/s100240010121. [DOI] [PubMed] [Google Scholar]
- 34.Horn L-C, Langner A, Stiehl P, Wittekind C, Faber R. Identification of the causes of intrauterine death during 310 consecutive autopsies. Eur J Obstet Gynecol Reprod Biol. 2004;113:134–138. doi: 10.1016/S0301-2115(03)00371-3. [DOI] [PubMed] [Google Scholar]
- 35.Pinar H. Postmortem findings in term neonates. Semin Neonatol. 2004;9:289–302. doi: 10.1016/j.siny.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Hern WM. Correlation of fetal age and measurements between 10 and 26 weeks of gestation. Obstet Gynecol. 1984;63:26–32. [PubMed] [Google Scholar]
- 37.Maroun LL, Graem N. Autopsy standards of body parameters and fresh organ weights in nonmacerated and macerated human fetuses. Pediatr Dev Pathol. 2005;8:204–217. doi: 10.1007/s10024-004-7084-0. [DOI] [PubMed] [Google Scholar]
- 38.Singer DB, Sung CJ, Wigglesworth JS. Fetal growth and maturation: with standards for body and organ development. In: Wigglesworth JS, Singer DB, editors. Textbook of Fetal and Perinatal Pathology. 2. Boston: Blackwell Scientific Publications; 1998. pp. 8–40. [Google Scholar]
- 39.Archie JG, Collins JS, Lebel RR. Quantitative standards for fetal and neonatal autopsy. Am J Clin Pathol. 2006;126:256–265. doi: 10.1309/FK9D-5WBA-1UEP-T5BB. [DOI] [PubMed] [Google Scholar]