Abstract
AIM: To investigate the effect of Jianweiyuyang (JWYY) granule on gastric ulcer recurrence and its mechanism in the treatment of gastric ulcer in rats.
METHODS: Gastric ulcer in rats was induced according to Okeba’s method with minor modification and the recurrence model was induced by IL-1β. The expression of vascular endothelial growth factor mRNA (VEGF mRNA) was examined by reverse transcription polymerase chain reaction in gastric ulcer and microvessel density (MVD) adjacent to the ulcer margin was examined by immunohistochemistry.
RESULTS: MVD was higher in the JWYY treatment group (14.0±2.62) compared with the normal, model and ranitidine treatment groups (2.2±0.84, 8.8±0.97, 10.4±0.97) in rats (P<0.01). The expression level of VEGF mRNA in gastric tissues during the healing process of JWYY treatment group rats significantly increased compared with other groups (normal group: 0.190±0.019, model group: 0.642±0.034, ranitidine group: 0.790±0.037, P<0.01).
CONCLUSION: JWYY granules can stimulate angiogenesis and enhance the expression of VEGF mRNA in gastric ulcer rats. This might be the mechanism for JWYY accelerating the ulcer healing, and preventing the recurrence of gastric ulcer.
Keywords: Jianweiyuyang granule, Gastric ulcer, VEGF mRNA
INTRODUCTION
Peptic ulcer (PU) is one of the common clinical diseases. The incident rate of PU has been on the rise over the recent two decades[1]. Some latest studies showed that H2 antagonists and the proton pump inhibitor omeprazole had about 80-100% of healing rate and produced a good therapeutic effect on gastroduodenal ulcer after a 4-8-wk administration. However, gastroduodenal ulcer has 40-80% of recurrent frequency, once the drug is withdrawn within a year. Moreover, Helicobacter pylori eradication ameliorates symptoms and improves the quality of lives of patients, but many of them still cannot avoid ulcer relapses[2-4].
The repair of gastric ulcers requires the reconstitution of epithelial structures and underlying connective tissue, including vessels and muscle layers. This complex sequence of events requires a high degree of coordination among different cell types, which is regulated by several factors. Of them, some growth factors, such as vascular endothelial growth factor (VEGF) and the platelet-derived growth factor (PDGF), have received much attention in recent years, because they play a pivotal role in gastric ulcer repair[5]. Previous studies indicated that the Chinese traditional medicine Jianweiyuyang granules (JWYY) can accelerate ulcer healing, improve scar quality, and prevent ulcer recurrence[6,7]. However, the mechanisms responsible for these actions are not fully elucidated. In this study, we examined the microvessel density (MVD) in the ulcer margin by immunohistochemistry and demonstrated the specific expression of VEGF mRNA at the gastric ulcer margin tissue using reverse transcription polymerase chain reaction (RT-PCR). The aim of this study was to determine whether JWYY accelerated ulcer healing by enhancing the expression of VEGF mRNA and angiogenesis.
MATERIALS AND METHODS
Medicine and reagents
JWYY granules were purchased from Hunan Xiangya Pharmaceutical Co., Ltd (No. Z10960018). Ranitidine capsule (purity 98%) was from Hangzhou Sanofi-Synthélabo Minsheng Pharmaceutical Co., Ltd (No. H33021741). TRIzol reagent was from GIBCO BRL Co., Ltd (MRI, USA). Factor VIII antibody, revert AidTM first strand cDNA synthesis kit and marker were provided by Fermentas Life Sciences Co. (MBI, Lithuania). DEPC was from Sigma Co. (St. Louis, USA). SABC kit and DAB kit were from Wuhan Boster Biotechnology Co., Ltd.
Animals and ulcer induction
Ninety Sprague-Dawley rats, half male and half female, weighing 180-220 g, were chosen for use in this study. All the rats were obtained from Experimental Animal Center of Xiangya Medical School of Central South University. The rats were kept individually in wire-bottom cages with free access to standard rat diet and water. The animal room was illuminated with a 12-h light-dark cycle. The room temperature was kept at 18-22 °C and the humidity at 60-70%. Rats underwent laparotomy under pentobarbital anesthesia.
Ninety rats were divided into six groups: normal group, sham operation group, ulcer model group, ulcer recurrence group, ranitidine group, and JWYY treatment group. Gastric ulcers were induced by a minor modification of the previously described method[8]. In control group, the operation procedures of rats were similar procedures except the application of acetic acid (sham operation).
Rats with healed ulcers were subjected to experiments of induction of ulcer recurrence. The rats in the ulcer model group were killed without treatment with interleukin 1β (IL-1β). The ulcer index, MVD and the expression of VEGF mRNA were measured. Recombinant human IL-1β (1 μg/kg) diluted to 1 mL with isotonic saline was injected intraperitoneally to the remaining animals to induce the ulcer recurrence model[9].
Drug treatment
Each of the rats in JWYY group were treated with a single dose of 0.162 g/(mL·100 g) JWYY granules daily; each rat in the ranitidine control group was treated with a single dose of 5.4 mg/(mL·100 g) of ranitidine daily. Other groups were treated with distilled water.
Tissues preparation
Rats were killed 7 or 92 d later after they received drug treatment or feed, then the gastric walls of rats were removed. A portion of the wall was put into 4% formaldehydum polymerisatum for 1 h to examine the ulcer index by the method designed by us. Another portion of the wall was embedded into the paraffin wax for HE staining. The gastric ulcer margin tissue was removed for MVD and VEGF mRNA expression measurement separately.
Measurement of ulcer index
The length, width, and depth of the ulcer were measured by a compass. The area of the ulcer equals to the width of the ulcer times the length of the ulcer. The ulcer was divided into two grades by its depth more than or less than 2 mm. The ulcer index was classified into 10 degrees[6] (Table 1).
Table 1.
Method of measuring the gastric ulcer index
| Area (mm2) |
Depth |
|
| <2 mm | >2 mm | |
| 0 | _ | _ (ulcer healing) |
| 1–4 | 11 | 2 |
| 5–9 | 2 | 3 |
| 10–14 | 3 | 4 |
| 15–19 | 4 | 5 |
| 20–24 | 5 | 6 |
| 25–29 | 6 | 7 |
| 30–34 | 7 | 8 |
| 35–39 | 8 | 9 |
| 40 | 9 | 10 |
11–10 = grade of the ulcer index.
Determination of MVD
The consecutive 5-μm sections were cut and mounted onto glass slides. Sections were stained with HE. The microvessel was examined using immunohistochemistry method with an anti-VIII mouse monoclonal antibody with a dilution of 1:80. Negative controls were detected by PBS substitution for the primary antibodies. All the procedures were carried out according to the manufacturer’s instruction.
MVD was evaluated according to the previous method as described by Wang and Liao[10,11] . The stained sections were screened under low power, and five areas with the most intense neovascularization were selected. Microvessel counting of these areas was performed at the ulcer margin (×400). Any brown-stained endothelial cell or endothelial cell cluster was counted as a vessel. Vascular counts of each case were calculated. The mean microvessel count of the five richest vascular areas was taken as MVD.
Determination of expression of VEGF mRNA
Total RNA was isolated from gastric biopsy tissues by using TRIzol reagent according to the manufacturer’s instruction.
RT-PCR was performed according to the manufacturer’s instruction. RT-PCR reaction mixture containing 2 μL of total RNA, 1 μL oligo (dT)18 (0.5 μg/μL), 9 μL ddH2O in a tube was mixed gently and then spinned down 3-5 s in a microcentrifuge. The mixture was incubated at 70 °C for 5 min and chilled on ice and the drops were collected by brief centrifugation. Four microliters of 5× buffer, 2 μL 10 mmol/L dNTP, and 1 μL (20 U) Rnasin were added to the above collected drops and then the mixture was incubated at 37 °C for 5 min. Then 1 μL (200 U/μL) M-MuLV reverse transcriptase was added to the above mixture and incubated at 42 °C for 60 min. The reaction was stopped by heating at 70 °C for 10 min. The first strand cDNA synthesized was chilled on ice and stored at -20 °C until next analysis.
The specific primers were designed by software primer premier 5.0 and purchased from Shanghai DNA Biotechnology Corporation Ltd. The primers for VEGF were 5’-CCTGGTGGACATCTTCCAGGAGTACC-3’ (sense) and 5’-GAAGCTCATCTCTCCTATGTGCTGGC-3’ (anti-sense), and the size of the amplified fragment conserved in all of the variant spliced forms was 104 bp. PCR products for β-actin were used as a positive control and internal standard. The primers for β-actin were 5’-GAGGGAAATCGTGCGTGAC-3’ (sense) and 5’-CTGGAAGGTGGACAGTGAG-3’ (anti-sense). The length was 445 bp. PCR was carried out in a final volume of 25 μL reaction mixture containing 9.5 μL ddH2O, 2.5 μL 10×buffer, 1.5 μL MgCl2, 0.5 μL 10 mmol/L dNTP, 4 μL VEGF primer, 4 μL β-actin primer, 0.5 μL Taq DNA polymerase (1.5 μL) and 2.5 μL cDNA. The final concentration of each primer was 0.1 μmol/L. Amplification conditions included an initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 45 s, annealing at 63 °C for 45 s, and 1 min extending at 72 °C, and a final extension at 72 °C for 10 min. Negative reaction mixture had no cDNA. PCR products were electrophoretically separated on 1.5% agarose gel in 1×TBE buffer, after which the gel was stained with ethidium bromide (0.5 μg/mL). The PCR instrument was purchased from Perkin Elmer Company.
For quantitative assessment of the PCR products, a computerized video analysis system was used. The results are expressed as the VEGF/β-actin ratio.
Statistical analysis
Results are expressed as the mean±SD. The Student’s t-test was used to determine statistical significance of differences between two treatment groups. Comparisons of data between groups were performed with analysis of variance followed by F-test. P<0.05 was taken as significant. Statistical software SPSS10.0 was used in this study.
RESULTS
Effect of JWYY on gastric ulcer recurrence
After rats were induced by acetic acid, ulcers were detected in all rats by macroscopic examination except for the normal and sham operation groups (Figures 1 and 2). Moreover, after rats were induced by IL-1β, no ulcers in the normal and sham operation groups were detected by macroscopic examination (Figure 3). However, as shown in Figures 4-6 and Table 2, 9 of 10 rats in the model recurrence group had a gastric ulcer recurrence. In contrast, only 1 of 10 rats in the model group without IL-1β treatment was observed with ulcer recurrence. Moreover, 8 of 10 rats in the ranitidine group and 3 of 10 rats in the JWYY group with IL-1β treatment had gastric ulcer recurrence.
Figure 1.
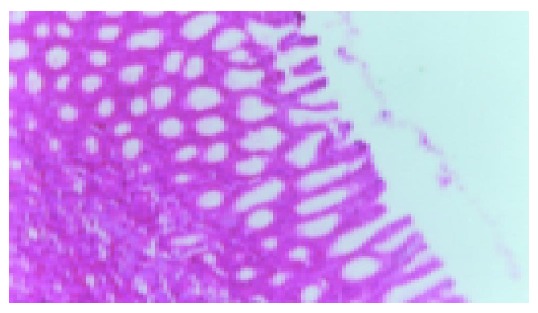
Normal gastric mucous (×200).
Figure 2.
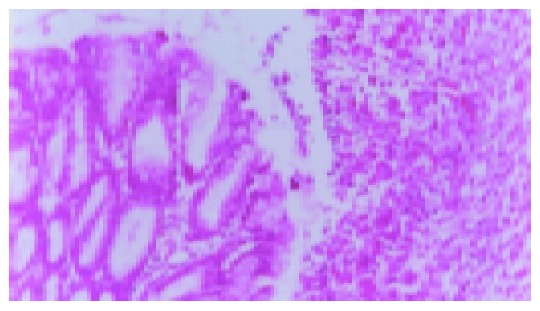
Model group ulcer formation (7 d, ×200).
Figure 3.
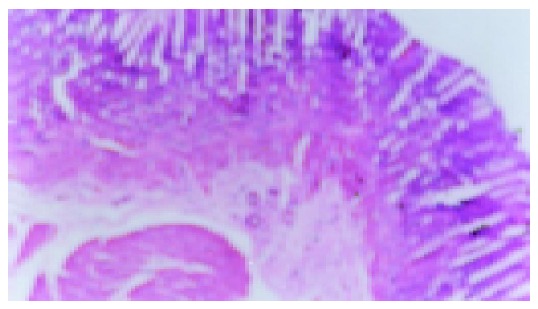
Model group without IL-b (92 d), scar tissue under the thinned gastric mucosa (×200).
Figure 4.
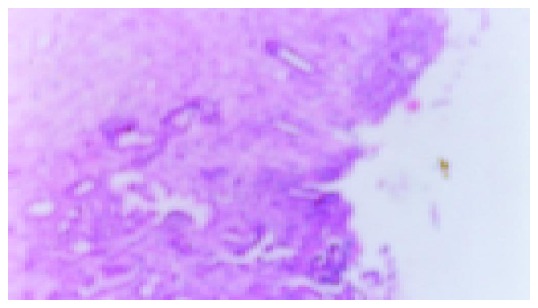
Ranitidine group with IL-b, small ulcer can be seen (×200).
Figure 6.
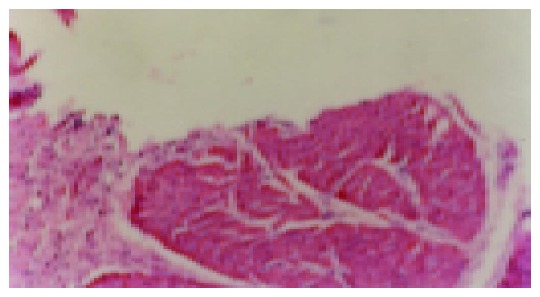
Model recurrence group, after ip IL-b, ulcer relapses (×200).
Table 2.
Ulcer index and the rate of recurrence (mean±SD)
| Group | n | Recurrence | Ulcer index | Ulcer index |
| IL-1β | rate | (7 d) | (92 d) | |
| Normal | 5 | 0 | 0 | 0 |
| Sham operation | 5 | 0 | 0 | 0 |
| Model | 10 | 10% | 8.7±0.48 | 0.12±0.32 |
| Model recurrence | 10 | 90%b | 8.7±0.48 | 6.8±0.48b |
| Ranitidine | 10 | 80%e | 6.3±0.57a | 5.8±0.57e |
| JWYY | 10 | 30%f | 3.8±0.86c,d | 0.82±0.84f |
aP<0.05 vs the model group; bP<0 01 vs the model group; cP<0.05, dP<0 01 vs the ranitidine group; eP>0.05 vs the model recurrence group; fP<0.01 vs the ranitidine group.
Figure 5.
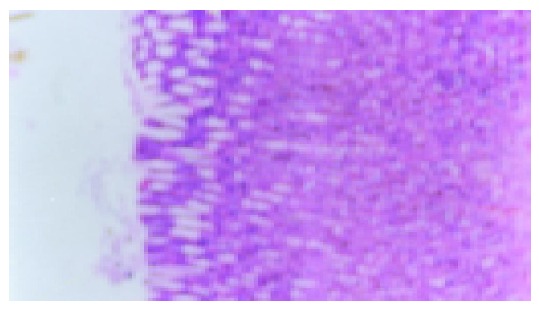
JWYY group, after ip IL-b, no ulcer recurrence (×200).
Effect of JWYY on MVD
As shown in Figure 7 and Table 3, microvessel was stained brown. MVD in the normal and sham operation groups were relatively few. MVD in the model group was increased compared with normal group, and MVD in the JWYY group was significantly increased vs other groups.
Figure 7.
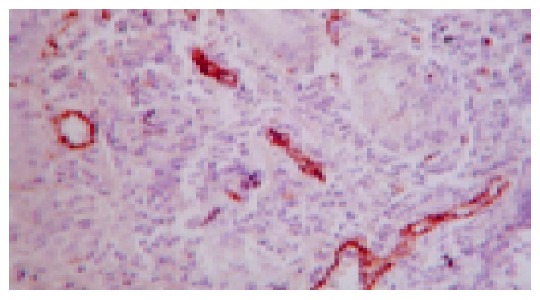
Microvessel of gastric ulcer margin in the rat stained by VIII factor (method of SABC ×400).
Table 3.
Change of MVD in tissues 7 d later (mean±SD)
| Group | n | MVD (7 d) |
| Normal | 5 | 2.2±0.84 |
| Sham operation | 5 | 2.6±1.14 |
| Model | 10 | 8.8±0.97b |
| Ranitidine | 10 | 10.4±0.97b,a |
| JWYY | 10 | 14.0±2.62a,d,f |
aP<0.05 vs the model group; bP<0.01 vs the normal and sham operation groups; 32P<0.01 vs the model group; fP<0.01 vs ranitidine group.
Expression of VEGF mRNA
In this study, the extracted total RNAs were of high purity (a range of A260/A280 of RNA is from 1.807 to 1.913).
The result showed that the expression level of VEGF mRNA in gastric tissues of rats was low in the normal and sham operation groups. However, the expression level of VEGF mRNA in model, model recurrence, ranitidine, and JWYY groups were significantly increased after 7 d of treatment.
As shown in Table 4, the same results of VEGF expression in gastric ulcer were observed in all groups except for the model group after treatment for 92 d. Taken together, JWYY could enhance the expression of VEGF mRNA in gastric ulcer rats.
Table 4.
VEGF mRNA expression in each group (mean±SD)
|
VEGF mRNA |
|||
| Group | n | 7 d | 92 d |
| Normal | 5 | 0.190±0.019 | 0.186±0.028 |
| Sham operation | 5 | 0.188±0.019 | 0.184±0.028 |
| Model | 10 | 0.642±0.034b | 0.188±0.012h |
| Model recurrence | 10 | 0.794±0.02g | |
| Ranitidine | 10 | 0.790±0.037b,d,f | 0.794±0.025h |
| JWYY | 10 | 0.898±0.046b,d | 0.899±0.043h |
bP<0.01 vs the normal and sham operation groups; dP<0.01 vs the model group; fP<0.01 vs the ranitidine group; hP<0.01 vs itself on the 7th d; gP>0.01 vs itself on the 7th d.
The result in black figure is considered as the result of the model recurrence group as well as the model group.
DISCUSSION
IL-1β is secreted by monocytes, macrophages, and other inflammatory cells. It can cause acute damage of gastric mucosa[12]. Recently, Watanabe et al[9,13], have developed a rat model of gastric ulcer recurrence induced by IL-1β and the pathologic characterization is very similar to that of the human chronic gastric ulcer.
In this experiment, we observed gastric ulcer in all rats after they underwent operation by Okabe’s method. Nine of ten healed ulcers in the group treated with IL-1β relapsed in the model recurrence group. In contrast, only 1 of 10 relapsed in the model group and no ulcer was observed in the normal and sham operation groups. Taken together, this model of ulcer recurrence was very successful.
The repair of gastric ulcers is a complicated process. It is associated with the reconstitution of epithelial structures and the underlying connective tissue, including vessels and muscle layers. It has been reported that the recurrence of ulcer is associated with the quality of ulcer healing (QOUH)[14], which is related to angiogenesis. Some studies have shown that several growth factors and their receptors are involved in ulcer healing process. Of those, VEGF has received considerable attention, since it is considered to be the only factor that specifically acts on endothelial cells. The delay of ulcer healing and the poor QOUH may result from the lower expression or absence of some growth factors and their receptors[15,16].
VEGF, previously described as a vascular permeability factor, is a growth factor that is involved in the mucosal protection and the angiogenic response taking place during ulcer healing. VEGF is a peptide which is secreted as dimeric glycoproteins containing characteristic regularly spaced eight-cysteine residues. Multiple isoforms can be generated by alternative splicing of a single gene transcript. VEGF has a variety of effects on vascular endothelium, including the ability to promote endothelial cell viability, mitogenesis, chemotaxis, and vascular permeability. Expression of VEGF and its receptors has been demonstrated in the ulcer margin of the human PU disease. In experimental models of acute gastric damage, the expression of VEGF increases during healing, while the pretreatment of rats with a single dose of oral VEGF may exert a protective effect against acute ethanol damage in the gastric mucosa[17]. Furthermore, the daily administration of VEGF has been found to promote the healing of cysteamine duodenal ulcer in rats by stimulation of angiogenesis and formation of granulation tissue. These data suggest that VEGF might play a dual role in mucosal protection and repair. On the one hand, it might improve mucosal resistance by an increase of vascular permeability that dilutes gastrotoxic agents and reduces the area of the hemorrhagic lesions. On the other hand, it might contribute to the development of the angiogenic response together with other growth factors[5,17]. The recent research findings indicate that the gene therapy with either VEGF or PDGF may be a rapid approach to achieve duodenal ulcer healing[18].
JWYY enhanced the expression of VEGF mRNA by stimulation of angiogenesis in the human gastric mucosa in our study. As a result, it improved the QOUH, accelerated the healing of ulcer and prevented its recurrence.
JWYY granules consist of Chinese thorowax root, pilose asiabell root, white peony root, corydalis tuber, common bletilla tuber, natural indigo, pearl powder and liquorice root. It can soothe the liver and strengthen the spleen, it has anti-spasm and pain relieving effect, stop bleeding, and regenerate muscle, and disperse the visceral heat. Previously, we demonstrated that JWYY accelerated the healing of acetic acid-induced gastric ulcer in rats and had good effect for the PU patients with stagnation of liver Qi and deficiency of spleen Qi syndrome or inharmonic liver and gastric syndrome. At the same time, it could prevent ulcer recurrence[6,7].
In conclusion, our results show that JWYY enhances the expression of VEGF mRNA in gastric ulcer in rats. This may be the mechanism by which JWYY improves the QOUH, and prevents its recurrence in the treatment of PU.
Footnotes
Supported by the Foundation of Traditional Chinese Medicine of the Bureau of Health of Hunan Province, No. 202053
Science Editor Zhu LH and Guo SY Language Editor Elsevier HK
References
- 1.Szabo S, Vincze A. Growth factors in ulcer healing: lessons from recent studies. J Physiol Paris. 2000;94:77–81. doi: 10.1016/s0928-4257(00)00146-7. [DOI] [PubMed] [Google Scholar]
- 2.Verma S, Giaffer MH. Helicobacter pylori eradication ameliorates symptoms and improves quality of life in patients on long-term acid suppression. A large prospective study in primary care. Dig Dis Sci. 2002;47:1567–1574. doi: 10.1023/a:1015823320831. [DOI] [PubMed] [Google Scholar]
- 3.Forrest EH, MacKenzie JF, Stuart RC, Morris AJ. Helicobacter pylori eradication for peptic ulceration: an observational study in a Scottish primary care setting. Scott Med J. 2002;47:28–33. doi: 10.1177/003693300204700202. [DOI] [PubMed] [Google Scholar]
- 4.Luo JC, Shin VY, Liu ES, Ye YN, Wu WK, So WH, Chang FY, Cho CH. Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur J Pharmacol. 2004;485:275–281. doi: 10.1016/j.ejphar.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 5.Milani S, Calabrò A. Role of growth factors and their receptors in gastric ulcer healing. Microsc Res Tech. 2001;53:360–371. doi: 10.1002/jemt.1104. [DOI] [PubMed] [Google Scholar]
- 6.Li JB, Tian YL, Cheng WH, Xiong GL. Jian-Wei-Yu-Yang granules in treatment of experimental gastric ulcer in rats. Shijie Huaren Xiaohua Zazhi. 2002;10:1282–1287. [Google Scholar]
- 7.Li JB, Jin YQ, Hu SY, YI ZJ, Cao GF, Zhou JH, Liu ZX. Curative effect of Chinese drugs for peptic ulcer is related to dosage form. Shijie Huaren Xiaohua Zazhi. 1998;6:310–312. [Google Scholar]
- 8.Pai R, Ohta M, Itani RM, Sarfeh IJ, Tarnawski AS. Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology. 1998;114:706–713. doi: 10.1016/s0016-5085(98)70584-0. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Arakawa T, Fukuda T, Higuchi K, Kobayashi K. Role of neutrophils in a rat model of gastric ulcer recurrence caused by interleukin-1 beta. Am J Pathol. 1997;150:971–979. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Dou KF. Expression of hPTTG1 and bFGF in gallbladder carcinoma tissue and their correlation with angiogenesis. Shijie Huaren Xiaohua Zazhi. 2004;12:680–684. [Google Scholar]
- 11.Liao XF, Yi JL, Li XR, Deng W, Yang ZF, Tian G. Angiogenesis in rabbit hepatic tumor after transcatheter arterial embolization. World J Gastroenterol. 2004;10:1885–1889. doi: 10.3748/wjg.v10.i13.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulligan MS, Ward PA. Immune complex-induced lung and dermal vascular injury. Differing requirements for tumor necrosis factor-alpha and IL-1. J Immunol. 1992;149:331–339. [PubMed] [Google Scholar]
- 13.Watanabe T, Higuchi K, Tominaga K, Fujiwara Y, Arakawa T. Acid regulates inflammatory response in a rat model of induction of gastric ulcer recurrence by interleukin 1beta. Gut. 2001;48:774–781. doi: 10.1136/gut.48.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarnawski A, Stachura J, Krause WJ, Douglass TG, Gergely H. Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol. 1991;13 Suppl 1:S42–S47. doi: 10.1097/00004836-199112001-00007. [DOI] [PubMed] [Google Scholar]
- 15.Baatar D, Jones MK, Tsugawa K, Pai R, Moon WS, Koh GY, Kim I, Kitano S, Tarnawski AS. Esophageal ulceration triggers expression of hypoxia-inducible factor-1 and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing. Am J Path. 2002;161:1449–1457. doi: 10.1016/s0002-9440(10)64420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi M, Kawabe T, Ogura K, Maeda S, Mikami Y, Kaneko N, Terano A, Omata M. Expression of vascular endothelial growth factor at the human gastric ulcer margin and in cultured gastric fibroblasts: a new angiogenic factor for gastric ulcer healing. Biochem Biophys Res Commun. 1997;234:493–498. doi: 10.1006/bbrc.1997.5974. [DOI] [PubMed] [Google Scholar]
- 17.Szabo S, Deng X, Khomenko T, Yoshida M, Jadus MR, Sandor Z, Gombos Z, Matsumoto H. Gene expression and gene therapy in experimental duodenal ulceration. J Physiol Paris. 2001;95:325–335. doi: 10.1016/s0928-4257(01)00045-6. [DOI] [PubMed] [Google Scholar]
- 18.Deng X, Szabo S, Khomenko T, Jadus MR, Yoshida M. Gene therapy with adenoviral plasmids or naked DNA of vascular endothelial growth factor and platelet-derived growth factor accelerates healing of duodenal ulcer in rats. J Pharmacol Exp Ther. 2004;311:982–988. doi: 10.1124/jpet.104.071464. [DOI] [PubMed] [Google Scholar]


