Abstract
AIM: Capsaicin, a pungent ingredient found in red pepper, has long been used in spices, food additives, and drugs. Cell death induced by the binding of capsaicin was examined in a human gastric adenocarcinoma cell line (AGS cells).
METHODS: By using XTT-based cytotoxicity assay, flow cytometry using the TUNEL method, and quantitation of DNA fragmentation, both cell death and DNA fragmentation were detected in AGS cells treated with capsaicin. By using Western blotting methods, capsaicin reduced the expression of Bcl-2, the antiapoptotic protein, in AGS cells in a concentration-dependent manner.
RESULTS: After incubation of AGS cells with capsaicin for 24 h, cell viability decreased significantly in a dose-dependent manner. After incubation of AGS cells with capsaicin for 24 h, apoptotic bodies also significantly increased, and were again correlated with the dose of capsaicin. When the concentration of capsaicin was 1 mmol/L, the amount of DNA fragments also increased. Similar results were also in the lower traces.
CONCLUSION: These results suggest that capsaicin-induced cell death might be via a Bcl-2 sensitive apoptotic pathway. Therefore, capsaicin might induce protection from gastric cancer.
Keywords: Capsaicin, Human gastric adenocarcinoma, Apoptosis, Bcl-2
INTRODUCTION
Carcinoma of the stomach is currently the leading cause of cancer-related death among the Chinese. The two most likely factors for such a high incidence of stomach cancer has been thought to be either Helicobacter pylori[1] or diet. Capsaicin (8-methyl-N-vanillyl-6-nonenamide), the main ingredient of hot chili peppers, has long been used in spices, food additives, and drugs[2]. Capsaicin has been shown to protect the gastric mucosa of animals and humans against various kinds of damage[3-6]. It is generally considered that capsaicin’s effect results from the activation of sensory afferent neurons in the stomach, and is mediated by various physiological functions, such as mucosal blood flow[7-10], mucus secretion[11], and bicarbonate secretions[12]. Vanilloid receptor subtype 1 (VR1), a receptor responsible for capsaicin action, has been cloned[13] and demonstrated to be located in both neural and non-neural cells[14]. VR1 has also been found to be expressed peripherally in gastric mucosal epithelial cells, playing a role in cell protection[15]. Recently, a series of studies have demonstrated that capsaicin inhibits mutagenicity and DNA binding of some chemical carcinogens, possibly by suppressing their metabolic activation[16-18]. With cells in culture, capsaicin-inhibited proliferation of HeLa, ovarian carcinoma, and mammary adenocarcinoma by decreasing NADH oxidase activity[19]. Capsaicin can also alter the expression of tumor forming-related genes by mediating the overexpression of p53 and/or c-myc genes in a Korean stomach cancer cell line[20]. Capsaicin was found to induce apoptosis in T cells by increasing the reactive oxygen species and by a subsequent mitochondrial transmembrane potential[21]. In this report, we have examined the underlying mechanism by which capsaicin induces apoptotic cell death in a human gastric adenocarcinoma cell line (AGS).
MATERIALS AND METHODS
Cell line
A human gastric adenocarcinoma cell line (AGS) was obtained from American Type Culture Collection. The cells were maintained in RPMI 1640 medium (Life Technologies, Inc., Melbourne, Australia) supplemented with 10% heat-inactivated fetal bovine serum with penicillin (100 U/mL) and streptomycin (100 μg/mL). Cultures in 75- or 25-mL culture flasks were incubated at 37 °C in a humidified gas mixture containing 50 mL/L CO2 balanced with air.
XTT-based cytotoxicity assay
Unlabeled AGS cells (1×104/well) were distributed on to a 96-well flat-bottomed plate. Appropriate numbers of cells were added, resulting in triplicate wells, and a final volume of 100 μL of RPMI medium with 10% bovine serum was obtained. After incubation of AGS cells for 24 h, the medium was then replaced with serum-free RPMI medium containing capsaicin (0.05 μmol/L-10 mmol/L) at 37 °C for 24 h. The quantity of viable cell was determined using a cell proliferation ELISA kit from Boehringer Mannheim (No. 1465015), according to the recommendations of the supplier. Each sample was tested in triplicate.
Detection of apoptotic cells by flow cytometry using TUNEL method
For demonstration of apoptosis, TUNEL assay was performed with an in situ cell death detection kit (POD, Boehringer Mannheim) according to the manufacturer’s recommendations. After incubation of AGS cells for 24 h, cells were analyzed by Epics Elite ESP flow cytometer. Apoptosis was analyzed using the Wincycle Software (Coulter). Adherent cells were harvested and stained in hypotonic fluorochrome solution (propidium iodide 50 μg/mL in sodium citrate plus 0.1% Triton X-100, Sigma). Apoptotic nuclei were identified as a subgenomic DNA peak and were distinguished from cell debris on the basis of both forward light scatter and fluorescence of propidium iodide.
Quantitation of DNA fragmentation
Apoptosis was also evaluated with a cell death ELISA kit (Boehringer Mannheim, Indianapolis, IN, USA) which utilizes a monoclonal antibody against histone to detect DNA fragments in the cytosolic fraction of lysed cells. Cells treated with or without capsaicin were harvested and lysed according to the manufacturer’s instructions. The samples were transferred into 96-well dishes coated with a mouse monoclonal antibody against histone. After incubation and washing, anti-DNA-peroxidase was added to the wells. The reaction was developed with the substrate supplied by the manufacturer and the absorbance of the wells was read at 410 nm. The ratio of the absorbance of the treated cells to the untreated cells was calculated as an enrichment factor, which provides a qualitative assessment of apoptosis. Each sample was tested in triplicate.
Western blotting
After incubation of AGS cells for 24 h, about 107 cells were washed in PBS and solubilized buffer (50 mmol/L Tris-HCl pH 7.4, 125 mmol/L NaCl, 0.1% NP-40, 5 mmol/L NaF, 1 mmol/L PMSF, 1 ng/mL leupeptin, 10 ng/mL soybean trypsin inhibitor, 1 ng/mL aprotinin, 10 ng/mL N-tosyl-L-phenylalanyl chloromethyl ketone) for 60 min on ice. Lysates were centrifuged at 2 500 g for 5 min. Protein concentration was determined by means of the Bradford protein assay (BioRad Lab., Richmond, CA, USA) using bovine serum albumin as the standard. Thirty micrograms of protein was resolved by electrophoresis on 10% polya-crylamide gels, electrotransferred to a polyvinylidene difluoride filter (Millipore, Bedford, MA, USA), and then blotted with mouse monoclonal antibody for Bcl-2 (1:500 dilution). Blots were developed with peroxidase-labeled anti-mouse IgG (1:400 dilution) using a Lumi-Light Plus Western Blotting Kit (Boehringer Mannheim).
Statistical analysis
All values in the text and figures are expressed as mean±E. Statistical differences were evaluated by Student’s t-test in unpaired samples, by paired t-test in paired samples, or by Wilcoxon’s sum of ranks test. When evaluating multiple values, the Bonferroni correction was used. Probability values less than 0.05 were considered to be significant in all experiments. Analysis of the data and plotting of the figures were done with the aid of software (SigmaStat and SigmaPlot, Version 5.0, Jandel, USA; PHARM/PCS, Version 4.2, MCS, USA) run on an IBM PC-AT computer. Protein blot images were captured by an Imaging Densitometer with the aid of software (Bio-ID, V.97 software for Windows 95, Vilber Laurmat, France). Comparisons were made only between averaged values of bands within the same gel.
RESULTS
Based on the XTT assay, DNA fragmentation and flow cytometric analysis, induction of apoptosis by capsaicin was reconfirmed in stomach tumor cell line AGS. In this study, after incubation of AGS cells with capsaicin for 24 h, cell viability decreased significantly in a dose-dependent manner (Figure 1A). This reduction in cell viability induced by treatment with capsaicin was ascribed to apoptotic DNA fragmentation, and the amounts of fragmented DNA were also dependent on the dose of capsaicin (Figure 1B). After incubation of AGS cells with capsaicin for 24 h, apoptotic bodies also significantly increased, and were again correlated with the dose of capsaicin. The upper traces of Figure 2 show capsaicin-induced, concentration-dependent positive TUNEL staining. When the concentration of capsaicin was 1 mmol/L, the amount of DNA fragments also increased. Similar results were also in the lower traces of Figure 2, where apoptotic bodies increased due to the application of capsaicin. Figure 3 shows that the expression of Bcl-2, the antiapoptotic protein, was significantly reduced in AGS cells by capsaicin in a concentration-dependent manner.
Figure 1.
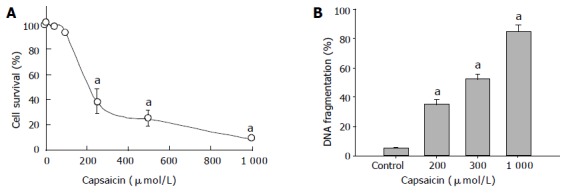
A: Effect of capsaicin on cell survival in the cultured gastric cancer cell line, AGS. Survival was analyzed by a cell proliferation ELISA kit from Boehringer Mannheim. The results are the mean±SE of three experiments. aP<0.05 vs solvent control; B: Effect of capsaicin on the amount of DNA fragmentation in the cultured gastric cancer cell line, AGS. The amount of fragmented DNA produced was determined with an ELISA kit from Boehringer Mannheim. The results are the mean±SEM of three experiments. aP<0.05 vs solvent control.
Figure 2.
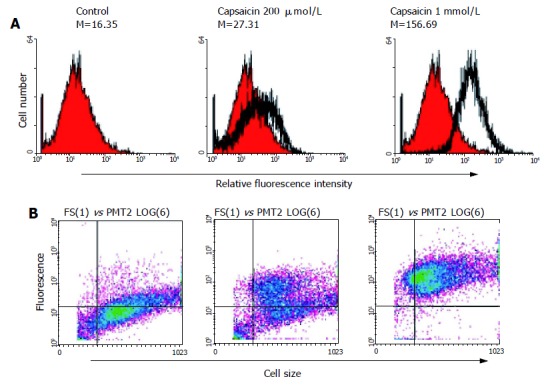
Flow cytometry analysis for capsaicin-induced apoptosis in AGS gastric carcinoma cells (A,B).
Figure 3.
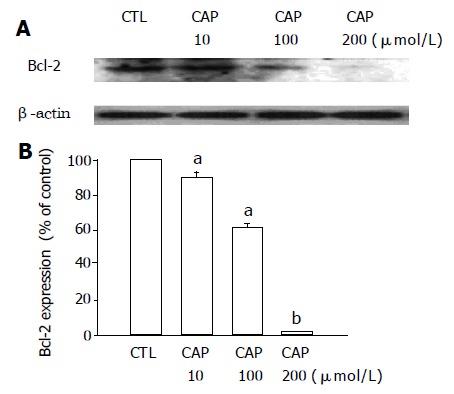
Effect of capsaicin (CAP) on the expression of Bcl-2 in AGS cells. Each bar represents means±SE of three experiments(A,B). aP<0.05, bP<0.01 vs solvent control (CTL).
DISCUSSION
Apoptosis, or programmed cell death, is a natural form of cell death controlled by a constitutively expressed machinery that induces condensation of the nucleoplasm and cytoplasm, blebbing of cytoplasmic membranes, and fragmentation of the cell into apoptotic bodies that are rapidly recognized and eliminated by adjacent cells[22-24]. Induction of apoptosis by the vanilloid compound capsaicin has been reported in several studies. Vanilloid compounds are quinone analogs that inhibit the NADH-plasma membrane electron transport system and induce apoptosis in transformed cells[21]. Capsaicin (3.5-10 μmol/L) induces apoptotic cell death in an in vitro Korean stomach tumor cell (SNU-1), which may possibly be mediated by overexpression of p53 and/or c-myc genes, but not by overexpression of those of c-erbB-2, c-jun and bcl-2 genes[20], based on XTT assay, DNA fragmentation and flow cytometric analysis. In our study, induction of apoptosis by capsaicin was reconfirmed in another stomach tumor cell line, AGS. In this study, cell viability significantly decreased in a dose-dependent manner after incubation of AGS cells with capsaicin for 24 h. This reduction in cell viability induced by treatment with capsaicin was ascribed to apoptotic DNA fragmentation, and the amounts of fragmented DNA were also dependent on the dose of capsaicin. After incubation of AGS cells with capsaicin for 24 h, apoptotic bodies increased significantly in a dose-dependent manner.
We examined the role of Bcl-2 in capsaicin-induced cell death. In this study, the expression of Bcl-2, the antiapoptotic protein, was significantly reduced by capsaicin. Savill J et al[25] were the first to report that bcl-2 can prolong cell survival. Recently, the bcl-2 gene has emerged as a critical regulator of programmed cell death in a variety of physiological and pathological contexts[26]. Bcl-2 has also been reported to regulate transmembrane calcium fluxes[27,28]. It is of interest to note that when AGS cells were exposed to higher doses (10-200 μmol/L) of capsaicin, the expression of Bcl-2 protein was reduced, which differs from findings reported by Kim et al[20], and suggests that vanilloid compound-induced cell death might be via a Bcl-2 sensitive apoptotic pathway in the AGS cells.
In conclusion, capsaicin reduced the expression of Bcl-2 in a concentration-dependent manner in AGS cells, which suggests that Bcl-2 may play an important role in capsaicin-induced apoptosis.
Footnotes
Supported by Grants from the National Science Council of the ROC, No. NSC 89-2314-B-037-073 and NSC-89-2315-B-037-004
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Marchetti M, Aricò B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 2.Cordell GA, Araujo OE. Capsaicin: identification, nomenclature, and pharmacotherapy. Ann Pharmacother. 1993;27:330–336. doi: 10.1177/106002809302700316. [DOI] [PubMed] [Google Scholar]
- 3.Szolcsanyi J, Bartho L. Impaired defense mechanism to peptic ulcer in the capsaicin-desensitized rat. In: Mozsik G, Hannien O, Javor T, editors. Gastrointestinal defense mechanisms. Oxford, Budapest: Pergamon Press and Akademiai Kiado; 1981. p. 39-51. Mozsik G HO, Javor T, editor. Oxford: Budapest: Pergamon Press and Akademiai Kiado,1981.p.39-51; 1981. p. 39-51 [Google Scholar]
- 4.Holzer P, Sametz W. Gastric mucosal protection against ulcerogenic factors in the rat mediated by capsaicin-sensitive afferent neurons. Gastroenterology. 1986;91:975–981. doi: 10.1016/0016-5085(86)90702-x. [DOI] [PubMed] [Google Scholar]
- 5.Holzer P, Lippe IT. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience. 1988;27:981–987. doi: 10.1016/0306-4522(88)90201-1. [DOI] [PubMed] [Google Scholar]
- 6.Yeoh KG, Kang JY, Yap I, Guan R, Tan CC, Wee A, Teng CH. Chili protects against aspirin-induced gastroduodenal mucosal injury in humans. Dig Dis Sci. 1995;40:580–583. doi: 10.1007/BF02064374. [DOI] [PubMed] [Google Scholar]
- 7.Holzer P, Pabst MA, Lippe IT, Peskar BM, Peskar BA, Livingston EH, Guth PH. Afferent nerve-mediated protection against deep mucosal damage in the rat stomach. Gastroenterology. 1990;98:838–848. doi: 10.1016/0016-5085(90)90005-l. [DOI] [PubMed] [Google Scholar]
- 8.Holzer P, Livingston EH, Saria A, Guth PH. Sensory neurons mediate protective vasodilatation in rat gastric mucosa. Am J Physiol. 1991;260:G363–G370. doi: 10.1152/ajpgi.1991.260.3.G363. [DOI] [PubMed] [Google Scholar]
- 9.Lippe IT, Pabst MA, Holzer P. Intragastric capsaicin enhances rat gastric acid elimination and mucosal blood flow by afferent nerve stimulation. Br J Pharmacol. 1989;96:91–100. doi: 10.1111/j.1476-5381.1989.tb11788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto J, Takeuchi K, Okabe S. Characterization of gastric mucosal blood flow response induced by intragastric capsaicin in rats. Jpn J Pharmacol. 1991;57:205–213. doi: 10.1254/jjp.57.205. [DOI] [PubMed] [Google Scholar]
- 11.Kang JY, Teng CH, Wee A, Chen FC. Effect of capsaicin and chilli on ethanol induced gastric mucosal injury in the rat. Gut. 1995;36:664–669. doi: 10.1136/gut.36.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi K, Ueshima K, Matsumoto J, Okabe S. Role of capsaicin-sensitive sensory nerves in acid-induced bicarbonate secretion in rat stomach. Dig Dis Sci. 1992;37:737–743. doi: 10.1007/BF01296432. [DOI] [PubMed] [Google Scholar]
- 13.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 14.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 15.Kato S, Aihara E, Nakamura A, Xin H, Matsui H, Kohama K, Takeuchi K. Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem Pharmacol. 2003;66:1115–1121. doi: 10.1016/s0006-2952(03)00461-1. [DOI] [PubMed] [Google Scholar]
- 16.Teel RW. Effects of capsaicin on rat liver S9-mediated metabolism and DNA binding of aflatoxin. Nutr Cancer. 1991;15:27–32. doi: 10.1080/01635589109514108. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Hamilton SM, Stewart C, Strother A, Teel RW. Inhibition of liver microsomal cytochrome P450 activity and metabolism of the tobacco-specific nitrosamine NNK by capsaicin and ellagic acid. Anticancer Res. 1993;13:2341–2346. [PubMed] [Google Scholar]
- 18.Teel RW. Effects of different inducers of cytochrome P450 on the mutagenesis of the tobacco-specific nitrosamine 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanone (NNK) in Salmonella typhimurium TA1535. Anticancer Res. 1992;12:1287–1290. [PubMed] [Google Scholar]
- 19.Morré DJ, Chueh PJ, Morré DM. Capsaicin inhibits preferentially the NADH oxidase and growth of transformed cells in culture. Proc Natl Acad Sci USA. 1995;92:1831–1835. doi: 10.1073/pnas.92.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JD, Kim JM, Pyo JO, Kim SY, Kim BS, Yu R, Han IS. Capsaicin can alter the expression of tumor forming-related genes which might be followed by induction of apoptosis of a Korean stomach cancer cell line, SNU-1. Cancer Lett. 1997;120:235–241. doi: 10.1016/s0304-3835(97)00321-2. [DOI] [PubMed] [Google Scholar]
- 21.Macho A, Blázquez MV, Navas P, Muñoz E. Induction of apoptosis by vanilloid compounds does not require de novo gene transcription and activator protein 1 activity. Cell Growth Differ. 1998;9:277–286. [PubMed] [Google Scholar]
- 22.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 23.Willmott NJ, Choudhury Q, Flower RJ. Functional importance of the dihydropyridine-sensitive, yet voltage-insensitive store-operated Ca2+ influx of U937 cells. FEBS Lett. 1996;394:159–164. doi: 10.1016/0014-5793(96)00939-8. [DOI] [PubMed] [Google Scholar]
- 24.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 26.Weil M, Jacobson MD, Coles HS, Davies TJ, Gardner RL, Raff KD, Raff MC. Constitutive expression of the machinery for programmed cell death. J Cell Biol. 1996;133:1053–1059. doi: 10.1083/jcb.133.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 28.Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


