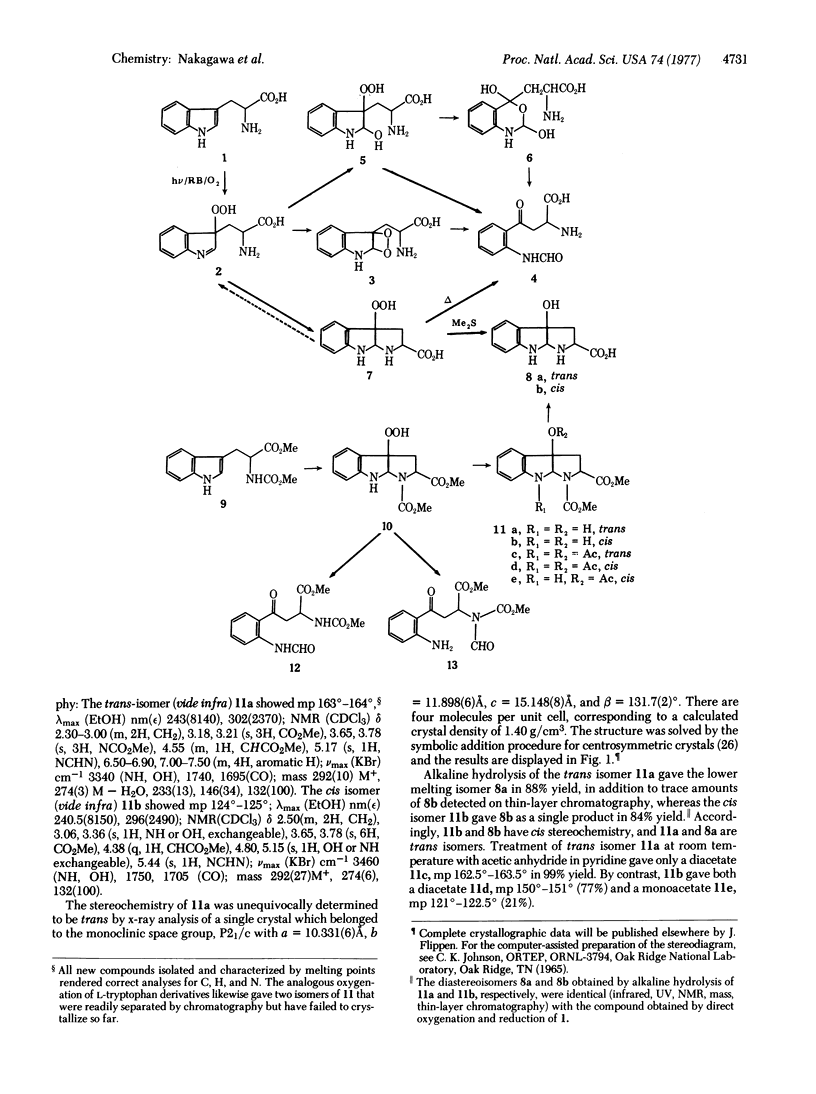
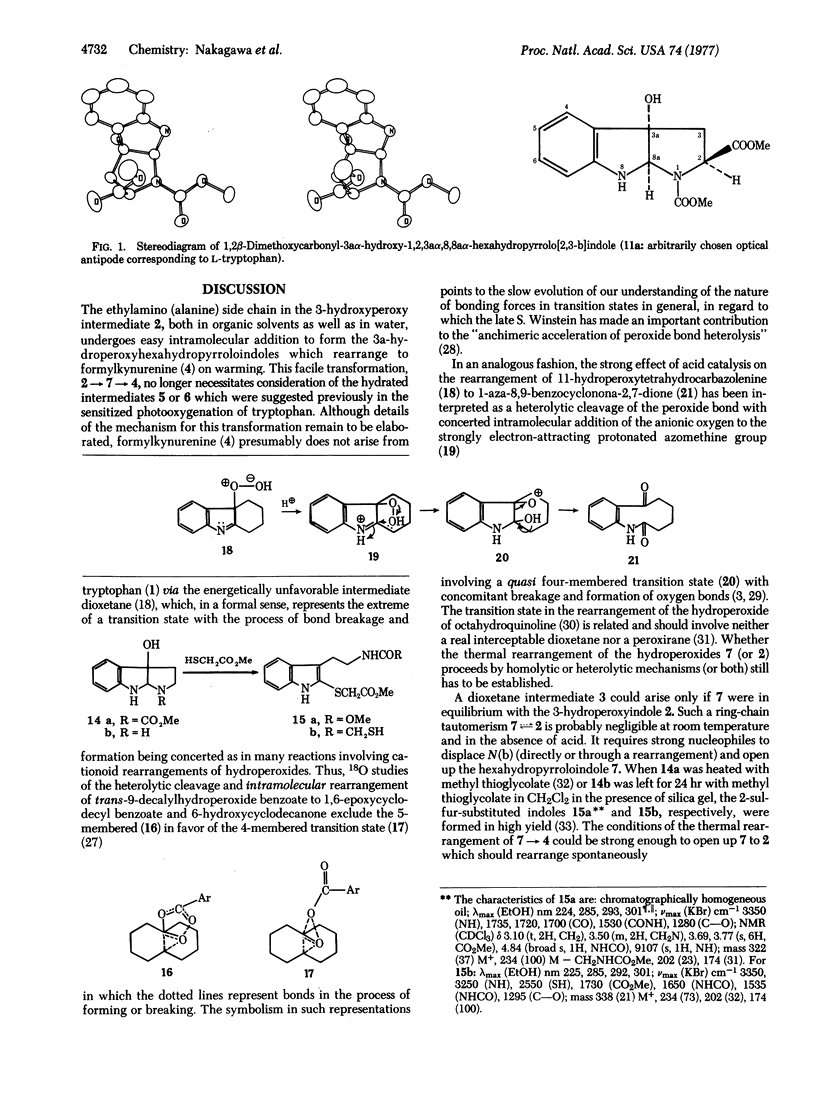
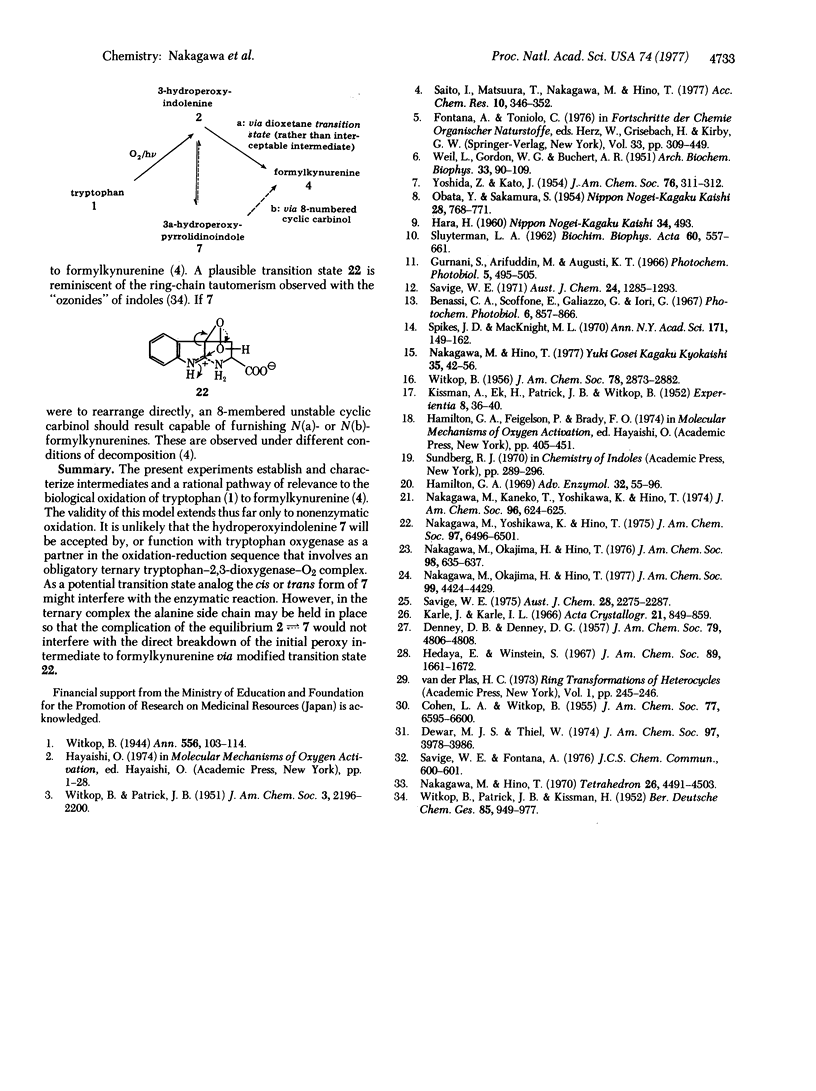
Abstract
The dye-sensitized photooxygenation of DL-tryptophan in aqueous solution leads to the tricyclic compound 2-carboxy-3a-hydroperoxy-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indole which, on reduction with dimethyl sulfide, furnishes two diastereoisomeric alcohols separable by fractional crystallization into a higher melting (mp 254°-256°) and a lower melting (mp 228°) diastereoisomer. Each of these alcohols was correlated with one of the analogous pair of isomeric 1,2-dicarbomethoxy analogs by alkaline hydrolysis and by x-ray analysis. In this way, the 3a-hydroxy-1,2-dimethoxycarbonyl- 1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indole, mp 163°-164°, was shown to have the trans configuration with regard to the relative positions of the hydroxyl and carbomethoxy groups and that, on alkaline hydrolysis, it produced the isomer with mp 228°, which therefore also has the trans configuration. The mechanism of the smooth thermal rearrangement of the (presumably ring-chain tautomeric) tryptophan hydroperoxy intermediates to formylkynurenine is discussed with its implications for the biological oxidation by tryptophan 2,3-dioxygenase.
Keywords: dye-sensitized photooxygenation, labile hydroperoxy tryptophan metabolites, x-ray analysis
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EK A., KISSMAN H., PATRICK J. B., WITKOP B. Chemical contributions to the mechanism of the biological oxidation of tryptophan. Experientia. 1952 Jan 15;8(1):36–40. doi: 10.1007/BF02168898. [DOI] [PubMed] [Google Scholar]
- Gurnani S., Arifuddin M., Augusti K. T. Effect of visible light on amino acids. I. Tryptophan. Photochem Photobiol. 1966 Jul;5(7):495–505. doi: 10.1111/j.1751-1097.1966.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Hamilton G. A. Mechanisms of two- and four-electron oxidations catalyzed by some metalloenzymes. Adv Enzymol Relat Areas Mol Biol. 1969;32:55–96. doi: 10.1002/9780470122778.ch3. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Kaneko T., Yoshikawa K., Hino T. Photosensitized oxygenation of tryptophan methyl ester and Nb-methyl-tryptamine. Isolation and identification of 3a-hydroxypyrroloindole and 4a-hydroxy-1,2-oxazinoindole. J Am Chem Soc. 1974 Jan 23;96(2):624–625. doi: 10.1021/ja00809a071. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Okajima H., Hino T. Photosensitized oxygenation of Nb-methoxycarbonyltryptamines. A new pathway to kynurenine derivatives. J Am Chem Soc. 1977 Jun 22;99(13):4424–4429. doi: 10.1021/ja00455a035. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Yoshikawa K., Hino T. The photosensitized oxygenation of Nb-methyltryptamine. J Am Chem Soc. 1975 Oct 29;97(22):6496–6501. doi: 10.1021/ja00855a035. [DOI] [PubMed] [Google Scholar]
- SLUYTERMAN L. A. Photo-oxidation, sensitized by proflavine, of a number of protein constituents. Biochim Biophys Acta. 1962 Jul 16;60:557–561. doi: 10.1016/0006-3002(62)90874-0. [DOI] [PubMed] [Google Scholar]
- WEIL L., GORDON W. G., BUCHERT A. R. Photooxidation of amino acids in the presence of methylene blue. Arch Biochem Biophys. 1951 Aug;33(1):90–109. doi: 10.1016/0003-9861(51)90084-7. [DOI] [PubMed] [Google Scholar]


