Abstract
AIM: To study the different gene expression profiles in rats with Barrett’s esophagus (BE) and esophageal adenocarcinoma (EA) induced by gastro-duodeno-esophageal reflux.
METHODS: Esophagoduodenostomy was performed in 8-wk old Sprague-Dawley rats to induce gastro-duodeno-esophageal reflux, and a group of rats that received sham operation served as control. Esophageal epithelial pathological tissues were dissected and frozen in liquid nitrogen immediately. The expression profiles of 4 096 genes in EA and BE tissues were compared to normal esophagus epithelium in normal control (NC) by cDNA microarray.
RESULTS: Four hundred and forty-eight genes in BE were more than three times different from those in NC, including 312 upregulated and 136 downregulated genes. Three hundred and seventy-seven genes in EA were more than three times different from those in NC, including 255 upregulated and 142 downregulated genes. Compared to BE, there were 122 upregulated and 156 downregulated genes in EA. In the present study, the interested genes were those involved in carcinogenesis. Among them, the upregulated genes included cathepsin C, aminopeptidase M, arachidonic acid epoxygenase, tryptophan-2,3-dioxygenase, ubiquitin-conjugating enzyme, cyclic GMP-stimulated phosphodiesterase, tissue inhibitor of metalloproteinase-1, betaine-homocysteine methyltra-nsferase, lysozyme, complement 4b binding protein, complement 9 protein, insulin-like growth factor binding protein, UDP-glucuronosyltransferase, tissue inhibitor of metalloproteinase-3, aldolase B, retinoid X receptor gamma, carboxylesterase and testicular cell adhesion molecule 1. The downregulated genes included glutathione synthetase, lecithin-cholesterol acyltransferase, p55CDC, heart fatty acid binding protein, cell adhesion regulator and endothelial cell selectin ligand.
CONCLUSION: Esophageal epithelium exposed excessively to harmful ingredients of duodenal and gastric reflux may develop into BE and even EA gradually. The gene expression level is different between EA and BE, and may be related to the occurrence and progression of EA.
Keywords: Gastroduodenoesophageal reflux, Barrett’s esophagus, Esophageal adenocarcinoma, Gene expression
INTRODUCTION
The incidence of esophageal adenocarcinoma (EA) has increased considerably in the past few years and has already exceeded any other kind of malignant tumor in USA and the incidence of EA in Caucasians has exceeded squamous carcinoma[1]. A Dundee inquisition discovered that the incidence of EA has increased 49% in the last few years[2]. No detailed clinical epidemiological data in this respect can be acquired in Asia. However, some gastroenterology specialists also thought that the incidence of EA has increased in their country[3]. This may be related to the increasing incidence of gastro-esophageal reflux diseases, especially Barrett’s esophagus (BE)[4,5]. Gastro-esophageal reflux diseases are caused by mucosa exposed excessively to harmful ingredients of duodenal and gastric reflux. It is usually considered that gastro-esophageal reflux can lead to esophagitis, BE and even EA. However, the exact mechanism especially the molecular biological mechanism of EA is unknown. In the present study, the same surgical procedures were performed in Sprague-Dawley rats to induce gastro-duodeno-esophageal reflux and the difference in gene expression profiles between BE and EA was investigated by cDNA microarray.
MATERIALS AND METHODS
Experimental animal and animal model
One hundred and twenty healthy Sprague-Dawley rats weighing 200-250 g were purchased from the Experimental Animal Center of Xi’an Jiaotong University. The rats were housed in rat cages at 22-25 °C with free access to standard rat pellet food and water for 40 wk. Rats were treated following the Guidelines for the Care and Use of Laboratory Animals of National Animal Welfare Committee. The male pairing female rats were divided into two groups. Esophago-duodenostomy was performed on 90 rats to produce gastro-duodeno-esophageal reflux, and sham operation was performed on 30 rats[6]. The animals were fed with a standard chow.
Tissues and specimens
All the tissue specimens including BE and EA were taken from animals 40 wk after esophago-duodenostomy, by which gastro-duodeno-esophageal reflux animal model was induced. Normal esophageal epithelium at the same anatomical site served as normal control (NC). Inner part of each sample was cut, frozen in liquid nitrogen immediately after surgical resection, at the same time about 0.2 cm×0.2 cm×0.2 cm tissue of outer marginal part was used for histopathological examination to ensure all the frozen tissue specimens and their corresponding histological morphology.
Chip preparation
Four thousand and ninety-six target cDNA clones were used in cDNA microarray (United Gene Ltd). These genes were amplified by PCR, using universal primers and then purified by standard method. The quality of PCR was monitored by agarose gel electrophoresis. The obtained genes were dissolved in 3×SC spotting solution and then spotted on silylated slides (Telechem Inc.) by Cartesian 7500 spotting robotics (Cartesian, Inc.). Each target gene was dotted twice. After spotting, the slides were hydrated (2 h) and dried (0.5 h, room temperature). The samples were cross-linked with UV light and treated with 0.2% SDS, H2O and 0.2% NaNBH4 for 10 min respectively. Then the slides were dried in cold condition for use.
Probe preparation
Total sample RNA was extracted by single step method. Briefly, after being taken out from liquid nitrogen, specimens were ground completely into tiny powders, while liquid nitrogen was added in ceramic mortar and then the powders were homogenized in D solution plus 1% mercaptoethanol. After centrifugation, the supernatant was extracted with phenol:chloroform (1:1), NaAC and acidic phenol:chloroform (5:1), respectively. The aqueous phase was precipitated by an equal volume of isopropanol and centrifuged. The precipitates were dissolved with Millie-Q H2O. After further purification by LiCl precipitating method, RNA was obtained by UV analysis and electrophoresis. mRNA was isolated and purified with Oligotex mRNA Midi kit (Qiagen, Inc.). The fluorescent-labeled cDNA probe was prepared through retro-transcription as previously described[7]. The probes from NC were labeled with Cy3-dUTP, while those from BE and EA with Cy5-dUTP respectively. The probes were mixed (Cy3-dUTP NC+Cy5-dUTP BE and Cy3-dUTP NC+ Cy5-dUTP EA respectively) and precipitated by ethanol, and then resolved in 20 mL hybridization solution (5×SC+0.2% SDS).
Hybridization and washing
Probes and the chip were denatured respectively in 95 °C bath for 5 min, and then the probes were added onto the chip. They were hybridized in a sealed chamber at 60 °C for 15-17 h and washed in turn with solutions of 2×SC+0.2% SDS, 0.1×SC+0.2% SDS and 0.1% SSC for 10 min each, then dried at room temperature.
Fluorescent scanning and result analysis
The chip was read by a Scan Array 3000 Scanner (General Scanning Inc.). The overall intensities of Cy3 and Cy5 were normalized and corrected by a coefficient, according to the ratios of the located 40 h keeping genes. Cy3 was normalized as Cy3*. The acquired image was further analyzed by Gene Pix Pro 3.0 software with digital computer to obtain the intensities of fluorescent signals and the Cy5/Cy3* ratio. The data were an average of the two repeated spots. The differentially expressed genes were defined as the absolute value of the Cy5/Cy3* natural logarithm being more than 1.10 (the variation of gene expression was more than threefold); either Cy3 or Cy5 signal value required being more than 800, or both signal values being more than 200, or else being thought as an ineffective gene dot; PCR results being satisfactory.
RESULTS
Features of esophagus pathological changes
Sixty-nine rats survived in animal model group in the end and all animals that had undergone sham operation survived. Incidences of BE and EA in esophago-duodenostomy animal group were 37.5% and 2.8% respectively, significantly higher than that in the sham operation group.
Total sample RNA extraction
Total sample RNA from normal esophagus epithelium, BE and EA was extracted and the A260/A280 was 2-2.6, indicating that pure mRNA was acquired.
Scatter spots of hybridization signals on gene chip
The scatter spots plotted with Cy3 and Cy5 fluorescent signal values displayed quite a disperse pattern in distribution. Most spots gathered around a 45 angle diagonal line, in which blue spots represented the area, where the signal intensities ranged 0.33-3-folds compared to those of the control. Some yellow spots distributed beyond or far from 45 angle diagonal line, indicating the existence of abnormal gene expression in BE or EA and their signal intensities were three times more or less than those in NC (Figures 1A and B).
Figure 1.
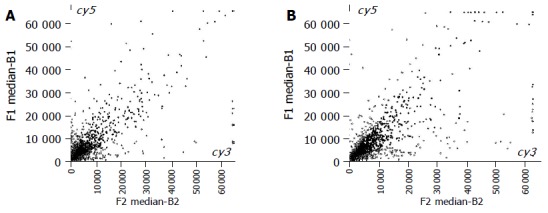
Scatter spots of hybridization signals on gene chip. A: Esophagus adenocarcinoma and normal control; B: Barrett’s esophagus and normal control.
Results and gene expression pattern by scanning analysis
In BE epithelia, 448 genes exhibited expression variations more than three times compared to the control, the up- and down-regulated genes were 312 and 136 respectively. In EA, 377 genes showed expression variations more than three times compared to NC, the up- and down-regulated genes were 255 and 122 respectively. Difference in gene expression between EA and BE was acquired by subtracting genes in common from total genes expressed differently in EA and BE compared to NC (any ineffective dot of hybridization was excluded from analysis). A total of 268 genes expressed differently in BE compared to those in EA, the up- and down-regulated genes were 112 and 156 respectively. These genes might be divided into 12 groups (Table 1) according to their functions. Some genes involved in carcinogenesis are listed in Table 2.
Table 1.
Different expressions of functional genes in BE and EA
| Functional genes | Number | Upregulated | Downregulated |
| Oncogenes and tumor | 23 | 13 | 10 |
| suppression genes | |||
| Ion channel and transporters | 26 | 13 | 13 |
| Cell cycle proteins | 13 | 4 | 9 |
| Extra-pressure reaction proteins | 20 | 8 | 12 |
| Cell regulatory proteins | 15 | 5 | 10 |
| Cell apoptosis-related proteins | 19 | 11 | 8 |
| DNA synthesis, repair, | 20 | 6 | 14 |
| recombinant factors | |||
| DNA binding, transcription factors | 23 | 7 | 16 |
| Cell receptors | 23 | 7 | 16 |
| Immunity-related proteins | 29 | 12 | 17 |
| Cell signal transducing proteins | 28 | 9 | 19 |
| Metabolism-related proteins | 29 | 17 | 12 |
| Total | 268 | 112 | 156 |
Table 2.
Different expressions of some genes in BE and EA
| Upregulated | Downregulated |
| Cathepsin C | Glutathione synthetase |
| Aminopeptidase M | Lecithin-cholesterol acyltransferase |
| Arachidonic acid epoxygenase | p55CDC |
| Tryptophan-2,3-dioxygenase | Heart fatty acid binding protein |
| Ubiquitin conjugating enzyme | Cell adhesion regulator |
| Cyclic GMP-stimulated | Selectin, endothelial cell, ligand |
| phosphodiesterase | |
| Tissue inhibitor of metalloproteinase 1 | |
| Betaine-homocysteine methyltransferase | |
| Lysozyme | |
| Complement 4 binding protein, alpha | |
| Insulin-like growth factor binding protein | |
| UDP-glucuronosyltransferase | |
| Tissue inhibitor of metalloproteinase 3 | |
| Rat liver aldolase B | |
| Retinoid X receptor gamma | |
| Complement component 9 | |
| Rat liver carboxylesterase | |
| Testicular cell adhesion molecule 1 |
DISCUSSION
Research has confirmed that BE is closely related to EA, every year the danger of BE developing into EA increases by 0.5% and the former is regarded as precancerous of the latter[5]. However, adenocarcinoma in the gastro-esophageal junction is thought as EA and its diagnosis can be made according to the fact that more than 50% carcinomas are situated in the esophageal lateral, mixing up the tissue origin of EA. Some clinical epidemiologic data on cardiac adenocarcinoma and EA indicate that the two diseases are similar in respects of age, sex, clinical, and pathological characteristics so that the origin of the two diseases is thought as the same tissue[8] and the two diseases are known as adenocarcinoma at the gastro-esophageal junction[9].
Miwa et al[6], reported that EA arises from BE, which is induced by the contents of gastro-duodeno-esophageal reflux. The role of single genes in esophageal adenocarcinogenesis is reported[10]. However, the exact mechanism especially the molecular biological mechanism of EA is unknown. The carcinogenesis involved in more steps and more factors is caused by abnormal expression of tumor-associated genes including activation of oncogenes or inactivation of tumor suppression genes or both. Elucidation of the gene expression differences in malignant, precancerous and normal tissue is a crucial procedure for cancer control study. It is generally accepted that although the number of genes with mutation is limited in cancer, a large number of genes in related pathways may be affected at the expression level, and this aberrant gene transcriptional expression network plays an essential role in the initiation/maintenance of the malignant phenotype. With the advances in molecular biological techniques, cDNA microarray has been used to detect gene expression difference in various specimens by parallel analysis on a large scale[11]. In this research, animal model of gastro-duodeno-esophageal reflux was made and the difference in gene expression profiles between BE and EA was investigated by cDNA microarray. In the present study, we were interested in a group of genes involved in carcinogenesis.
Among the upregulated genes in EA, 18 genes were involved in carcinogenesis and carcinomatous progression including cathepsin C, aminopeptidase M, arachidonic acid epoxygenase, tryptophan-2,3-dioxygenase, ubiquitin-conjugating enzyme, cyclic GMP-stimulated phosphodiesterase, tissue inhibitor of metalloproteinase-1, tissue inhibitor of metalloproteinase-3, betaine-homocysteine methyltransferase, lysozyme, complement 4b binding protein, complement 9 protein, insulin-like growth factor (IGF) binding protein, UDP-glucuronosyltransferase, aldolase B, retinoid X receptor gamma, carboxylesterase and testicular cell adhesion molecule-1. These genes might participate in carcinogenesis and carcinomatous progression of EA by various mechanisms.
Cathepsin C is a kind of cysteine protease. Cysteine proteases are negatively correlated with those inhibitors in effect and there is a balance in effect between them in normal tissues. However, in tumor tissue, genes expressing cysteine proteases are upregulated and inhibitors of cysteine protease are downregulated, thus breaking the balance in normal tissue and leading to extracellular matrix proteins decompounding, which might be related to invasion and metastasis of tumor[12]. Aminopeptidase M causes conversion of angiotensin III into angiotensin IV[13], which might be related to lesions of gastrointestinal tract mucosa[14]. The 5-upstream region of the arachidonic acid epoxygenase gene was isolated by amplification of a 2 341-bp fragment and putative regulatory elements that resembled activator protein-1-like sequences were identified. Activator protein-1 as a nucleolus transcription factor, combined DNA by leucine zipper, is a pivotal factor of cellular malignant transformation in various oncogene signal-transducing pathways[15]. T lymphocytes undergo proliferation arrest when exposed to tryptophan shortage, which can be provoked by tryptophan 2,3-dioxygenase, an enzyme that catalyzes tryptophan degradation and is expressed in most human tumors and normal placenta[16]. Ubiquitin-conjugating enzyme is a key component of ubiquitin-proteasome-proteolytic pathway that is associated with tumorous cachexia and upregulated expression in some tumor tissues[17]. The cGMP phosphodiesterase decreases cGMP in SW480 and HT29 colon tumor cells, which inhibit apoptosis[18]. Tissue inhibitor of metalloproteinase inhibits the activity of metalloprotease and the latter is associated with tumor invasion and metastasis. In common, the former is downregulated and the latter is upregulated in tumor tissue. However, some researches indicate that some tissue inhibitor of metalloproteinase can also stimulate cell proliferation and angiogenesis, thus leading to tumorigenesis[19]. For example, overexpression of tissue inhibitor of metalloproteinase-1 is associated with poor prognosis and relapse in patients with tumor[19]. New data suggest that high levels of tissue inhibitor of metalloproteinase-3 mRNA in human breast tumors are associated with successful adjuvant endocrine therapy[20]. Betaine-homocysteine-methyltransferases catalyze synthesis of methionine from homocysteine, which provides methyl for DNA synthesis. Methionine, obtained in the diet and synthesized by several reactions in the body, is the sole precursor of S-adenosylmethionine, the primary methyl donor in the body. Enzymatic DNA methylation is an important component of gene control and may serve as a silencing mechanism for gene function. Disruption in methionine metabolism and methylation reactions may be involved in cancer process[21]. Lysozyme is an innate non-immunologic antibacterial enzyme produced by the Paneth cells of the upper intestinal tract. Lysozyme is not normally secreted in the alimentary tract without Paneth cells, where intense lysozyme production might herald a possible dysplastic evolution and even neoplasia[22]. Complement 4b binding protein and complement 9 are crucial ingredients of complement immune system and multi-complement 9 unites into a polymer as formation of the membrane attack complex, which mediates tumor cell lysis[23]. IGF-I and its main binding protein modulate cell growth and survival, and are important in tumor development and progression. Circulating concentrations of IGF and its binding protein are associated with an increased risk for common cancers, but the association is modest and varies between sites[24]. Uridine diphospho-glucuronosyltransferases are a family of drug metabolizing enzymes contributing to hepatic drug metabolism and protection against environmental toxins. They are involved in catalyzing estrogen, which may be associated with an increased risk for breast cancer[25]. Aldolase B is a kind of glycolytic enzyme. There is a direct relationship between the proliferation of tumor cells and the activities of glycolytic and gluconeogenic enzymes. A significant rise in glycolytic enzyme activities and a simultaneous fall in gluconeogenic enzyme activities are found in mammary carcinoma[26]. Retinoids are natural and synthetic compounds related to retinoic acid, that act through interaction with two basic types of nuclear receptors: retinoic acid receptors and retinoid X receptors (RXR alpha, RXR beta, and RXR gamma) as retinoid-inducible transcription factors. Thus, retinoid receptors are considered to be ligand-activated, DNA-binding, trans-acting, transcription-modulating proteins involved in a general molecular mechanism responsible for transcriptional responses in target genes. They exert both beneficial and detrimental activity related to the selective receptors[27]. Haugen et al[28], reported that differential tumor expression of RAR beta and RXR gamma and lack of the RXR gamma isoform are found in normal thyroid tissue. Cell lines expressing both RAR beta and RXR gamma demonstrate significant growth suppression when treated with retinoids, whereas cell lines lacking these isoforms are unaffected. Carboxylesterase isoenzyme RL2 shows hydrolyzing activity towards activators of protein kinase C. Since protein kinase C is involved in carcinogenesis and cell proliferation, up-regulated carboxylesterase in adenocarcinoma might be involved in carcinogenesis[29]. Testicular cellular adhesion molecule is a cellular adhesion molecule expressed normally in testicle, which plays a role in cancer angiogenesis, progression, and metastasis[30].
Among the downregulated genes in EA, the downregulated genes involved in carcinogenesis include glutathione synthetase, lecithin-cholesterol acyltransferase, p55CDC, heart fatty acid binding protein, cell adhesion regulator and endothelial cell selectin ligand. The cellular defense system (including glutathione and glutathione-related enzymes) plays a crucial role in cell survival and growth. Glutathione and glutathione synthetase are associated with tumorigenesis and apoptosis[31]. An important factor that determines the movement of cholesterol in and out of the cells is the free cholesterol/esterified cholesterol ratio in plasma. The ratio increases in several types of malignancies both in humans and in experimental animals, which might be related to decreased activity of lecithin-cholesterol acyltransferase[32]. p55CDC, a mitotic checkpoint protein, plays a crucial role in carcinogenesis and decreased expression of the proteins is associated with progression of some cancers[33]. Heart fatty acid binding protein is also known as a mammary-derived growth inhibitor, which is a tumor growth suppressor gene capable of inhibiting tumor cell proliferation[34]. Cell adhesion regulator can inhibit tumor cellular growth and regulate tumor angiogenesis. Its expression in some tumor tissues is downregulated and associated with progression of tumor[35]. Endothelial cell selectin ligand mediates lymphocytes interacting with endothelial cells or tumor cells and plays an important role in the metastasis of carcinoma[36], indicating that the ability to protect against esophagus tumorigenesis, decreases with the development of normal esophageal epithelium into BE, by overexposure to contents of gastric and duodenal reflux.
Many up- or down-regulated genes in EA compared to BE indicate that many genes in related pathways may affect development of BE into carcinoma and its molecular mechanism is very complex. The difference in gene expression between BE and EA indicates the molecular characteristics of the onset and promotion of BE and its progression into EA. The exact pathogenesis of EA and the relationship between EA and differently expressed genes need further study.
Footnotes
Supported by the Major Program of Clinical Medicine of Ministry of Public Health, No. 20012130
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Barrett MT, Yeung KY, Ruzzo WL, Hsu L, Blount PL, Sullivan R, Zarbl H, Delrow J, Rabinovitch PS, Reid BJ. Transcriptional analyses of Barrett's metaplasia and normal upper GI mucosae. Neoplasia. 2002;4:121–128. doi: 10.1038/sj.neo.7900221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rana PS, Johnston DA. Incidence of adenocarcinoma and mortality in patients with Barrett's oesophagus diagnosed between 1976 and 1986: implications for endoscopic surveillance. Dis Esophagus. 2000;13:28–31. doi: 10.1046/j.1442-2050.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Chen XL, Wang KM, Guo XD, Zuo AL, Gong J. Barrett's esophagus and its correlation with gastroesophageal reflux in Chinese. World J Gastroenterol. 2004;10:1065–1068. doi: 10.3748/wjg.v10.i7.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 5.Geboes K. Barrett's esophagus: the metaplasia-dysplasia-carcinoma sequence: morphological aspects. Acta Gastroenterol Belg. 2000;63:13–17. [PubMed] [Google Scholar]
- 6.Miwa K, Sahara H, Segawa M, Kinami S, Sato T, Miyazaki I, Hattori T. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer. 1996;67:269–274. doi: 10.1002/(SICI)1097-0215(19960717)67:2<269::AID-IJC19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci USA. 1996;93:10614–10619. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruol A, Parenti A, Zaninotto G, Merigliano S, Costantini M, Cagol M, Alfieri R, Bonavina L, Peracchia A, Ancona E. Intestinal metaplasia is the probable common precursor of adenocarcinoma in barrett esophagus and adenocarcinoma of the gastric cardia. Cancer. 2000;88:2520–2528. doi: 10.1002/1097-0142(20000601)88:11<2520::aid-cncr13>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Byrne JP, Mathers JM, Parry JM, Attwood SE, Bancewicz J, Woodman CB. Site distribution of oesophagogastric cancer. J Clin Pathol. 2002;55:191–194. doi: 10.1136/jcp.55.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bektas N, Donner A, Wirtz C, Heep H, Gabbert HE, Sarbia M. Allelic loss involving the tumor suppressor genes APC and MCC and expression of the APC protein in the development of dysplasia and carcinoma in Barrett esophagus. Am J Clin Pathol. 2000;114:890–895. doi: 10.1309/L1Q3-E3AQ-APU9-NA0A. [DOI] [PubMed] [Google Scholar]
- 11.Huang GS, Yang SM, Hong MY, Yang PC, Liu YC. Differential gene expression of livers from ApoE deficient mice. Life Sci. 2000;68:19–28. doi: 10.1016/s0024-3205(00)00912-7. [DOI] [PubMed] [Google Scholar]
- 12.Krepela E, Procházka J, Kárová B, Cermák J, Roubková H. Cysteine proteases and cysteine protease inhibitors in non-small cell lung cancer. Neoplasma. 1998;45:318–331. [PubMed] [Google Scholar]
- 13.Prieto I, Hermoso F, Gasparo M, Vargas F, Alba F, Segarra AB, Banegas I, Ramírez M. Angiotensinase activities in the kidney of renovascular hypertensive rats. Peptides. 2003;24:755–760. doi: 10.1016/s0196-9781(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez S, Alarcón de la Lastra C, Ortiz P, Motilva V, Martín MJ. Gastrointestinal tolerability of metamizol, acetaminophen, and diclofenac in subchronic treatment in rats. Dig Dis Sci. 2002;47:2791–2798. doi: 10.1023/a:1021077810548. [DOI] [PubMed] [Google Scholar]
- 15.Marden NY, Fiala-Beer E, Xiang SH, Murray M. Role of activator protein-1 in the down-regulation of the human CYP2J2 gene in hypoxia. Biochem J. 2003;373:669–680. doi: 10.1042/BJ20021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishio T, Goto S, Tahara K, Tone S, Kawano K, Kitano S. Immunoactivative role of indoleamine 2,3-dioxygenase in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:319–326. doi: 10.1111/j.1440-1746.2003.03259.x. [DOI] [PubMed] [Google Scholar]
- 17.El-Nady GM, Ling R, Harrison TJ. Gene expression in HCV-associated hepatocellular carcinoma-upregulation of a gene encoding a protein related to the ubiquitin-conjugating enzyme. Liver Int. 2003;23:329–337. doi: 10.1034/j.1478-3231.2003.00862.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Li H, Underwood T, Lloyd M, David M, Sperl G, Pamukcu R, Thompson WJ. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J Pharmacol Exp Ther. 2001;299:583–592. [PubMed] [Google Scholar]
- 19.Miyata Y, Kanda S, Nomata K, Hayashida Y, Kanetake H. Expression of metalloproteinase-2, metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in transitional cell carcinoma of upper urinary tract: correlation with tumor stage and survival. Urology. 2004;63:602–608. doi: 10.1016/j.urology.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Edwards DR. TIMP-3 and endocrine therapy of breast cancer: an apoptosis connection emerges. J Pathol. 2004;202:391–394. doi: 10.1002/path.1548. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu K, Abe M, Tsukada K. Deficiency of methionine synthesis enzyme activity in ascites tumor cells. Biochem Int. 1990;20:1105–1110. [PubMed] [Google Scholar]
- 22.Rubio CA. Colorectal adenomas produce lysozyme. Anticancer Res. 2003;23:5165–5171. [PubMed] [Google Scholar]
- 23.Bomstein Y, Fishelson Z. Enhanced sensitivity of P-glycoprotein-positive multidrug resistant tumor cells to complement-mediated lysis. Eur J Immunol. 1997;27:2204–2211. doi: 10.1002/eji.1830270913. [DOI] [PubMed] [Google Scholar]
- 24.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 25.Adegoke OJ, Shu XO, Gao YT, Cai Q, Breyer J, Smith J, Zheng W. Genetic polymorphisms in uridine diphospho-glucuronosyltransferase 1A1 (UGT1A1) and risk of breast cancer. Breast Cancer Res Treat. 2004;85:239–245. doi: 10.1023/B:BREA.0000025419.26423.b8. [DOI] [PubMed] [Google Scholar]
- 26.Sujatha V, Sachdanandam P. Recuperative effect of Semecarpus anacardium linn. nut milk extract on carbohydrate metabolizing enzymes in experimental mammary carcinoma-bearing rats. Phytother Res. 2002;16 Suppl 1:S14–S18. doi: 10.1002/ptr.777. [DOI] [PubMed] [Google Scholar]
- 27.Brtko J, Thalhamer J. Renaissance of the biologically active vitamin A derivatives: established and novel directed therapies for cancer and chemoprevention. Curr Pharm Des. 2003;9:2067–2077. doi: 10.2174/1381612033454144. [DOI] [PubMed] [Google Scholar]
- 28.Haugen BR, Larson LL, Pugazhenthi U, Hays WR, Klopper JP, Kramer CA, Sharma V. Retinoic acid and retinoid X receptors are differentially expressed in thyroid cancer and thyroid carcinoma cell lines and predict response to treatment with retinoids. J Clin Endocrinol Metab. 2004;89:272–280. doi: 10.1210/jc.2003-030770. [DOI] [PubMed] [Google Scholar]
- 29.Maki T, Hosokawa M, Satoh T, Sato K. Changes in carboxylesterase isoenzymes of rat liver microsomes during hepatocarcinogenesis. Jpn J Cancer Res. 1991;82:800–806. doi: 10.1111/j.1349-7006.1991.tb02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perabo F, Sharma S, Gierer R, Wirger A, Fimmers R, Steiner G, Adam M, Schultze-Seemann W. Circulating intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin in urological malignancies. Indian J Cancer. 2001;38:1–7. [PubMed] [Google Scholar]
- 31.Lee YY, Kim HG, Jung HI, Shin YH, Hong SM, Park EH, Sa JH, Lim CJ. Activities of antioxidant and redox enzymes in human normal hepatic and hepatoma cell lines. Mol Cells. 2002;14:305–311. [PubMed] [Google Scholar]
- 32.Subbaiah PV, Liu M, Witt TR. Impaired cholesterol esterification in the plasma in patients with breast cancer. Lipids. 1997;32:157–162. doi: 10.1007/s11745-997-0020-5. [DOI] [PubMed] [Google Scholar]
- 33.Xu K, Wang X, Xue W, Wang X, Hou S. Expressions of MAD2 and p55CDC in prostate cancer and their correlations with the prostate cancer grading. Beijing DaXue XueBao. 2003;35:586–590. [PubMed] [Google Scholar]
- 34.Tang MK, Kindler PM, Cai DQ, Chow PH, Li M, Lee KK. Heart-type fatty acid binding proteins are upregulated during terminal differentiation of mouse cardiomyocytes, as revealed by proteomic analysis. Cell Tissue Res. 2004;316:339–347. doi: 10.1007/s00441-004-0881-y. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Jovanovic B, Pins M, Lee C, Bergan RC. Over expression of endoglin in human prostate cancer suppresses cell detachment, migration and invasion. Oncogene. 2002;21:8272–8281. doi: 10.1038/sj.onc.1206117. [DOI] [PubMed] [Google Scholar]
- 36.Mathieu S, Prorok M, Benoliel AM, Uch R, Langlet C, Bongrand P, Gerolami R, El-Battari A. Transgene expression of alpha(1,2)-fucosyltransferase-I (FUT1) in tumor cells selectively inhibits sialyl-Lewis x expression and binding to E-selectin without affecting synthesis of sialyl-Lewis a or binding to P-selectin. Am J Pathol. 2004;164:371–383. doi: 10.1016/s0002-9440(10)63127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


