Abstract
AIM: To determine the tolerability and safety profile of a low-dose maintenance therapy with 6-TG in azathioprine (AZA) or 6-mercaptopurine (6-MP) intolerant inflammatory bowel disease (IBD) patients over a treatment period of at least 1 year.
METHODS: Database analysis.
RESULTS: Twenty out of ninety-five (21%) patients discontinued 6-TG (mean dose 24.6 mg; mean 6-TGN level 540 pmol/8×108 RBC) within 1 year. Reasons for discontinuation were GI complaints (31%), malaise (15%) and hepatotoxicity (15%). Hematological events occurred in three patients, one discontinued treatment. In the 6-TG-tolerant group, 9% (7/75) could be classified as hepatotoxicity. An abdominal ultrasound was performed in 54% of patients, one patient had splenomegaly.
CONCLUSION: The majority of AZA or 6-MP-intolerant IBD patients (79%) is able to tolerate maintenance treatment with 6-TG (dosages between 0.3 and 0.4 mg/kg per d). 6-TG may still be considered as an escape maintenance immunosuppressant in this difficult to treat group of patients, taking into account potential toxicity and efficacy of other alternatives. The recently reported hepatotoxicity is worrisome and 6-TG should therefore be administered only in prospective trials.
Keywords: 6-Thioguanine, Azathioprine, 6-Mercaptopurine, Crohn’s disease, Ulcerative colitis, Side effects, Tolerance, Safety
INTRODUCTION
Until now, no definite medical or surgical therapy is available for the treatment of inflammatory bowel disease (IBD) as the etiology remains essentially unknown. Therefore, the aims of medical therapy in IBD are relief of symptoms and to prevent long-term complications. Maintenance therapy should preferably be efficient, safe, and cost-effective. The immune modulating thiopurines 6-mercaptopurine (6-MP) and its pro-drug azathioprine (AZA) have proven efficacy in active IBD and in maintenance of an induced clinical remission[1]. These thiopurines are prescribed on a large scale in IBD and are considered as a mainstay treatment option. However, issues concerning delayed onset of activity, refractoriness, and toxicity have limited the general use of AZA and 6-MP. In previous reports, up to 20% of patients discontinued AZA or 6-MP prematurely due to adverse events[2]. The metabolism of thiopurines has been partly elucidated in recent years (Figure 1). The toxicity profile depends at least partly on the generated metabolites of AZA and 6-MP[3]. At first, AZA is converted to 6-MP by a non-enzymatic reaction, which is then converted by a multi-step enzymatic pathway into a number of active, inactive, or toxic metabolites. The efficacy of AZA and 6-MP appears to be correlated with the formation of the 6-thioguanine nucleotides (6-TGNs). During this complex metabolization process, possible hepatotoxic metabolites are generated under the influence of the enzyme thiopurine methyltransferase (TPMT). The methylation products of 6-MP, 6-methylmercaptopurine (6-MMP) and their ribonucleotides, may be associated with hepatotoxicity[3]. Furthermore, flu-like symptoms, rashes, pancreatitis, and neutropenia induced by AZA or 6-MP have recently been related to mutations in the inosine triphosphate pyrophosphatase (ITPase) gene leading to accumulation of the proposed metabolite thio inosine triphosphate[4]. A possible strategy to avoid AZA or 6-MP-induced toxicity is the administration of a thiopurine, which is metabolically closer to the 6-TGNs. The thiopurine 6-thioguanine (6-TG) is an attractive candidate, which has the advantage of being directly converted to 6-TGNs[5]. Therefore, treatment and safety outcomes might be less sensitive for intra- and inter-individual metabolic variations. Furthermore, due to the relative simple metabolization, the number of possible toxic metabolites is strongly reduced. Several open label studies in patients with IBD have shown promising efficacy and acceptable short-term toxicity of 6-TG[6,7]. More recently, the use of 6-TG has been associated with the induction of nodular regenerative hyperplasia (NRH) of the liver. The aim of the present study was to determine the tolerability and safety profile of a low-dose maintenance therapy with 6-TG in AZA or 6-MP intolerant IBD patients over a treatment period of at least 1 year.
Figure 1.
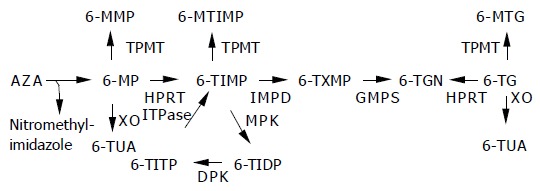
Metabolization process of thiopurines.
AZA is degraded to 6-MP and nitromethyl-imidazole, via a non-enzymatic step. Three competing enzymes metabolize 6-MP. Xanthine oxidase (XO) inactivates 6-MP to 6-thiouric acid (6-TUA). TPMT methylates 6-MP, the formed 6-MMP is associated with hepatotoxicity. By hypoxanthine guanine phosphoribosyl transferase (HPRT), 6-MP is catalyzed to 6-thioinosine monophosphate (6-TIMP), then via inosine monophosphate dehydrogenase to 6-thioxanthosine monophosphate, ultimately leading to the pharmacologically active 6-TGN via the enzyme guanosine monophosphate synthetase. 6-TIMP can also be methylated to 6-methyl-TIMP. In a normal useless cycle, 6-TIMP is phosphorylated by monophosphate kinase to 6-thioinosine diphosphate, subsequently by diphosphate kinase to 6-thioinosine triphosphate (6-TITP) and ultimately back to 6-TIMP due to the ITPase. When ITPase activity is impaired or lacking, 6-TITP accumulates. 6-TG is directly converted by HPRT to 6-TGN. XO and TPMT metabolize 6-TG to 6-methyl-TG and 6-TUA, respectively. ITPase has no known role in the metabolism of 6-TG.
MATERIALS AND METHODS
Patient selection
The study is a retrospective database analysis exploring the tolerability and safety of 6-TG over at least a 1-year treatment period in AZA or 6-MP-intolerant IBD patients, treated in participating university and district hospitals in the Netherlands. In each patient, the attending physician judged the indication for administration of 6-TG. Some patients have been described earlier in a short safety assessment of 6-TG[6]. Patients were eligible for the study, if they met the following in- and exclusion criteria. Inclusion criteria were: age between 18 and 75 years, presence of confirmed CD or UC with an indication for immunosuppressive therapy, but in whom standard AZA or 6-MP therapy failed, due to adverse events. Immunosuppression was indicated in case of chronic active disease, corticosteroid dependency or recurrent disease. Exclusion criteria were pregnancy, lactation, presence of active infection, history of tuberculosis, HIV, hepatitis B or C, history of severe pancreatitis (necrotizing pancreatitis or pancreatitis leading to multi-organ-failure), ongoing treatment with concomitant immunosuppressive drugs (e.g., cyclosporine, methotrexate (MTX), thalidomide or infliximab), impaired renal function (serum creatinine >2 times normal upper limit), impaired hepatic function (>2 times normal upper limit) and persistent bone marrow suppression.
Study design
In all patients, 6-TG (Lanvis, tablet 40 mg, Glaxo Wellcome) was administered orally in a dose of 20-40 mg once daily based on the decision of the attending physician. The following data were collected: patient demographics, history of thiopurine exposure, type of thiopurine intolerance, the use of concomitant medication, blood cell counts, and liver enzymes were recorded. Dose of 6-TG, duration of 6-TG, occurrence of adverse events, blood cell counts, and liver enzymes were reviewed after a minimum of 12 mo after initiation of 6-TG treatment. During the described period, the genotyping of TPMT became available and in a subgroup of patients the TPMT status was determined. RBC 6-TGN levels were determined at least 4 wk after giving a stable dose in order to obtain steady-state levels of 6-TGN. Dose adjustments were left to the discretion of the attending physician, but when 6-TGN levels were above 1 000-1 500 pmol/8×108 RBC, dose reduction was contemplated. In case of a leukocyte count below 3.5×109/L, dose reduction was advocated and in case of severe leukopenia (below 1.0×109/L) 6-TG had to be discontinued. Additionally, in order to explore signs of hepatotoxicity, an abdominal ultrasonography was advised after at least 1-year 6-TG administration. Special attention was paid to splenomegaly, signs of portal hypertension, and hepatic changes.
Outcome measurements
Primary outcome measures were the ability to tolerate 6-TG and the occurrence of adverse events leading to discontinuation of 6-TG over the 1-year period. The relationship of any adverse event with the use of 6-TG was established by the following method: unrelated, no temporal relation and other etiology likely; possibly related, potential temporal relation and other etiologies possible; probably related, potential temporal relation and other etiologies unlikely; related, clear temporal relation not otherwise explained. Secondary outcome measures were the occurrence of hematological events (defined as leukocyte count <4.0×109/L or platelet count <100×109/L), the occurrence of hepatotoxicity (defined as a rise of at least two times the upper normal limit of a single liver enzyme level), pancreaticotoxicity (serum amylase >220 U/L) and signs of liver-related abnormalities on the abdominal ultrasound. The following data concerning disease activity were analyzed as well: ESR, C-reactive protein (CRP), serum albumin and a global physician assessment score (features were same, better or worse).
6-TG metabolite monitoring and genotyping of TPMT
Blood samples for 6-TGN measurements were obtained at least 4 wk after the onset of a stable 6-TG dose. The samples were centrifuged to isolate erythrocytes and after washing with PBS, erythrocyte counts were done. Samples were stored at -20 °C until required. RBC 6-TGN levels were measured in the laboratory of the Department of Clinical Pharmacy, Maasland Hospital Sittard, using a proprietary modified previously published assay[8]. The lower limit of quantification of the assay was 30 pmol/8108 RBC for 6-TGN levels with a run-to-run coefficient of variation of 6.6%. Extra blood samples were drawn once during the 1-year period to assess TPMT (G238C, G460A and A719G, i.e., TPMT*1, TPMT*2, TPMT*3A, TPMT*3B, TPMT*3C) genotypes in a sub-group of patients, independent of the biochemical or clinical status. The genotyping was performed at the Department of Gastroenterology and Hepatology at the Academic Medical Centre in Amsterdam.
Statistical analysis
Data are expressed as mean±SD. Significance was evaluated by a t test for paired or independent data; P values of less than 0.05 were considered significant. Pearson’s correlation was used to determine relationships between parameters. A χ2 test was used to determine the significance between the TPMT genotype and 6-TG intolerance. SPSS for Windows version 11.0 was used for statistical analysis.
RESULTS
Patients
In 95 patients, treatment with 6-TG was initiated in the period from June 2001 to July 2003. Fifty-eight patients (61%) were female (mean age: 40 years, range: 20-74 years) and 37 (39%) were male (mean age: 47 years, range: 20-71 years). Forty-two (44%) patients were diagnosed with UC compared to 53 (56%) patients with CD. All patients were intolerant to AZA, 6-MP or both. The adverse events leading to discontinuation of AZA or 6-MP use were gastrointestinal complaints, general malaise, allergic reactions, hepatotoxicity or myelotoxicity. The patient’s characteristics are given in detail in Table 1. The mean initial 6-TG dose was 24.6 mg (range 20-40 mg). The mean initial 6-TG dose adjusted to bodyweight was 0.37 mg/kg (SD 0.16 mg/kg).
Table 1.
Patients’ characteristics
| n = 95 patients | |
| Female:male | 58:37 patients |
| Age (yr) | 43 years (range 20–74 yr) |
| UC:CD | 42:53 patients |
| Duration of IBD at start 6-TG | 10.4 yr (SD 9.4 yr) |
| AZA intolerance | 78 patients (82%) |
| 6-MP intolerance | 4 patients (4%) |
| AZA and 6-MP intolerance | 13 patients (14%) |
| AZA or 6-MP rechallenge | 36 patients (38%) |
| Adverse events on AZA or 6MP: | 30% gastrointestinal complaints |
| 20% general malaise | |
| 14% allergic reactions | |
| 10% pancreaticotoxicity | |
| 6% hepatotoxicity | |
| 5% myelotoxicity | |
| 14% rest (e.g., myalgia or alopecia) | |
| 6-TG dosage at the start | 24.6 mg (range 20-40 mg) |
Primary outcome
Seventy-five (79%) of the ninety-five patients were able to tolerate 6-TG during 1-year use. In 20 (21%) patients, the administration was discontinued due to side effects. The 20 intolerant patients (9 females and 11 males) encountered 26 side effects. The data concerning the adverse events leading to withdrawal and the relationship with 6-TG use are summarized in Table 2. No mortality was reported. The mean 6-TG dosage was 0.30 mg/kg in the tolerant group compared to 0.34 mg/kg in the intolerant group (NS).
Table 2.
Adverse events (n = 26) leading to discontinuation of 6-TG use in 20 patients (AE, adverse event; 6-TG, 6-thioguanine; GI complaints, gastrointestinal complaints)
| AE on 6-TG | Frequency (%) | Mean time to AE (d) | Relationship with 6-TG |
| GI complaints | 8/26 (31) | 43 | Probably 6/8 possibly 2/8 |
| Hepatotoxicity | 4/26 (15) | 182 | Probably 2/4 possibly ¼unrelated 1/4 |
| Myelodepression | 1/26 (4) | 50 | Related |
| Pancreaticotoxicity | 1/26 (4) | 103 | Possibly |
| General malaise | 4/26 (15) | 51 | Probably 3/4 possibly ¼ |
| Allergic reaction | 1/26 (4) | 1 | Related |
| Other AE | 7/26 (27) | 39 | Related 1/7 probably |
| 2/7 possibly 4/7 |
Secondary outcomes
Hematological events Three patients had signs of myelosuppression. One patient had a leukocyte count of 3.2×109/L and a platelet count of 70×109/L after 50 d of 6-TG use (20 mg). Subsequently, 6-TG was discontinued. The second patient had a leukopenia (leukocytes 3.5×109/L) and a third patient had a thrombocytopenia (platelets 87×109/L) at 1-year measurements, both did not discontinue 6-TG. All other patients, both tolerant and intolerant to 6-TG, had platelet and leukocyte counts above the set lower limits. However, the mean platelet count decreased significantly from baseline (309×109/L) to 1 year of 6-TG treatment (290×109/L, P = 0.033). The mean leukocyte count decreased as well (from 11.9 to 7.8×109/L) though not significantly. The 6-TGN level did not correlate with the decrease in both leukocytes or platelets counts. Hemoglobin levels remained unchanged in both patient groups.
Hepatotoxicity and pancreaticotoxicity In the 6-TG tolerant group the ASAT, ALAT, GGT, and AP levels were determined in 65%, 71%, 60%, and 68% of patients at 1-year 6-TG use, respectively. The mean levels of ASAT, ALAT, GGT, and AF were 24.7 U/L (range 6-118 U/L), 24.5 U/L (range 5-206 U/L), 48 U/L (range 6-535 U/L), and 84 U/L (range 42-322 U/L), respectively. A significant increase in liver enzymes during treatment in the tolerant group was not established. At 1-year use, in 9% of patients (7/75) hepatotoxicity occurrence on 6-TG treatment could be classified. Four patients (4/20) of the intolerant group discontinued 6-TG use due to hepatotoxicity. All had normal liver tests at entry and three of these patients encountered allergic reactions but no hepatotoxicity on the prior AZA therapy. Three patients had a serum amylase level above 220 U/L before the start of 6-TG that all became normal during treatment. One patient developed a transitory symptomatic pancreatitis with a serum amylase level of 430 U/L during 6-TG treatment and discontinued treatment.
Abdominal ultrasonography In 51 patients (54%), an abdominal ultrasonography was performed after at least 1 year 6-TG use. Five ultrasounds (10%) were considered as abnormal; three patients with steatosis, one patient had a choledocholithiasis and the last patient had a hydrops of the gallbladder and a splenomegaly (spleen size 13 cm). The latter two patients were classified as hepatotoxicity on 6-TG treatment.
Disease activity parameters No significant decrease in CRP (15-11 mg/L) or ESR level (15-14 mm) was established in the 6-TG tolerant group. The albumin level increased (37.6-40.1 g/L) significantly in the tolerant group (P = 0.002). A significant decrease of albumin level (38.5-36.6 g/L) was established in the intolerant group (P = 0.03). The global physician score was determined at 1-year 6-TG use in 85% (64/75) of the tolerant patients. Forty-seven patients (73%) were classified as better, 15 patients (23%) as the same and 4 patients (6%) as worse during 6-TG treatment.
6-TG metabolite monitoring and genotyping of TPMT The mean steady state 6-TGN level, determined in 63% (47/75) of the tolerant patients, was 540 pmol/8108 RBC (SD 245, range 0-1 404). In the eight intolerant patients in whom metabolite levels were measured (40%), the 6-TGN level was 725 pmol/8108 RBC (SD 422, range 229-1 563). The difference in 6-TGN levels between tolerant and intolerant patients was not significant. No statistical significant correlations were established between laboratory parameters (leukocytes, platelets, hemoglobin, AF, GGT, ASAT, and ALAT) and 6-TGN level. The TPMT genotyping was performed in 51 patients (54%). Two patients (3.9%) had one mutant non-functional allele (TPMT*3A and TPMT*3C) and both patients were intolerant for 6-TG (myelodepression and hepatotoxicity) (significant, P = 0.003). The 6-TGN level was not determined in both patients. Patients with two mutant non-functional alleles of TPMT (homozygous mutants) were not found in our population.
DISCUSSION
In this group of AZA or 6-MP-intolerant IBD patients, we demonstrated that 6-TG in dosages of 0.3-0.4 mg/kg daily was well tolerated over a period of 1 year as a maintenance immunosuppressant in 79% of the patients. This result suggests that during the metabolization process of AZA or 6-MP metabolites are generated that induce side effects, which at least in part are not generated during the 6-TG metabolism. No mortality was reported due to 6-TG use. When 6-TG was discontinued, the side effects leading to withdrawal resolved spontaneously. One patient developed a myelodepression that may be explained by the impaired TPMT activity, probably shunting 6-TG away from methylation by TPMT toward the formation of 6-TGNs, which have been associated with myelotoxicity. Unfortunately, no 6-TGN level was determined in this patient. The real incidence of 6-TG-related histological liver abnormalities like NRH or veno-occlusive disease (VOD) in the present study remains unknown, as liver biopsies were not performed. The incidence of 6-TG related increase in liver enzymes probably will be lower than 7 out of 75 patients, as 2 patients already had abnormal liver tests before the start of 6-TG and 1 patient had symptomatic choledocholithiasis.
In the present study, we found a mean 6-TGN level of 540 pmol/8108 RBC in the tolerant group, which is above the proposed therapeutic threshold of 250 pmol/8108 RBC and even higher than the proposed upper limit of efficacy (450 pmol/8108 RBC) under AZA or 6-MP therapy[3,9]. This is consistent with previous observations[6,7]. It remains to be elucidated whether the same 6-TGN limits should be used when 6-TG is administered. In our study, 73% clinically benefited from 6-TG by using the global physician score and the mean albumin level increased significantly. However, due to the retrospective nature of the study, we must be reserved in drawing firm conclusions as no standard efficacy parameters as the Crohn’s disease activity index or Truelove-Witts index were used. Despite the fact that we have not demonstrated a significant relationship between 6-TGN levels, laboratory results and adverse events, we believe that therapeutic drug monitoring may be a helpful tool in dosing 6-TG. Extremely high 6-TGN levels, which have been associated with an increased risk of developing myelotoxicity, can be prevented and compliance can be monitored.
The TPMT status has been associated with the ability to tolerate AZA or 6-MP. Patients with impaired TPMT activity are more prone to develop a myelodepression[10]. However, our study demonstrates that in AZA or 6-MP-intolerant patients only two patients (3.9%) had one mutant TPMT allele, which is even lower than the incidence in the normal Caucasian population[11]. Interestingly, both patients did not develop a myelodepression on AZA therapy. This indicates that other metabolic pathways may lead to toxicity of AZA or 6-MP as was recently demonstrated for the ITPase routing (Zelinkova et al, submitted for publication). However, both patients with one mutant TPMT allele discontinued the use of 6-TG. Unfortunately, in these patients no 6-TGN concentrations were measured before discontinuation of 6-TG. Possibly, an impaired TPMT activity leads to more 6-TG being metabolized by the enzymes HGPRT or XO leading to higher 6-TGN and 6-TUA levels, respectively (Figure 1). The accumulation of these metabolites may lead to intolerance of 6-TG.
Recently, the use of 6-TG in IBD patients has been associated with the induction of NRH and VOD[12]. In the present study, we performed an abdominal ultrasonography after at least 1-year 6-TG use to screen for possible hepatotoxicity in 51 patients. Only one patient showed signs of portal hypertension indicated by an enlarged spleen. NRH has been associated with thrombocytopenia[16] and in our evaluation only two patients had platelets counts below 100 U/L. We are well aware of the fact that by using biochemistry and ultrasound outcomes, we probably underestimate the real incidence of 6-TG-induced NRH, as the golden standard is based on histology. Additionally, liver tests abnormalities were shown not to be indicative for NRH. Conversely, it should be taken into account that AZA and 6-MP can induce NRH or VOD as well, but performing liver biopsies during treatment with these compounds is not recommended in clinical practice.
Currently, the use of 6-TG in IBD patients is abandoned due to potential hepatotoxicity[12]. However, low-dose 6-TG may still be considered as an escape maintenance strategy in AZA- or 6-MP-intolerant IBD patients whom are refractory for alternative therapies. The number of proven effective medical maintenance options is scarce for these patients. MTX has a reasonable toxicity profile and seems effective in CD, comparable with AZA or 6-MP[13] but the potential use of MTX in UC patients has yet to be proven. In addition, long-term use of MTX may be limited[14]. Cyclosporine seems to have no role as a maintenance immunosuppressive alternative in IBD[15]. Infliximab can be administered as a maintenance option in CD with acceptable toxicity and may be effective in treating UC. However, concomitant immunosuppressive therapy with thiopurines or MTX next to infliximab therapy seems mandatory to reduce the immunogenic response[16].
Treatment with 6-TG is well tolerated in AZA and 6-MP-intolerant IBD patients and seems to be effective. We believe that low-dosed 6-TG (0.3-0.4 mg/kg per d) maintenance therapy may still be an escape option for this difficult to treat group of patients. The benefit of 6-TG use in this sub-group may balance its toxicity profile, especially taking into account the toxicity and efficacy of other therapeutic alternatives. Still, the reported hepatotoxicity is worrisome and 6-TG should therefore be administered only in prospective clinical trials.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol. 1996;91:423–433. [PubMed] [Google Scholar]
- 2.Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641–649. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- 3.Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 4.Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM, Shobowale-Bakre E, Escuredo E, Fairbanks LD, Sanderson JD. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase) Pharmacogenetics. 2004;14:181–187. doi: 10.1097/00008571-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 5.de Jong D, Mulder CJ, van Sorge AA. Why measure thiopurine methyltransferase activity? Direct administration of 6-thioguanine might be the alternative for 6-mercaptopurine or azathioprine. Gut. 2001;49:874. doi: 10.1136/gut.49.6.874a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derijks LJ, de Jong DJ, Gilissen LP, Engels LG, Hooymans PM, Jansen JB, Mulder CJ. 6-Thioguanine seems promising in azathioprine- or 6-mercaptopurine-intolerant inflammatory bowel disease patients: a short-term safety assessment. Eur J Gastroenterol Hepatol. 2003;15:63–67. doi: 10.1097/00042737-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Dubinsky MC, Feldman EJ, Abreu MT, Targan SR, Vasiliauskas EA. Thioguanine: a potential alternate thiopurine for IBD patients allergic to 6-mercaptopurine or azathioprine. Am J Gastroenterol. 2003;98:1058–1063. doi: 10.1111/j.1572-0241.2003.07413.x. [DOI] [PubMed] [Google Scholar]
- 8.Derijks LJ, Gilissen LP, Engels LG, Bos LP, Bus PJ, Lohman JJ, Curvers WL, Van Deventer SJ, Hommes DW, Hooymans PM. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: implications for therapy. Ther Drug Monit. 2004;26:311–318. doi: 10.1097/00007691-200406000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Cuffari C, Hunt S, Bayless T. Utilisation of erythrocyte 6-thioguanine metabolite levels to optimise azathioprine therapy in patients with inflammatory bowel disease. Gut. 2001;48:642–646. doi: 10.1136/gut.48.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansari A, Hassan C, Duley J, Marinaki A, Shobowale-Bakre EM, Seed P, Meenan J, Yim A, Sanderson J. Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:1743–1750. doi: 10.1046/j.1365-2036.2002.01353.x. [DOI] [PubMed] [Google Scholar]
- 11.Weinshilboum R. Thiopurine pharmacogenetics: clinical and molecular studies of thiopurine methyltransferase. Drug Metab Dispos. 2001;29:601–605. [PubMed] [Google Scholar]
- 12.Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, Martin P, Vierling JM, Geller SA, Targan SR, et al. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003;125:298–303. doi: 10.1016/s0016-5085(03)00938-7. [DOI] [PubMed] [Google Scholar]
- 13.Ardizzone S, Bollani S, Manzionna G, Imbesi V, Colombo E, Bianchi PG. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn's disease: a randomised, investigator-blind study. Dig Liver Dis. 2003;35:619–627. doi: 10.1016/s1590-8658(03)00372-4. [DOI] [PubMed] [Google Scholar]
- 14.Bell SJ, Kamm MA. Review article: the clinical role of anti-TNFalpha antibody treatment in Crohn's disease. Aliment Pharmacol Ther. 2000;14:501–514. doi: 10.1046/j.1365-2036.2000.00777.x. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer SB, Present DH. The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord. 2003;3:81–92. [PubMed] [Google Scholar]
- 16.Rutgeerts P, Van Assche G, Vermeire S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology. 2004;126:1593–1610. doi: 10.1053/j.gastro.2004.02.070. [DOI] [PubMed] [Google Scholar]


