Abstract
AIM: To search the pathophysiological mechanism of diarrhea based on daily stool weights, fecal electrolytes, osmotic gap and pH.
METHODS: Seventy-six patients were included: 51 with microscopic colitis (MC) [40 with lymphocytic colitis (LC); 11 with collagenous colitis (CC)]; 7 with MC without diarrhea and 18 as a control group (CG). They collected stool for 3 d. Sodium and potassium concentration were determined by flame photometry and chloride concentration by titration method of Schales. Fecal osmotic gap was calculated from the difference of osmolarity of fecal fluid and double sum of sodium and potassium concentration.
RESULTS: Fecal fluid sodium concentration was significantly increased in LC 58.11±5.38 mmol/L (P<0.01) and CC 54.14±8.42 mmol/L (P<0.05) than in CG 34.28±2.98 mmol/L. Potassium concentration in LC 74.65±5.29 mmol/L (P<0.01) and CC 75.53±8.78 mmol/L (P<0.05) was significantly less compared to CG 92.67±2.99 mmol/L. Chloride concentration in CC 36.07±7.29 mmol/L was significantly higher than in CG 24.11±2.05 mmol/L (P<0.05). Forty-four (86.7%) patients had a secretory diarrhea compared to fecal osmotic gap. Seven (13.3%) patients had osmotic diarrhea.
CONCLUSION: Diarrhea in MC mostly belongs to the secretory type. The major pathophysiological mechanism in LC could be explained by a decrease of active sodium absorption. In CC, decreased Cl/HCO3 exchange rate and increased chloride secretion are coexistent pathways.
Keywords: Lymphocytic colitis, Collagenous colitis, Secretory diarrhea
INTRODUCTION
Microscopic colitis is a new form of idiopathic inflammatory bowel disease. First studies dealing with microscopic colitis appeared in the late seventies[1-3]. Clinical manifestations are substantially milder than other forms of idiopathic inflammatory bowel diseases. The main characteristics include chronic watery diarrhea and abdominal cramps. Endoscopic[4-8] and radiological examination of gastrointestinal tract are normal. The major criteria for diagnosis are set on the basis of histological findings of colonic mucosal biopsies[9] and stool examinations. There are two basic types of microscopic colitis: collagenous and lymphocytic colitis. They could be differentiated on the basis of pathohistological findings. Collagenous colitis is defined by the appearance of diffuse thickening of subepithelial collagenous band (>10 μm)[10], desquamation and degeneration of surface epithelium, increased number of intraepithelial lymphocytes and mild or moderate mononuclear infiltration of lamina propria[10,11-13]. Collagenous colitis shows striking predominance in women (F:M 7:1) in their fifth and sixth decades[4,10,14]. They are often associated with other autoimmune diseases: rheumatoid arthritis[15-17], systemic and discoid lupus erythematosus[18,19], juvenile sclerodermia[20], CREST syndrome[21], abnormal function of thyroid gland[14]. Lymphocytic colitis is defined by increased number of intraepithelial lymphocyte of colonic mucosa (>20 IEL per 100 epithelial cells)[10]. Other histological features include flattened surface epithelium with mucine depletion, mononuclear infiltration of lamina propria, and minimal crypt distortion or cryptitis[10,11]. Lymphocytic colitis typically occurs in the sixth decade with roughly equivalent female to male ratio[10,14,19-21]. According to data from the literature there is an association between lymphocytic colitis and other autoimmune diseases: sicca syndrome[15], celiac disease[24-26] idiopathic pulmonary fibrosis, uveitis, and idiopathic thrombocytopenic purpura[12]. Ethiopathogenesis of microscopic colitis still remains unclear. The long-term use of NSAID[27], and other medication (cyclo3fort[28], ranitidine[29], ticlodipin) could be important. A good number of authors[19,20,30] claim that autoimmune process is the cause of prime importance in ethiopathogenetic mechanism.
Intraepithelial lymphocytes in lymphocytic and collagenous colitis belong to T suppressor cells, CD8 group[19]. Stimulated by some luminal antigen they can cause cross reaction with endogen antigen present in the epithelial cells, damage them or reveal direct toxic action on the enterocytes[31]. The hypothesis of the infective etiology of microscopic colitis is based on the results of success of antibiotic therapy[4]. Bile acids also may play a role as shown by malabsorption of bile acids and proper therapeutic response to the cholestiramine[32], which is also suggested by combined manifestation with primary ileal villous atrophy[33]. Deregulation of a collagen synthesis[19,33] has also been mentioned as a possible etiological factor. The precise pathophysiological mechanism of secretory diarrhea typical for microscopic colitis has not been clarified. Most of the authors[31,34,35] believe that the following processes prevail in the development of secretory diarrhea:
- Reduced active sodium absorption
- Inhibited chloride and bicarbonate exchange
- Increased electrogenic chloride secretion followed by passive sodium and water transport.
- Decreased passive permeability of colonic mucosa.
Evidence for each of these hypotheses is contained in a Bo-Linn perfusion study[34]. Results of net and unidirectional electrolyte fluxes and electrical potential difference suggested that colonic fluid absorption was abnormal. The main reasons for these are: reduced active and passive sodium and chloride absorption and reduced Cl/HCO3 exchange. Zeroogian and Chopra thought that mediators secreted by inflammatory cells in lamina propria may play an important role in disturbances of absorptive colonic capacity and reducing passive transport[21]. Other authors consider that watery diarrhea is a consequence of increased active chloride intraluminal secretion and accompanying passive sodium and water transport[31,35]. Increased concentration of prostaglandin E2 secreted by pericryptal fibroblasts may stimulate active chloride secretion. Pericryptal fibroblasts compose pericryptal sheets which can cause functional abnormalities. In some cases an increased concentration of prostaglandin E2 is found in jejunal aspirate and feces[31]. The same group considers that injured surface epithelium and collagen deposit band of colonic mucosa may prevent absorption of luminal water and electrolytes. The most important pathophysiological role could be played by inflammatory cells infiltrated in the lamina propria, common characteristic for both lymphocytic and collagenous colitis. Precise mechanism of diarrhea remains unclear due to unknown etiological factors yet to be discovered. It is possible that different mechanisms cause diarrhea in the course of the disease, depending on whether pericryptal absorption site are blocked by the collagen deposit or inflammatory cells infiltrate.
MATERIALS AND METHODS
The study was performed in the Center for Gastroenterology and Hepatology (Zvezdara, University Clinical Center, Belgrade) between 1993 and 2004. Seventy-six people were included in the study (58 with diagnoses of microscopic colitis and 18 healthy people composing a control group).
The following diagnostic procedures were performed in both groups: routine biochemical examinations with electrolyte status, lactose tolerance test, thyroid status, presence of antinuclear antibody (ANA), antimitochondrial antibody (AMA) and antibodies to tyreoglobulin; standard and specific (Yersinia enterocolitica and Campylobacter jejuni) stool examinations for bacteria and parasites; duodenal tube with quantitative and qualitative bacterial culture of aspirate; small bowel barium enema, enteroscopy with small bowel biopsies; colonoscopy with terminal ileoscopy and serial biopsies of colonic and terminal ileum mucosa. Biopsy specimens were fixed with 40 g/L formaldehyde and embedded in paraffin. Five micrometer sections were stained with hematoxylin and eosin. Thickness of the subepithelial collagen layer was measured with light microscopy using screw micrometer by an experienced pathologist. Number of intraepithelial lymphocytes was determined with regard to 100 epithelial cells of colonic mucosa.
Functional examinations of stool
Stool samples All patients collected stool for 3 d (72 h) in preweight plastic bucket that held 3-5 L. Buckets with stools were kept cold under refrigeration (+4 °C) during the collection period. During that time patients were on a diet with 70-100 g fats per day.
Determination of sodium, potassium and chloride concentration in fecal fluid Concentration of sodium and potassium were determined by flame photometry (Corning 480 Flame Photometer)[36,37] and chloride concentration by the titration method of Schales[38]. Data of sodium, potassium and chloride were multiplicities with water dilution factor. Daily losses of electrolytes by stool were calculated from the concentration of electrolytes in fecal fluid. Fecal osmotic gap was calculated from the difference of measured osmolality of fecal fluid and double sum of the sodium and potassium concentration [290-(Na+K) ×2].
Estimation of pH of fecal fluid For estimating fecal pH we used balanced Beckman Expandomatic pH meter[37].
Determination of daily fecal fat The amount of fat in daily stool was quantified by titration method of Van de Kamer[39]. Normal values of fecal fat in our laboratory are less than 6 g/d. Normal values by the data of Fine are 6.4 g/d[40,43].
Statistical analysis
For all tested parameters we calculated mean and standard error (SE). For the statistical significant difference (P<0.05) among different group of patients, unpaired Student’s t-test was used.
RESULTS
There were 51 patients with complete conditions for diagnosis of microscopic colitis [40 patients with lymphocytic colitis (LC) and 11 patients with collagenous colitis (CC)]. Seven patients had histological diagnoses of MC (5 patients with LC and 2 with CC) without diarrhea at that moment. Control group consisted of 18 healthy persons.
Based upon the status of the collagen plate and the number of IEL of biopsy specimen of right colon, patients are classified as a group with LC or CC or CG (Table 1). In the group of patients with LC the median number of IEL/100 epithelial cells was (32±12) and the mean thickness of collagen plate was (8±2) μm with moderate inflammation of lamina propria. Patients with CC had average thickness of collagen band (25±9) μm and (14±4) IEL/100 epithelial cells with severe inflammation of lamina propria. Control group of patients had (41) IEL/100 epithelial cells and mean thickness of collagen plate (8±3) μm.
Table 1.
Mean±SD of number of IEL/100 EC and thickness of collagen plate in patients with LC and CC
| n | No. of IEL/100 EC (mean±SD) | Thickness of collagen band (mean±SD) | Inflammation of propria | |
| Lymphocytic colitis | 46 | 32±12 | 8±2 | Moderate |
| Collagenous colitis | 13 | 14±4 | 25±9 | Severe |
| Control group | 18 | 4±1 | 8±3 | Mild/none |
Physical examination, all biochemical tests, radiological and endoscopic findings were normal in all patients. Diagnoses of microscopic colitis was established based on the data of verified diarrhea [daily stool weight (DSW)> 200 g/24 h] and histopathology analysis of biopsy specimens of colonic mucosa. Mean values of DSW (mean±SE) in the group of patients with LC and CC were statistically significantly higher than in the other two groups: CG and group of 7 patients with histological signs of microscopic colitis without diarrhea (P<0.01, Table 2). The average values of sodium concentration in the patients with LC (P<0.01) and CC (P<0.05) were statistically significantly different than in the CG. Daily fecal loss of this electrolyte patient with LC and CC was statistically significantly different than among the healthy people (P<0.05, Figure 1) The mean values of potassium concentration in patients with LC (P<0.01) and CC (P<0.05) were statistically significantly different than CG. Daily fecal loss of potassium in patients with LC and CC was statistically significantly different with regard to CG (P<0.05). The average value of chloride concentration in patients with CC was statistically significantly different with regard to CG (P<0.05). Daily fecal loss of this electrolyte in patients with LC and CC was statistically significantly different than among the healthy people (P<0.05). We tried to clarify the mechanism of diarrhea by calculation of the fecal osmotic gap (FOG). Secretory diarrhea (FOG <50 memo/kg) was found in 44 (86.7%) of patients with microscopic colitis. Osmotic diarrhea was present in 7 (13.3%) of patients. The reason in determining the concentration of fecal fat in patients with LC and CC was to establish possible impact of some other disease (small bowel or pancreatic) on pathophysiological mechanism of diarrhea. Six (11.7%) patients with LC had slightly increased daily fecal fat (9-11 g/24 h). DSW in the group of patients [9 (17.46%)] with LC and accompanying disease (mean: 315.10 g/24 h) was not statistically significantly different than in the group of patients with LC (mean: 372.64 g/24 h). The average values of fecal pH in the patients with LC (6.31±0.14) and CC (6.36±0.11) were not statistically significantly different compared to CG.
Table 2.
mean±SE values of daily stool weight, electrolyte concentration in fecal fluid and fecal pH in examined group of patients
| DSW (g/24 h) | Conc Na+ (mmol/L) | Conc K+ (mmol/L ) | Conc Cl– (mmol/L) | pH | |
| Lympocytic colitis | 386.25±5.39b | 58.11±5.38b | 74.65±5.29b | 28.85±2.55 | 6.31±0.14 |
| Collagenous colitis | 245.66±18.43b | 54.14±8.42a | 75.53±8.78a | 36.07±7.29a | 6.36±0.11 |
| Control group | 118.34±8.79 | 34.28±2.98 | 92.67±2.99 | 24.11±2.05 | 6.36±0.15 |
| MC without diarrhea | 139.19±15.92 |
P<0.05 vs Control group; bP<0.01 vs control group.
Figure 1.
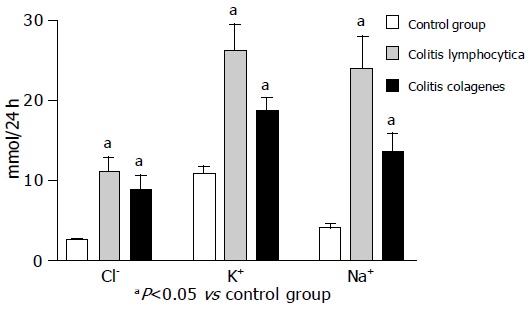
Average values of fecal daily loss of Na+, K+, Cl- (mean±SE).
DISCUSSION
Precise mechanism of diarrhea in patients with microscopic colitis still remains unclear. Most of the authors[31,34,35] believe that dominant processes in the development of diarrhea are: 1. Reduced active sodium absorption 2. Inhibited chloride and bicarbonate exchange 3. Increased electrogenic chloride secretion followed by passive sodium and water transport. 4. Decreased passive permeability of colonic mucosa. First data about mechanism of secretory diarrhea were established by Bo-Linn perfusion study[34]. The average values of DSW in this and other similar studies[21,33,34] were higher than values from our study (LC: 200, 2-1 400 g/24 h; CC: 206-350 g/24 h). Using “steady-state” perfusion method, the authors have proved that electrolyte and fluid absorption in colon were seriously disturbed in patients with microscopic colitis. The reasons for reduced electrolyte and fluid absorption are microscopic changes of colonic mucosa. Degenerative injuries of surface epithelium, subepithelial collagen deposit and persistence of inflammatory cells (prostaglandin E2)[31,34,35] infiltrate in lamina propria play the most important role in decreased absorption of luminal water and electrolyte. Pathohistological findings of colonic biopsy specimen in our patients are in accordance with the results of previous studies[8-10]. Decreased active sodium absorption is visible by a reduction of the flux lumen/plasma of sodium and chloride. Inhibited chloride and bicarbonate exchange is shown by the reduced bicarbonate secretion rate and lowered chloride absorption[34]. In our study, sodium concentration of the fecal fluid in patients with LC and CC was highly increased in relation to control group. These facts are in accordance with data of other authors. Potassium concentration in fecal fluid of patients with LC and CC was significantly less compared to CG. But the average values of potassium concentration were higher with regard to data from other studies. The mean values of chloride concentrations in patients with CC were statistically significantly higher with regard to CG. These results in our study were less than the data of other authors[34,35]. Daily fecal loss of electrolyte in patients with LC and CC were significantly higher compared to a group of healthy persons. These results were expected for sodium and chloride, due to great concentration of these electrolytes in fecal fluid. Despite smaller potassium fecal concentration, great potassium fecal loss appeared, due to daily stool weight. This fact could have clinical significance and could explain uncommon severe hypokalemia in some patients with microscopic colitis. Finally, our results of fecal electrolytes concentration and their daily fecal loss also confirm the hypothesis of disturbed active sodium absorption and absorption/secretion of chloride[31,34,35,41]. The dominant process in electrolyte malabsorption in patients with LC could be reduced by active sodium absorption. In the group of patients with CC prevailing mechanisms are decreasing the rate of Cl/HCO3 exchange and increased electrogenic Cl secretion, in addition to, reduced active sodium absorption. There were no significant differences between main values of fecal pH of examined patients with LC and CC with regard to healthy persons. Still it is interesting to note that all patients with proper therapeutic response to cholestyramine had slightly increased fecal pH (>6.8). This data could suggest possible co-factorial influence of bile acid malabsorption[17,33] on the mechanism of diarrhea in microscopic colitis. Only 6 (11.7%) patients had slightly increased daily fecal fat (9-11 g/24 h). All of them had lymphocytic colitis. In the group of patients with microscopic colitis and associated disease there were no differences in average values of daily stool weight compared to patients with microscopic colitis without accompanied disease. On the basis of our results it could be concluded that influence of accompanied disease on the mechanism of diarrhea is of secondary importance. Most of the authors[31,34,41-43,45] claim that secretory diarrhea is characteristic of microscopic colitis. By Erer’s criteria, the border value of fecal osmotic gap (FOG) for distinction between secretory and osmotic diarrhea is 50 mOsmol/kg[43,44]. In our study 86.7% of patients had secretory diarrhea and 13.3% osmotic diarrhea. All patients with secretory diarrhea belong to the group of patients with lymphocytic colitis. There was a group of 7 patients with histological diagnosis of microscopic colitis and without approved diarrhea. All of them had intermittent diarrhea. We recommended them to collect stool during the period of diarrhea. The schedule for future examination of these patients remains imprecise.
In conclusion, on the basis of our results it could be stated that diarrhea in microscopic colitis (LC, CC) belongs to the secretory type. The major pathophysiological mechanism in patients with LC may be a decrease of active sodium absorption. In collagenous colitis decreased Cl/HCO3 exchange rate and increased electrogenic chloride secretion is coexistent pathway in the genesis of diarrhea.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Lindström CG. 'Collagenous colitis' with watery diarrhoea--a new entity? Pathol Eur. 1976;11:87–89. [PubMed] [Google Scholar]
- 2.Read NW, Krejs GJ, Read MG, Santa Ana CA, Morawski SG, Fordtran JS. Chronic diarrhea of unknown origin. Gastroenterology. 1980;78:264–271. [PubMed] [Google Scholar]
- 3.Kingham JG, Levison DA, Ball JA, Dawson AM. Microscopic colitis-a cause of chronic watery diarrhoea. Br Med J (Clin Res Ed) 1982;285:1601–1604. doi: 10.1136/bmj.285.6355.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohr J, Tysk C, Eriksson S, Abrahamsson H, Järnerot G. Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Gut. 1996;39:846–851. doi: 10.1136/gut.39.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olesen M, Eriksson S, Bohr J, Järnerot G, Tysk C. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut. 2004;53:536–541. doi: 10.1136/gut.2003.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barta Z, Mekkel G, Csípo I, Tóth L, Szakáll S, Szabó GG, Bakó G, Szegedi G, Zeher M. Microscopic colitis: a retrospective study of clinical presentation in 53 patients. World J Gastroenterol. 2005;11:1351–1355. doi: 10.3748/wjg.v11.i9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baert F, Wouters K, D'Haens G, Hoang P, Naegels S, D'Heygere F, Holvoet J, Louis E, Devos M, Geboes K. Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut. 1999;45:375–381. doi: 10.1136/gut.45.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veress B, Löfberg R, Bergman L. Microscopic colitis syndrome. Gut. 1995;36:880–886. doi: 10.1136/gut.36.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazenby AJ, Yardley JH, Giardiello FM, Jessurun J, Bayless TM. Lymphocytic ("microscopic") colitis: a comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol. 1989;20:18–28. doi: 10.1016/0046-8177(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 10.Bogomoletz WV, Fléjou JF. Newly recognized forms of colitis: collagenous colitis, microscopic (lymphocytic) colitis, and lymphoid follicular proctitis. Semin Diagn Pathol. 1991;8:178–189. [PubMed] [Google Scholar]
- 11.Fernández-Bañares F, Salas A, Esteve M, Espinós J, Forné M, Viver JM. Collagenous and lymphocytic colitis. evaluation of clinical and histological features, response to treatment, and long-term follow-up. Am J Gastroenterol. 2003;98:340–347. doi: 10.1111/j.1572-0241.2003.07225.x. [DOI] [PubMed] [Google Scholar]
- 12.Robert ME. Microscopic colitis: pathologic considerations, changing dogma. J Clin Gastroenterol. 2004;38:S18–S26. doi: 10.1097/01.mcg.0000124027.92823.b5. [DOI] [PubMed] [Google Scholar]
- 13.Olesen M, Eriksson S, Bohr J, Järnerot G, Tysk C. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993-1998. Gut. 2004;53:346–350. doi: 10.1136/gut.2003.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zins BJ, Sandborn WJ, Tremaine WJ. Collagenous and lymphocytic colitis: subject review and therapeutic alternatives. Am J Gastroenterol. 1995;90:1394–1400. [PubMed] [Google Scholar]
- 15.Castanet J, Lacour JP, Ortonne JP. Arthritis, collagenous colitis, and discoid lupus. Ann Intern Med. 1994;120:89–90. doi: 10.7326/0003-4819-120-1-199401010-00023. [DOI] [PubMed] [Google Scholar]
- 16.Sowa JM. Arthritis and collagenous colitis. Ann Intern Med. 1994;121:237. doi: 10.7326/0003-4819-121-3-199408010-00020. [DOI] [PubMed] [Google Scholar]
- 17.Bowling TE, Price AB, Al-Adnani M. Interchange between collagenous and lymphocytic colitis in severs disease with autoimmune associations requiring colectomy: a case report. Gut. 1996;38:788–791. doi: 10.1136/gut.38.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heckerling P, Urtubey A, Te J. Collagenous colitis and systemic lupus erythematosus. Ann Intern Med. 1995;122:71–72. doi: 10.7326/0003-4819-122-1-199501010-00025. [DOI] [PubMed] [Google Scholar]
- 19.Schiller LR. Diagnosis and management of microscopic colitis syndrome. J Clin Gastroenterol. 2004;38:S27–S30. doi: 10.1097/01.mcg.0000123990.55626.ee. [DOI] [PubMed] [Google Scholar]
- 20.Bohr J, Tysk C, Yang P, Danielsson D, Järnerot G. Autoantibodies and immunoglobulins in collagenous colitis. Gut. 1996;39:73–76. doi: 10.1136/gut.39.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeroogian JM, Chopra S. Collagenous colitis and lymphocytic colitis. Annu Rev Med. 1994;45:105–118. doi: 10.1146/annurev.med.45.1.105. [DOI] [PubMed] [Google Scholar]
- 22.Tagkalidis P, Bhathal P, Gibson P. Microscopic colitis. J Gastroenterol Hepatol. 2002;17:236–248. doi: 10.1046/j.1440-1746.2002.02640.x. [DOI] [PubMed] [Google Scholar]
- 23.Pardi DS, Ramnath VR, Loftus EV, Tremaine WJ, Sandborn WJ. Lymphocytic colitis: clinical features, treatment, and outcomes. Am J Gastroenterol. 2002;97:2829–2833. doi: 10.1111/j.1572-0241.2002.07030.x. [DOI] [PubMed] [Google Scholar]
- 24.Freeman HJ. Collagenous colitis as the presenting feature of biopsy-defined celiac disease. J Clin Gastroenterol. 2004;38:664–668. doi: 10.1097/01.mcg.0000135363.12794.2b. [DOI] [PubMed] [Google Scholar]
- 25.Matteoni CA, Goldblum JR, Wang N, Brzezinski A, Achkar E, Soffer EE. Celiac disease is highly prevalent in lymphocytic colitis. J Clin Gastroenterol. 2001;32:225–227. doi: 10.1097/00004836-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016–2021. doi: 10.1111/j.1572-0241.2002.05917.x. [DOI] [PubMed] [Google Scholar]
- 27.Mulder CJ, Harkema IM, Meijer JW, De Boer NK. Microscopic colitis. Rom J Gastroenterol. 2004;13:113–117. [PubMed] [Google Scholar]
- 28.Beaugerie L, Luboinski J, Brousse N, Cosnes J, Chatelet FP, Gendre JP, Le Quintrec Y. Drug induced lymphocytic colitis. Gut. 1994;35:426–428. doi: 10.1136/gut.35.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaugerie L, Patey N, Brousse N. Ranitidine, diarrhoea, and lymphocytic colitis. Gut. 1995;37:708–711. doi: 10.1136/gut.37.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Järnerot G, Bohr J, Tysk C, Eriksson S. Faecal stream diversion in patients with collagenous colitis. Gut. 1996;38:154–155. doi: 10.1136/gut.38.1.154-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stampfl DA, Friedman LS. Collagenous colitis: pathophysiologic considerations. Dig Dis Sci. 1991;36:705–711. doi: 10.1007/BF01311225. [DOI] [PubMed] [Google Scholar]
- 32.Baert D, Coppens M, Burvenich P, De Cock G, Lagae J, Rasquin K, Vanderstraeten E. Chronic diarrhoea in non collagenous microscopic colitis: therapeutic effect of cholestyramine. Acta Clin Belg. 2004;59:258–262. doi: 10.1179/acb.2004.038. [DOI] [PubMed] [Google Scholar]
- 33.Marteau P, Lavergne-Slove A, Lemann M, Bouhnik Y, Bertheau P, Becheur H, Galian A, Rambaud JC. Primary ileal villous atrophy is often associated with microscopic colitis. Gut. 1997;41:561–564. doi: 10.1136/gut.41.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bo-Linn GW, Vendrell DD, Lee E, Fordtran JS. An evaluation of the significance of microscopic colitis in patients with chronic diarrhea. J Clin Invest. 1985;75:1559–1569. doi: 10.1172/JCI111861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ernest DL, Hixon LJ. Collagenous and lymphocytic colitis. In Sleisenger MN, Fordtran JS (Eds): Gastrointestinal disease. 5th ed. WB Saunders Co. Philadelphia; 1993. pp. 1563–1570. [Google Scholar]
- 36.Jesenovac N. Determination of sodium in fecal fluid. Special methods of biochemical laboratory analysis. Yugoslav society of medical biochemistries. Karlovac. 1988;1:192–193. [Google Scholar]
- 37.Jesenovac N. Determination of potassium in fecal fluid. Special methods of biochemical laboratory analysis. Yugoslav society of medical biochemistries. Karlovac. 1988:1: 182–183. [Google Scholar]
- 38.Jesenovac N. Determination of chloride in fecal fluid. Special methods of biochemical laboratory analysis. Yugoslav society of medical biochemistries. Karlovac. 1988:1: 257–258. [Google Scholar]
- 39.Van de Kamer JH. Quantitative determination of the saturated and unsaturated higher fatty acids in fecal fat. Scand J Clin Lab Invest. 1953;5:30–36. doi: 10.3109/00365515309093507. [DOI] [PubMed] [Google Scholar]
- 40.Fine KD, Fordtran JS. The effect of diarrhea on fecal fat excretion. Gastroenterology. 1992;102:1936–1939. doi: 10.1016/0016-5085(92)90316-q. [DOI] [PubMed] [Google Scholar]
- 41.Delgado J, Delgado B, Fich A, Odes S. Microscopic colitis. Isr Med Assoc J. 2004;6:482–484. [PubMed] [Google Scholar]
- 42.Koskela RM, Niemelä SE, Karttunen TJ, Lehtola JK. Clinical characteristics of collagenous and lymphocytic colitis. Scand J Gastroenterol. 2004;39:837–845. doi: 10.1080/00365520410006468. [DOI] [PubMed] [Google Scholar]
- 43.Fine KD, Krejs GJ, Fordtran JS. Diarrhea. In Sleisenger MH, Fordtran JS (eds. ): Gastrointestinal disease. 5th ed. WB Saunders Co. Philadelphia; 1993. pp. 1043–1072. [Google Scholar]
- 44.Eherer AJ, Fordtran JS. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545–551. doi: 10.1016/0016-5085(92)90845-p. [DOI] [PubMed] [Google Scholar]
- 45.Menduiña Guillén MJ, Alaminos García P, Valenzuela Barranco M. Microscopic colitis. A possible diagnosis in secretory diarrhea. An Med Interna. 2004;21:387–390. doi: 10.4321/s0212-71992004000800006. [DOI] [PubMed] [Google Scholar]


