Abstract
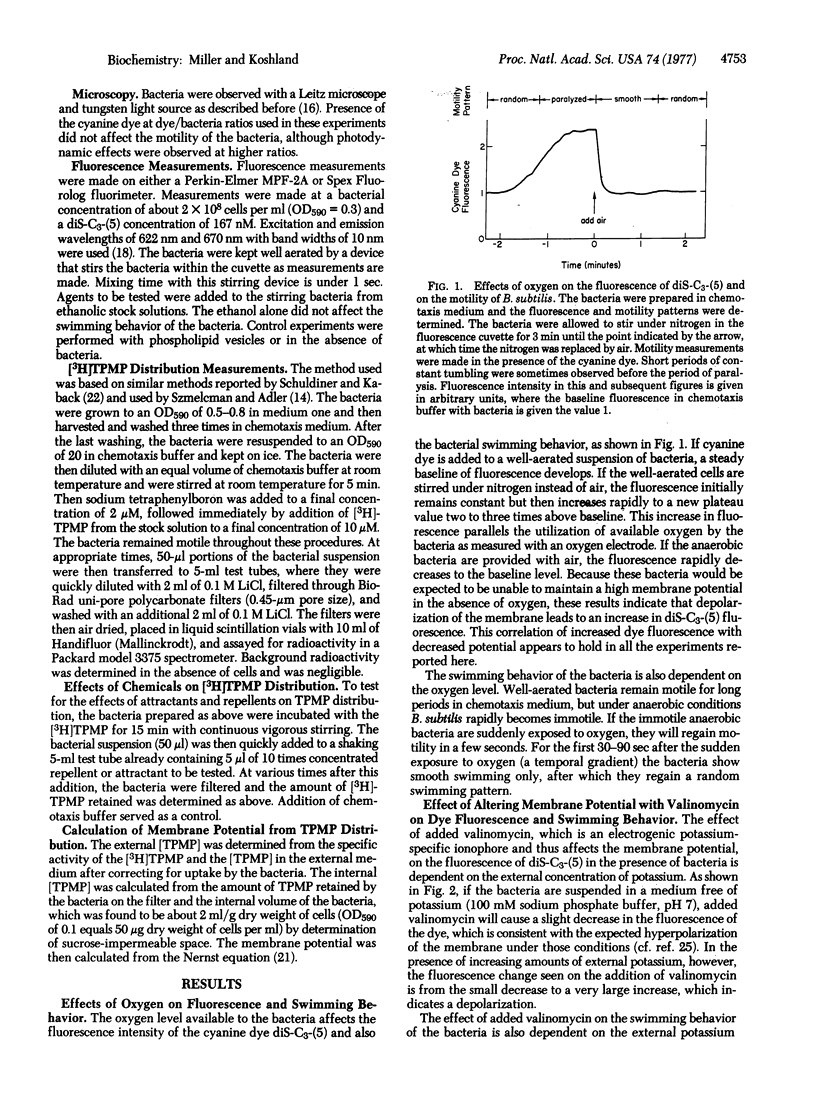
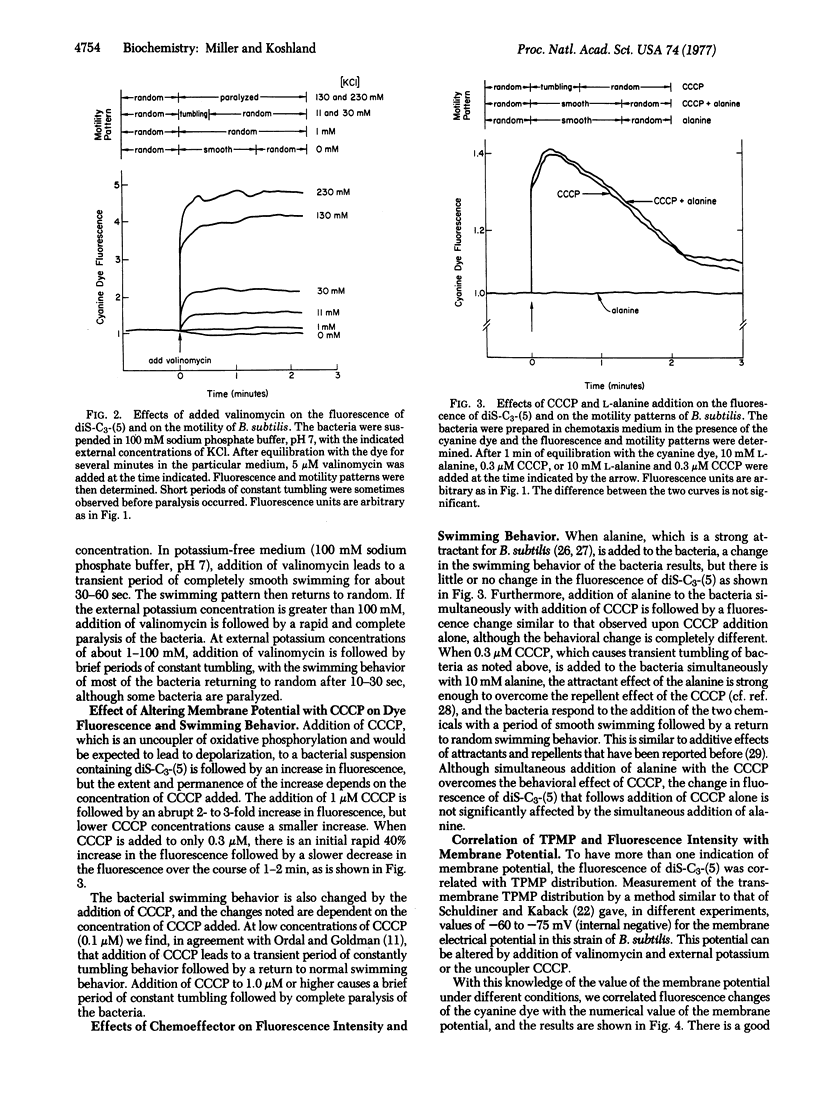
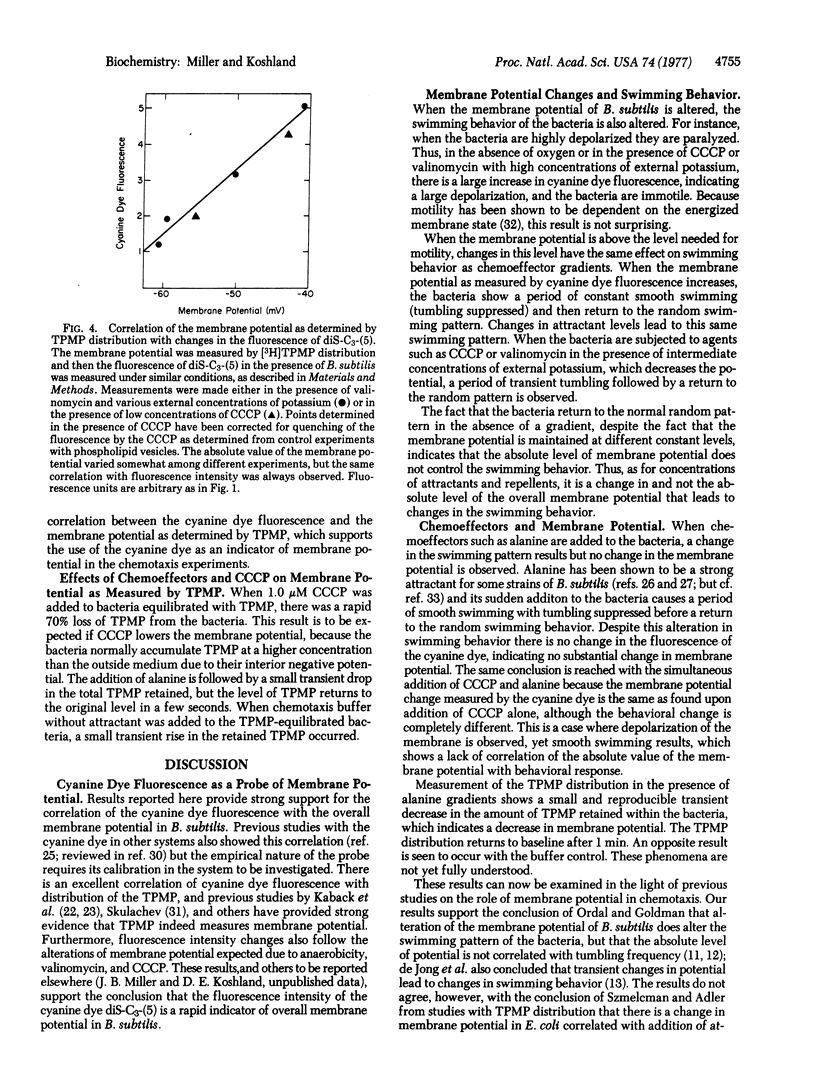
The relationship of membrane potential to motility and chemotaxis of Bacillus subtilis has been tested by using the fluorescence of a cyanine dye as a probe of the potential. The dye fluorescence was found to be an indicator of membrane potential by correlation with triphenylmethylphosphonium ion distribution and with changes due to anaerobicity and ionophore addition. When the potential was sufficient for motility and constant over time, it was found that the absolute level of the potential did not affect the swimming behavior of the bacteria. Transient alteration of the membrane potential did, however, lead to changes in swimming behavior. Attractants were found to alter the swimming behavior of the bacteria without altering the membrane potential. Thus, change of the overall membrane potential of a normal B. subtilis is not required for chemotaxis, but such a change is sensed by the bacteria just as changing levels of attractants and repellents are sensed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Aswad D. W., Koshland D. E., Jr Evidence for an S-adenosylmethionine requirement in the chemotactic behavior of Salmonella typhimurium. J Mol Biol. 1975 Sep 15;97(2):207–223. doi: 10.1016/s0022-2836(75)80035-0. [DOI] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Role of methionine in bacterial chemotaxis. J Bacteriol. 1974 May;118(2):640–645. doi: 10.1128/jb.118.2.640-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Chemotaxis in bacteria. Annu Rev Biophys Bioeng. 1975;4(00):119–136. doi: 10.1146/annurev.bb.04.060175.001003. [DOI] [PubMed] [Google Scholar]
- Caraway B. H., Krieg N. R. Uncoordination and recoordination in Spirillum volutans. Can J Microbiol. 1972 Nov;18(11):1749–1759. doi: 10.1139/m72-271. [DOI] [PubMed] [Google Scholar]
- Carls R. A., Hanson R. S. Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):848–855. doi: 10.1128/jb.106.3.848-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- De Jong M. H., van der Drift C., Vogels G. D. Proton-motive force and the motile behavior of Bacillus subtilis. Arch Microbiol. 1976 Dec 1;111(1-2):7–11. doi: 10.1007/BF00446543. [DOI] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Faust M. A., Doetsch R. N. Effect of drugs that alter excitable membranes on the motility of Rhodospirillum rubrum and Thiospirillum jenense. Can J Microbiol. 1971 Feb;17(2):191–196. doi: 10.1139/m71-033. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membr Biol. 1972;8(1):27–44. doi: 10.1007/BF01868093. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Protonmotive force in fermenting Streptococcus lactis 7962 in relation to sugar accumulation. Biochem Biophys Res Commun. 1974 Aug 5;59(3):879–886. doi: 10.1016/s0006-291x(74)80061-6. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R., Koshland D. E., Jr Bacterial motility and chemotaxis: light-induced tumbling response and visualization of individual flagella. J Mol Biol. 1974 Apr 15;84(3):399–406. doi: 10.1016/0022-2836(74)90448-3. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Membrane fluidity and chemotaxis: effects of temperature and membrane lipid composition on the swimming behavior of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1977 Apr;111(2):183–201. doi: 10.1016/s0022-2836(77)80122-8. [DOI] [PubMed] [Google Scholar]
- Ordal G. W. Control of tumbling in bacterial chemotaxis by divalent cation. J Bacteriol. 1976 May;126(2):706–711. doi: 10.1128/jb.126.2.706-711.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W. Effect of methionine on chemotaxis by Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1005–1012. doi: 10.1128/jb.125.3.1005-1012.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Gibson K. J. Chemotaxis toward amino acids by Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):151–155. doi: 10.1128/jb.129.1.151-155.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotactic repellents of Bacillus subtilis. J Mol Biol. 1976 Jan 5;100(1):103–108. doi: 10.1016/s0022-2836(76)80037-x. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotaxis away from uncouplers of oxidative phosphorylation in Bacillus subtilis. Science. 1975 Sep 5;189(4205):802–805. doi: 10.1126/science.808854. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Villani D. P., Gibson K. J. Amino acid chemoreceptors of Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):156–165. doi: 10.1128/jb.129.1.156-165.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):848–854. doi: 10.1021/bi00624a006. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal R., Lanyi J. K. Light-induced membrane potential and pH gradient in Halobacterium halobium envelope vesicles. Biochemistry. 1976 May 18;15(10):2136–2143. doi: 10.1021/bi00655a017. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Adler J. Change in membrane potential during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4387–4391. doi: 10.1073/pnas.73.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. L., Koshland D. E., Jr Intrinsic and extrinsic light responses of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1975 Aug;123(2):557–569. doi: 10.1128/jb.123.2.557-569.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang N., Macnab R., Koshland D. E., Jr Common mechanism for repellents and attractants in bacterial chemotaxis. Science. 1973 Jul 6;181(4094):60–63. doi: 10.1126/science.181.4094.60. [DOI] [PubMed] [Google Scholar]
- Van Der Drift C., De Jong M. H. Chemotaxis toward amino acids in Bacillus subtilis. Arch Mikrobiol. 1974 Mar 4;96(2):83–92. [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]
- Zukin R. S., Koshland D. E., Jr Mg2+, Ca2+-dependent adenosine triphosphatase as receptor for divalent cations in bacterial sensing. Science. 1976 Jul 30;193(4251):405–408. doi: 10.1126/science.132702. [DOI] [PubMed] [Google Scholar]



