Abstract
Autophagy is a process preserving the balance between synthesis, degradation and recycling of cellular components and is therefore essential for neuronal survival and function. Several key proteins govern the autophagy pathway including beclin1 and microtubule associated protein 1 light chain 3 (LC3). Here, we show a brain-specific reduction in beclin1 expression in postmortem hippocampus of schizophrenia patients, not detected in peripheral lymphocytes. This is in contrast with activity-dependent neuroprotective protein (ADNP) and ADNP2, which we have previously found to be deregulated in postmortem hippocampal samples from schizophrenia patients, but that now showed a significantly increased expression in lymphocytes from related patients, similar to increases in the anti-apoptotic, beclin1-interacting, Bcl2. The increase in ADNP was associated with the initial stages of the disease, possibly reflecting a compensatory effect. The increase in ADNP2 might be a consequence of neuroleptic treatment, as seen in rats subjected to clozapine treatment. ADNP haploinsufficiency in mice, which results in age-related neuronal death, cognitive and social dysfunction, exhibited reduced hippocampal beclin1 and increased Bcl2 expression (mimicking schizophrenia and normal human aging). At the protein level, ADNP co-immunoprecipitated with LC3B suggesting a direct association with the autophagy process and paving the path to novel targets for drug design.
Keywords: activity-dependent neuroprotective protein, beclin, microtubule-associated protein 1 light chain 3, BcL2, schizophrenia
Introduction
Autophagy is a highly regulated process, having a crucial impact on cellular homeostasis. While autophagy is constitutively active in cells, it also presents an adaptive tactic to survive stress conditions, such as starvation.1 Autophagy is particularly important in the highly sensitive cells of the nervous system—the neurons. By preserving the balance between synthesis/degradation and recycling of cellular components, autophagy is essential for neuronal survival. Blockade of autophagy in neurons led to cell death and neurodegeneration in rodents.2,3 Several studies pointed out that abnormality in autophagy may lead to activation of apoptosis4,5 as detected in the most prevalent neurodegenerative disease, Alzheimer's disease (AD). Schizophrenia and AD have similarities in the pattern of regional brain dysfunction, biochemical dysfunction and apoptotic processes.6 Postmortem brain neuropathology in schizophrenia is characterized by synaptic and dendritic deficits in the cerebral cortex and hippocampus, findings that may be related to neuroimaging evidence of reduced gray matter volume and a 40% volume reduction in the CA2 of the hippocampus in schizophrenic patient.7,8
The protein beclin1, central to the regulation of autophagy, is involved in formation of autophagosomes by membrane recruitment.9 Beclin1 functions in tumor suppression, life-span extension, cell death regulation, embryonic development, immune defense as well as in prevention of neurodegeneration and heart diseases.10 Beclin1 knockout is lethal to mouse embryos at day 7.5 in utero.11 Upregulation of beclin1 is essential for differentiation and for the autophagy process, whereas depletion of beclin1 may trigger apoptotic processes.11,12 In an amino-acid starvation model, beclin1 knockdown enhanced proapoptotic signals.13 An alteration in beclin1 expression has been reported in early AD.14 One of the main autophagy markers is the family of the microtubule-associated protein 1 light chain 3, LC3 proteins (LC3, LC3A and LC3B).15 LC3 is cleaved at the C-terminal to LC3-I and transformed by phosphatidylethanolamine (PE) to LC3-II. LC3II connects to the autophagosome membrane.16
Two-hybrid system analyses showed a potential direct interaction between LC3B and activity-dependent neuroprotective protein (ADNP).17 ADNP18 and the homologous protein ADNP2 provide cell protection.19 Both proteins are highly conserved in humans, mice and rats.20 These two-protein family members (ADNP and ADNP2) share structural domains including a homeobox domain profile and zinc finger domains,21 and may serve as transcription factors.22 ADNP was shown to regulate multiple gene families during embryonic development, including key transcription factors and essential genes associated with neurogenesis/organogenesis, lytic vacuoles and lipid transport. ADNP knockout in mice is embryonic lethal (at day 9.5) implicating ADNP as essential for neural tube closure and brain formation.23,24 ADNP haploinsufficient mice (ADNP+/−) showed a delay in neuronal tube closure compared to control mice and reduced astrocyte ability to provide neuroprotection coupled with age-related neuronal death. In addition, ADNP+/− mice showed cognitive deficits and social dysfunction.25 Interestingly, ADNP immunoreactivity is found in the nuclei as well as in the cytoplasm26 and in the extracellular space,27 and the ADNP sequence includes a nuclear localization signal, leucine-rich nuclear export sequence21 and cellular secretion/internalization sequences.27 In the nucleus, ADNP and ADNP2 are part of the chromatin remodeling complex, SWI/SNF.22,28 Recombinant ADNP provides neuroprotection29 and ADNP silencing/reduction results in increases in the proapoptotic P5321 and decreases in the neuronal/dendritic marker microtubule associated protein 2 (MAP2).26
ADNP contains a short eight amino-acid peptide sequence, NAPVSIPQ, called NAP.18 NAP impacts neuronal differentiation and survival in-vivo and in-vitro by interacting and stabilizing microtubule dynamics.30,31 NAP reduces the expression of P53,32 of activated caspase 3,33, 34, 35 the release of mitochondrial cytochrome C36 and DNA fragmentation,33 and protects the expression of MAP2,37 thereby inhibiting apoptosis.
Since ADNP presents clear developmental roles as well as a microtubule/neuroprotective role coupled with a phenotype of social and cognitive dysfunction in the haploinsufficient mouse,25 it was hypothesized that ADNP/ADNP2 expression may be deregulated in schizophrenia. Indeed, results showed ADNP and ADNP2 deregulation in postmortem hippocampus of schizophrenia patients compared to healthy matched controls. In addition, ADNP2 deregulation correlated with the disease progression.38 In a model of schizophrenia, the microtubule associated protein 6 (MAP6)-deficient mouse (also known as the stable tubule only (STOP) deficient mouse), the ADNP peptide fragment NAP provided protection against schizophrenia-like behaviors (hyperactivity and cognitive deficits).39 In a clinical trial, based on NAP efficacy in animal models, NAP (davunetide) protected functional capacity (activities of daily living) in schizophrenia patients40 and provided neuroprotection.41
Here, we propose a mechanism for cellular deregulation in schizophrenia associated with autophagy. Our results showed a significant reduction in beclin1 transcripts in the hippocampus but not in circulating lymphocytes of schizophrenia patients. The results suggested that brain autophagy has a role in the pathophysiology of schizophrenia. Our results further tied ADNP with the autophagic machinery by showing direct ADNP-LC3B interaction and decreased beclin1 RNA expression in the hippocampus of the ADNP+/− mouse. Furthermore, we found increased ADNP expression in circulating lymphocytes from schizophrenia patients compared to healthy controls and a negative correlation with disease duration, suggesting a possible compensatory mechanism/a biomarker for disease onset. We also found that ADNP2 expression is increased as a result of chronic clozapine (CLZ) treatment. Postmortem hippocampal transcript levels of Bcl2, a beclin1-interacting anti-apoptotic protein,42 correlated positively and significantly with age in normal controls but not in schizophrenia patients. A similar trend was observed in lymphocytes. In contrast to the pattern of changes in beclin1 transcript levels, Bcl-2 transcript level was found to be increased in lymphocytes from schizophrenia patients and in the ADNP+/− mouse hippocampus compared to controls. These results contribute to the understanding of the etiology of schizophrenia, towards potential future biomarkers en route to a novel target for drug design.
Materials and methods
Subjects
Postmortem hippocampal brain samples from 12 schizophrenia patients and 12 matched normal controls were obtained from the Victoria Brain Bank at the Florey Institute for Neuroscience and Mental Health, Australia. RNA extraction, reverse transcription and quantitative real time PCR were performed as described before and were under the same permission to use human materials (Ben Gurion University38). RNA integrity was routinely measured, as before.38 Lymphocyte RNA was isolated from controls (n=13) and schizophrenia (n=13) patients (age and sex matched without any other serious physical illness) and purified from blood specimens using Trizol reagent (Sigma, St Louis, MO, USA) with further purification of the RNA phase by the RNeasy Kit (Qiagen, Hilden, Germany). Purity and concentration of RNA samples were determined spectrophotometrically (GeneQuant, Pharmacia Biotech, England, UK). Additional verification of RNA quantity was determined by measuring OD260 with a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). RNA integrity was determined by fractionation on 1% agarose gel and staining with ethidium bromide. Reverse transcription and quantitative real time PCR were performed as described before.38
Beclin1 primers: 5′-gagaggagccatttattgaaac-3′ and 5′-ctccccaatcagagtgaagc-3′. ADNP primers: 5′-cttacgaaaaaccaggactatc-3′ and 5′-gacattgcggaaatgac-3′. ADNP2 primers: 5′-gaaagaaagtgagatatcgaacaaa-3′ and 5′-tggtcaatttcatcttcatgg-3′. Bcl2 primers: 5′-cctgtggatgactgagtacc-3′ and 3′-gagacagccaggagaaat-5′. BAX primers: 5′-agggtttcatccaggatcgag-3′ and 5′-cactcgctcagcttcttggt-3′ (human) and 5′-ggctggacactggacttcct-3′ and 5′-ggtgaggactccagccacaa-3′ (mouse). Primers for SQSMT1 (encoding for human p62): 5′-agaatcagcttctggtccatcg-3′ and 5′-cttcttttccctccgtgctc-3′. All genes were normalized to the TATA box binding protein gene (reference gene): 5′ggagccaagagtgaagaacag3′ and 5′cacagctccccaccatattc3′. The primers were designed using the primer 3 web interface (http://frodo.wi.mit.edu/primer3/) and synthesized by Sigma-Genosys (The Woodlands, TX, USA).
Animal experiments
All experiments were conducted according to the guidelines for care and use of laboratory animals at Tel-Aviv University, under governmental permission.
CLZ treatment of rats
Fourteen Sprague–Dawley (SD) rats (7 control and 7 treated rats, three per cage), (Harlan, Rehovot, Israel) weighing 200–250 g (age 3 months) were kept at 12 h light/dark cycles. Food and water was provided ad libitum. Clozapine (CLZ, Sigma, St Louis, MO, USA) was administered in the drinking water (25 g per liter) for 21 days.43 CLZ was dissolved in a minimum volume of 2 N HCl solution.44 The solution was further diluted with drinking water, and the pH was adjusted using 10 N NaOH to neutral pH (6–6.5). Rats were sacrificed on the last day of drug administration. Spleen samples were taken and stored at −80 °C.
Rats were decapitated and blood was collected. Rat lymphocytes were isolated from 10 ml of blood using the Ficoll-PaquePremium 1.084 density (GE Healthcare, Little Chalfont, UK) according to the manufacturer's instructions. RNA isolation was conducted using Trizol as described above. RNA from rat spleen was extracted using RNeasy Plus Micro Kit (Qiagen, Hilden, Germany). Purity, concentration and real time PCR were conducted as previously described20,35 (and as above).
ADNP+/− mice
ADNP knockout mice were developed at the Tel Aviv University laboratory of Professor Illana Gozes (corresponding author of the current paper).23,25 Nineteen mice (~6.5 months old) were decapitated, brains removed quickly, hippocampus was dissected, frozen in liquid nitrogen and maintained frozen (−80 °C) until further processing. RNA and protein were extracted using Macherey-Nagel NucleoSpin kit. Reverse transcription and quantitative real time PCR were performed as previously described45 (and as above).
Immunoprecipitation (IP)
Protein fractions were taken from the hippocampus of ~6.5-month-old ADNP+/− male mice and from wild-type mice (ADNP+/+) for further immunoprecipitation (IP) using the Co-IP kit (Pierce, Rockford, IL, USA) protocol as follows. 30 μl of A/G PLUS-Agarose beads were loaded to the IP column. Ten micrograms of anti-ADNP antibodies (Bethyl Laboratories Inc., Montgomery, TX, USA) were added to the beads followed by one hour incubation at 24 °C. To cross link the antibodies to the beads, 2.5 mM DSS (disuccinimidyl suberate) were added to the IP column for one hour incubation. After that, cleared hippocampal mouse lysates (500 μg) were added to the IP columns and incubated (16 h, 4 °C). Eluted antigen was identified by 15% polyacrylamide gel electrophoresis followed by western blot analysis for LC3B.28
For assessment of the involvement of the NAP epitope in ADNP–LC3B interaction, extracts of the neuroblastoma cell line SH-SY5Y were used. 106 cells per 75 cm2 flasks (Corning, NY, USA) were plated and incubated for 4 days in RPMI (Beit-Haemek, Israel) and was supplemented with 10% fetal calf serum (FCS, Beit-Haemek, Israel), 1% penicillin–streptomycin in a 5% CO2 atmosphere. Lysates of 500 μg or similar lysates in the presence of 3 mg NAP were precipitated with 10 μg ADNP antibody on IP column loaded with A/G agarose beads for 16 h, 4 °C and LC3B immunoreactive bands were detected as above.
Results
Human subject study
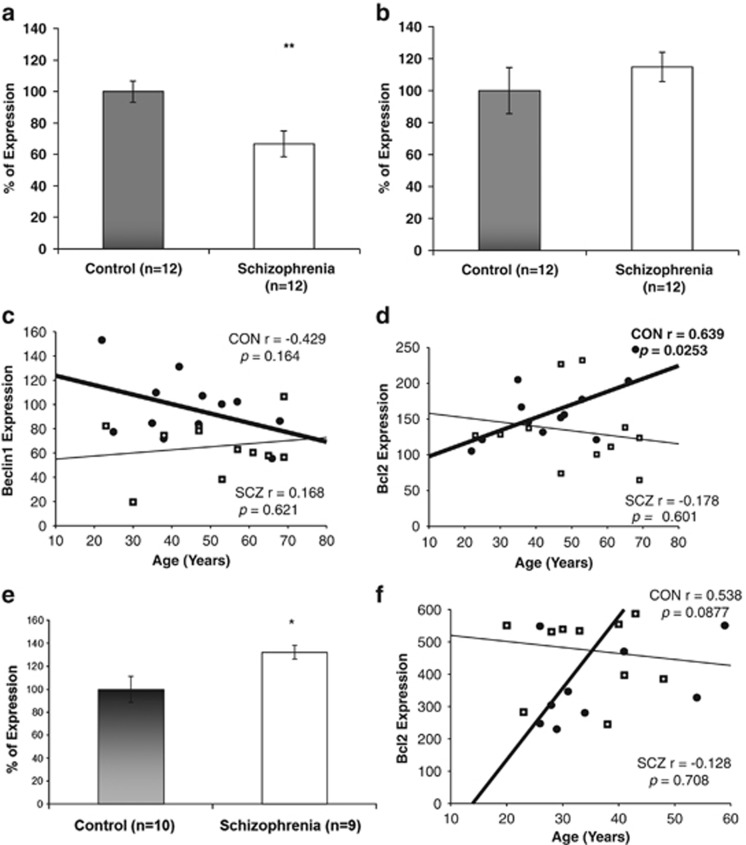
Beclin1 expression values were significantly decreased in postmortem hippocampus of schizophrenia patients (Figure 1a; **P<0.01). Results are depicted as percent of change of gene expression [2^ (-Δct)] with normal controls representing 100%. In fresh blood lymphocytes, no difference was detected in beclin1 expression between patients and normal controls, (Figure 1b; P=0.33). Hippocampal beclin1 expression did not correlate with age, in contrast to Bcl2, which specifically increased with normal but not with schizophrenia aging (Figures 1c and d). Bax and p62 transcript expression were similar in the hippocampal samples of controls and schizophrenia patients and did not correlate with age (Bax: control, r=0.245, P=0.398; schizophrenia, r=0.175, P=0.532; p62: control, r=0.241, P=0.406; schizophrenia, r=−0.122, P=0.665). In contrast, lymphocyte Bcl2 transcript was significantly increased (~30%) in schizophrenia patients and tended to increase with age in the healthy controls (Figure 1e and f).
Figure 1.
Beclinl transcript expression in postmortem hippocampus and lymphocytes of schizophrenia patients compared to healthy controls. (a) Hippocampus (**P<0.01). (b) Lymphocytes (no significant change). Results (% of control) are depicted as means +/− s.e.m (c, d). Age-dependence of the expression in postmortem hippocampus. (c) Beclinl did not correlate with age, although a trend of decrease with age was seen in the control subjects (Pearson's correlation, r=−0.429, P=0.164). (d) Bcl2 was significantly increased with age in the control subjects (Pearson's correlation, r=0.639, P<0.05). In contrast, Bcl2 expression did not correlate with age in hippocampal samples from the schizophrenia post-mortem brains. Control (CON)-closed circles, heavy line; schizophrenia (SCZ), squares with hairy +, light line. Expression levels=2−Δ Ct times 100. (e, f) Lymphocyte Bcl2 expression. (e) Bcl2 mRNA levels were significantly increased in schizophrenia patients compared to healthy age-matched controls (*P<0.05). Results were calculated as means +/−s.e.m, and relative expression as % of control. (f) A trend toward increased Bcl2 mRNA levels with age was observed only in the healthy cohort, similar to the results in the hippocampal samples (d) Control (CON)-closed circles, heavy line; schizophrenia (SCZ), squares with hairy +, light line. Expression levels=2−Δ Ct times.
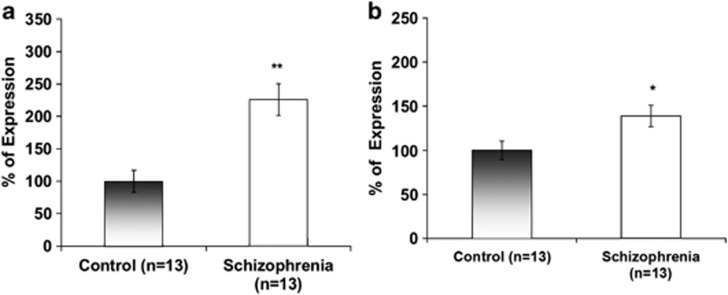
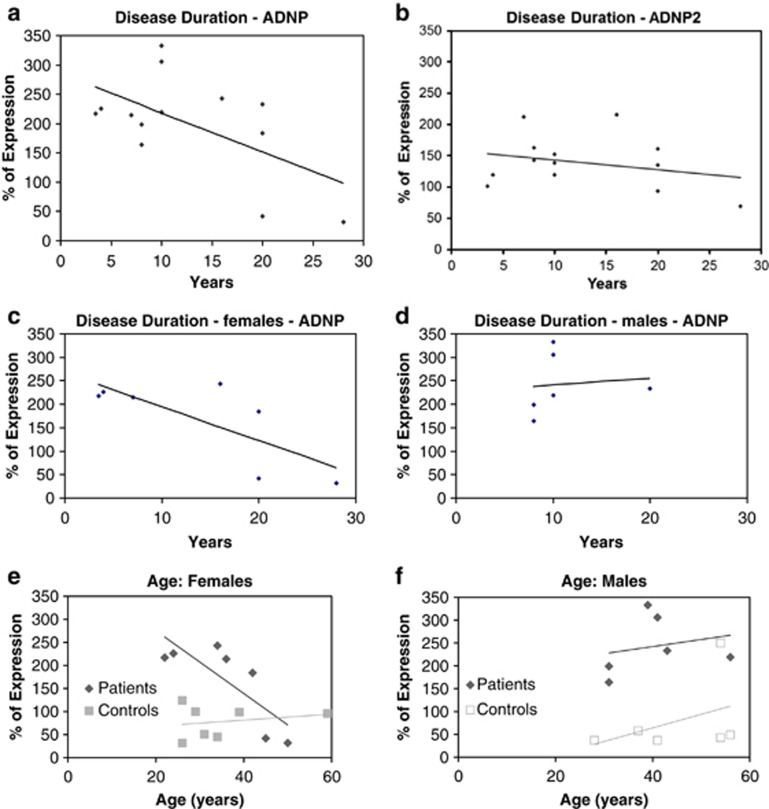
Previous results have shown a significantly reduced correlation between ADNP and ADNP2 in postmortem hippocampus of schizophrenia patients.38 Here, lymphocyte ADNP (like Bcl2) transcript expression was significantly increased in schizophrenia patients (Figure 2a; 150%, ***P<0.001). A 50% increase was observed in ANDP2 transcript in lymphocytes of schizophrenia patients (Figure 2b; *P<0.05). When correlating gene expression with disease duration, ADNP levels showed a significant negative correlation (Figure 3a; r=−0.581, P=0.037) which was not found for ADNP2 (Figure 3b) and was attributed to the female patients (Figure 3c and d, r=−0.76, P=0.049). Similar correlations were seen with age in the female patient population, but not in the normal controls, or in males (Figures 3e and f).
Figure 2.
ADNP and ADNP2 transcript expression in human lymphocytes. (a) ADNP and (b) ADNP2 expression was significantly increased in schizophrenia patients compared to healthy controls (**P<0.001 and *P<0.05, respectively). Expression levels were calculated as % of control.
Figure 3.
Effect of disease duration and sex on human lymphocyte ADNP and ADNP2 expression. (a) ADNP relative expression correlated negatively with disease duration (r=−0.581, P=0.037). (b) ADNP2 relative expression did not correlate with disease duration. (c) Female ADNP expression showed a significant negative correlation with disease duration (r=−0.76, P=0.049). (d) Male ADNP expression did not correlate with disease duration. Expression levels were calculated as % of control.
Taken together, we show that schizophrenia patients exhibit decreased hippocampal beclin1 transcript levels, along with the previously shown increase in ADNP2 levels (deregulation of ADNP/ADNP2 correlation)38 and correlation of Bcl2 expression only in normal aging, with contrasting results in peripheral lymphocytes, showing no change in beclin1 with a significant increase in ADNP, ADNP2 and Bcl2.
The effect of clozapine (CLZ) treatment
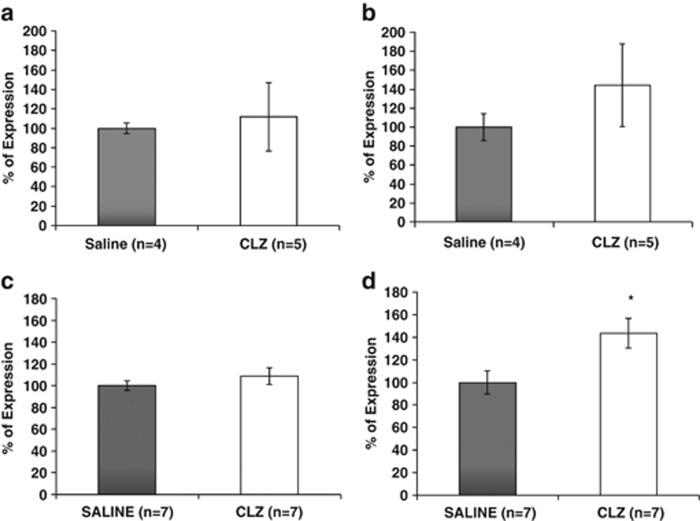
To evaluate whether the changes in ADNP and ADNP2 transcript levels in circulating lymphocytes reflect disease pathology or pharmaceutical intervention, rats were chronically treated with CLZ. No significant effect of CLZ treatment on ADNP and ADNP2 transcript levels was observed (Figure 4a and b, respectively). In parallel, rat spleen ADNP transcript level was not affected by CLZ treatment (Figure 4c), while that of ADNP2 was found to be significantly increased as a consequence of CLZ treatment (Figure 4d; *P<0.05).
Figure 4.
ADNP and ADNP2 expression in Sprague–Dawley rat lymphocytes and spleens following clozapine (CLZ) treatment (25 g per liter drinking water; 21 days). (a) ADNP transcript expression levels did not significantly change in lymphocytes of CLZ treated rats. (b) As in ADNP, no changes were detected in ADNP2 relative expression levels in lymphocytes of rats treated with CLZ. (c) ADNP expression values did not show any changes in spleens of rats treated with CLZ. (d) In contrast to ADNP, ADNP2 expression was significantly increased in spleens from CLZ-treated rats (*P<0.05).
Is there an association between beclin1, Bcl2 and ADNP expression?
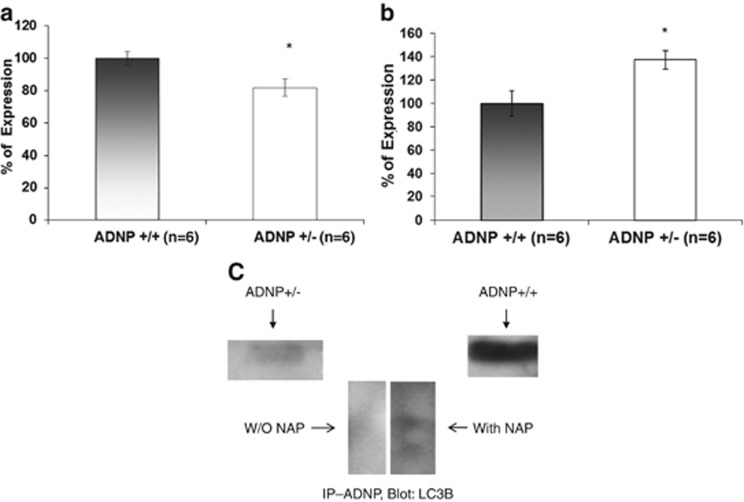
To examine whether the changes in beclin1 and ADNP are linked, we tested beclin1 RNA level of expression in ~6.5-month-old ADNP heterozygous (ADNP+/−) mouse hippocampus. It should be noted that previous studies did not show deregulation of ADNP2 transcripts in this model.28 Our results now showed that beclin1 RNA expression was decreased by ~20% in the ADNP-deficient mouse hippocampus (Figure 5a; *P<0.05) coupled to an ~30% increase in Bcl2 (Figure 5b; *P<0.05), suggesting an impact of ADNP deregulation on brain autophagy/apoptosis.
Figure 5.
Autophagy/apoptosis cascade markers in ADNP+/− mice. (a) Beclinl expression was significantly decreased in the hippocampus of the ADNP+/− mouse (*P<0.05). (b) Bcl2 mRNA expression was significantly increased in the hippocampus of the ADNP+/− mouse (*P<0.05). BAX levels did not significantly change (106% in ADNP+/− vs 100% in ADNP+/+, P=0.79, data not shown). 6.5-month-old male littermate ADNP+/− and ADNP+/+ mice were used. (c) Immunoprecipitation (IP) of ADNP revealed direct interaction with LC3B by western analysis. The ADNP–LC3B interaction correlated with the level of ADNP expression, a strong interaction in normal mice (ADNP+/+, right band) and a weaker interaction in ADNP+/− mice (left band). The interaction of ADNP with LC3B was augmented in the presence of NAP and two bands are seen in the SH-SY5Y neuronal model cells (middle panel).
Protein–protein interaction between ADNP and LC3B
To further assess the potential involvement of ADNP in autophagy, suggested by previous two hybrid system results, indicating that ADNP interacts with LC3B,17 we conducted ADNP immunoprecipitation followed by western blot analysis with anti-LC3B antibodies. Our results showed that hippocampal ADNP directly interacted with LC3B in ADNP+/+ mice (Figure 5c). The extent of the interaction was drastically decreased in the ADNP haploinsufficient (ADNP+/−) mice (Figure 5c) which exhibited a twofold reduction in ADNP expression.25 Furthermore, incubation of the LC3B–ADNP immune complex in the presence of NAP, the ADNP-derived drug candidate (davunetide) increased the LC3B–ADNP interaction, resulting in augmented LC3B-like immunoprecipitation by the ADNP antibody with two bands, representing the LC3 autophagy-related post-translational modification.
Conclusions
We found decreased beclin1 mRNA levels in the hippocampus of schizophrenia patients. Since beclin1 has a crucial role in the initiation of the autophagy process that is associated with the initiation of apoptosis, this protein may be regarded as a revolving door—with increased levels leading to autophagy and decreased levels leading to apoptosis. The finding is unique to the hippocampus as no changes were detected in fresh blood lymphocytes. In contrast to beclin1, Bcl2, ADNP and ADNP2 transcripts were significantly increased in lymphocytes from schizophrenia patients. The increase may reflect a compensatory mechanism coping with increasing pathology at the initial disease stage. In this respect, it should be borne in mind that ADNP can be found in the extracellular milieu and may be secreted to provide a protective environment.27 The negative correlation with disease progression, found particularly in female patients, might be related to the sexual dichotomy in ADNP expression, previously observed in the mouse hypothalamic arcuate nucleus that is regulated by estrogen levels.46 Indeed, sex differences in the incidence, onset and course of schizophrenia have led to the hypothesis that estrogens have a protective role in the pathophysiology of this disorder and that estradiol could be an effective augmentation strategy in the treatment of women with schizophrenia.47
Rat lymphocyte ADNP transcript levels did not significantly change following CLZ treatment, suggesting that the changes in ADNP expression may well be associated with the disease pathology. Interestingly, new findings show that mutations in ADNP are linked to autism.48 In contrast, ADNP2 levels were significantly increased by CLZ treatment, implicating ADNP2 as a possible peripheral biomarker responding to neuroleptic intervention.
ADNP deficiency in mice has previously been associated with learning and with social deficits, coupled with reduced neuronal protection, age-related enhanced cell death as well as tau/microtubule pathology.25 Here, we show for the first time that hippocampal ADNP deficiency paralleled reduced beclin1 expression which, in turn, parallels increased tauopathy and cell death.25 These results are in agreement with published data in a tauopathy model showing reduced neurodegeneration by trehalose-induced increased autophagy49 at the level of activation of LC3 cleavage. We now show that ADNP directly interacts with LC3B, implicating the requirement of a healthy ADNP system for the apoptotic/autophagy processes.
As autophagy has a role in synaptic and dendritic function, the present findings raise the possibility that normalizing beclin1 levels and compensating for ADNP/ADNP2/Bcl2 deregulation towards healthy values may provide novel therapeutic targets in schizophrenia. In this respect, NAP (davunetide), shown to have a positive impact on the activities of daily living, coupled to brain protection, in schizophrenia patients,40,41 may provide partial compensation for the deregulated ADNP system by increasing ADNP–LC3B interaction and, as recently shown, by restoring the autophagic function.50
Acknowledgments
Avia Merenlender–Wagner was an Eshkol Fellow and this work constitutes part of the requirements for a PhD thesis by Tel Aviv University; Professor Gozes' laboratory is supported by the AMN Foundation, CFTAU Montreal Circle of Friends, Joe and Grace Alter, Barbara and Don Seal, the Oberfeld and the Adams families, Adams Super Center for Brain Studies at Tel Aviv University. Initial studies in this research were also partially supported by Allon Therapeutics Inc. Professor Gozes is the incumbent of the Lily and Avraham Gildor Chair for the Investigation of Growth Factors at Tel Aviv University. Professor Agam is an incumbent of the Jack Dreyfus Chair in Psychiatry at the Ben-Gurion University of the Negev. BD is an NHMRC Senior Research Fellow (APP1002240). ES is an ARC Future Fellow (FT100100689). Postmortem brain samples were received from the Victorian Brain Bank Network, which is supported by the Florey Institute for Neuroscience and Mental Health, The Alfred, Victorian Forensic Institute of Medicine, and The University of Melbourne and funded by Australia's National Health & Medical Research Council, Victorian Government's Operational Infrastructure Support Program, Helen Macpherson Smith Trust, Parkinson's Victoria and Perpetual Philanthropic Services.
The authors declare no conflict of interest.
References
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, et al. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci USA. 2010;107:832–837. doi: 10.1073/pnas.0913170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death. Autophagy. 2005;1:66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- Sun Q, Fan W, Zhong Q. Regulation of Beclin 1 in autophagy. Autophagy. 2009;5:713–716. doi: 10.4161/auto.5.5.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008;4:947–948. doi: 10.4161/auto.6787. [DOI] [PubMed] [Google Scholar]
- Kim M, Fekadu J, Maronde E, Rami A.Alleviation of autophagy by knockdown of Beclin-1 enhances susceptibility of hippocampal neurons to proapoptotic signals induced by amino acid starvation Histochem Cell Biol 201313999–108. [DOI] [PubMed] [Google Scholar]
- Homma K, , , Suzuki K, , , Sugawara H. The Autophagy Database: an all-inclusive information resource on autophagy that provides nourishment for research. Nucleic Acids Res 2011; 39: D986–D990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Dang Y, Su W, Liu C, Ma H, Shan Y, et al. Molecular cloning and characterization of rat LC3A and LC3B—two novel markers of autophagosome. Biochem Biophys Res Commun. 2006;339:437–442. doi: 10.1016/j.bbrc.2005.10.211. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Vinayagam A, Stelzl U, Foulle R, Plassmann S, Zenkner M, Timm J, et al. A directed protein interaction network for investigating intracellular signal transduction. Sci Signal. 2011;4:rs8. doi: 10.1126/scisignal.2001699. [DOI] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- Kushnir M, Dresner E, Mandel S, Gozes I. Silencing of the ADNP-family member, ADNP2, results in changes in cellular viability under oxidative stress. J Neurochem. 2008;105:537–545. doi: 10.1111/j.1471-4159.2007.05173.x. [DOI] [PubMed] [Google Scholar]
- Sigalov E, Fridkin M, Brenneman DE, Gozes I. VIP-Related protection against lodoacetate toxicity in pheochromocytoma (PC12) cells: a model for ischemic/hypoxic injury. J Mol Neurosci. 2000;15:147–154. doi: 10.1385/JMN:15:3:147. [DOI] [PubMed] [Google Scholar]
- Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. 2001;276:708–714. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- Mandel S, Gozes I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J Biol Chem. 2007;282:34448–34456. doi: 10.1074/jbc.M704756200. [DOI] [PubMed] [Google Scholar]
- Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation Brain Res Dev Brain Res 200314483–90. [DOI] [PubMed] [Google Scholar]
- Mandel S, Rechavi G, Gozes I. Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol. 2007;303:814–824. doi: 10.1016/j.ydbio.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther. 2007;323:438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- Mandel S, Spivak-Pohis I, Gozes I. ADNP differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J Mol Neurosci. 2008;35:127–141. doi: 10.1007/s12031-007-9013-y. [DOI] [PubMed] [Google Scholar]
- Furman S, Steingart RA, Mandel S, Hauser JM, Brenneman DE, Gozes I. Subcellular localization and secretion of activity-dependent neuroprotective protein in astrocytes. Neuron Glia Biol. 2004;1:193–199. doi: 10.1017/S1740925X05000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresner E, Malishkevich A, Arviv C, Leibman Barak S, Alon S, Ofir R, et al. Novel Evolutionary-conserved Role for the Activity-dependent Neuroprotective Protein (ADNP) Family That Is Important for Erythropoiesis. J Biol Chem. 2012;287:40173–40185. doi: 10.1074/jbc.M112.387027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingart RA, Gozes I. Recombinant activity-dependent neuroprotective protein protects cells against oxidative stress. Mol Cell Endocrinol. 2006;252:148–153. doi: 10.1016/j.mce.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Divinski I, Mittelman L, Gozes I. A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem. 2004;279:28531–28538. doi: 10.1074/jbc.M403197200. [DOI] [PubMed] [Google Scholar]
- Khandhadia S, Cherry J, Lotety AJ.Age-Related Macular Degeneration. Neurodegener Diseases In: Ahmad SI, Landes Bioscience + Business Media (eds) 2012. Chapter 2, pp15–36.
- Gozes I, Steingart RA, Spier AD. NAP mechanisms of neuroprotection. J Mol Neurosci. 2004;24:67–72. doi: 10.1385/JMN:24:1:067. [DOI] [PubMed] [Google Scholar]
- Leker RR, Teichner A, Grigoriadis N, Ovadia H, Brenneman DE, Fridkin M, et al. NAP, a femtomolar-acting peptide, protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2002;33:1085–1092. doi: 10.1161/01.str.0000014207.05597.d7. [DOI] [PubMed] [Google Scholar]
- Idan-Feldman A, Ostritsky R, Gozes I. Tau and caspase 3 as targets for neuroprotection. Int J Alzheimers Dis. 2012;2012:493670. doi: 10.1155/2012/493670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idan-Feldman A, Schirer Y, Polyzoidou E, Touloumi O, Lagoudaki R, Grigoriadis NC, et al. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol Dis. 2011;44:327–339. doi: 10.1016/j.nbd.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Sapolsky R, Gozes I. NAP protects against cytochrome c release: inhibition of the initiation of apoptosis. Eur J Pharmacol. 2009;618:9–14. doi: 10.1016/j.ejphar.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Manley N, Sapolsky R, Gozes I. NAP protects hippocampal neurons against multiple toxins. Peptides. 2007;28:2004–2008. doi: 10.1016/j.peptides.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Dresner E, Agam G, Gozes I. Activity-dependent neuroprotective protein (ADNP) expression level is correlated with the expression of the sister protein ADNP2: deregulation in schizophrenia. Eur Neuropsychopharmacol. 2011;21:355–361. doi: 10.1016/j.euroneuro.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Merenlender-Wagner A, Pikman R, Giladi E, Andrieux A, Gozes I. NAP (davunetide) enhances cognitive behavior in the STOP heterozygous mouse—a microtubule-deficient model of schizophrenia. Peptides. 2010;31:1368–1373. doi: 10.1016/j.peptides.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF, et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res. 2012;136:25–31. doi: 10.1016/j.schres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Dong Z, Kangarlu A, Colibazzi T, Girgis RR, Kegeles LS, et al. Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology. 2013;38:1245–1252. doi: 10.1038/npp.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007;3:561–568. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Nadri C, Belmaker RH, Agam G. Lack of effect of mood stabilizers or neuroleptics on GSK-3 protein levels and GSK-3 activity. Int J Neuropsychopharmacol. 2003;6:117–120. doi: 10.1017/S1461145703003353. [DOI] [PubMed] [Google Scholar]
- Zink M, Schmitt A, May B, Muller B, Demirakca T, Braus DF, et al. Differential effects of long-term treatment with clozapine or haloperidol on GABAA receptor binding and GAD67 expression. Schizophr Res. 2004;66:151–157. doi: 10.1016/S0920-9964(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Dresner E, Agam G, Gozes I. Activity-dependent neuroprotective protein (ADNP) expression level is correlated with the expression of the sister protein ADNP2: Deregulation in schizophrenia. Eur Neuropsychopharmacol. 2011;21:355–361. doi: 10.1016/j.euroneuro.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Furman S, Hill JM, Vulih I, Zaltzman R, Hauser JM, Brenneman DE, et al. Sexual dimorphism of activity-dependent neuroprotective protein in the mouse arcuate nucleus. Neurosci Lett. 2005;373:73–78. doi: 10.1016/j.neulet.2004.09.077. [DOI] [PubMed] [Google Scholar]
- Begemann MJ, Dekker CF, van Lunenburg M, Sommer IE. Estrogen augmentation in schizophrenia: a quantitative review of current evidence. Schizophr Res. 2012;141:179–184. doi: 10.1016/j.schres.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Ben-David E, Shifman S. Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol Psychiatry. 2013;18:1054–1056. doi: 10.1038/mp.2012.148. [DOI] [PubMed] [Google Scholar]
- Schaeffer V, Lavenir I, Ozcelik S, Tolnay M, Winkler DT, Goedert M. Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain. 2012;135 (Pt 7:2169–2177. doi: 10.1093/brain/aws143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves AR, Gozes I, Cardoso SM. The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson's disease. Biochim Biophys Acta. 2013;1842:7–21. doi: 10.1016/j.bbadis.2013.10.003. [DOI] [PubMed] [Google Scholar]