Abstract
AIM: To evaluate the gastro-protective effect of capsaicin against the ethanol- and indomethacin (IND)-induced gastric mucosal damage in healthy human subjects.
METHODS: The effects of small doses (1-8 μg/mL, 100 mL) of capsaicin on the gastric acid secretion basal acid output (BAO) and its electrolyte concentration, gastric transmucosal potential difference (GTPD), ethanol- (5 mL 300 mL/L i.g.) and IND- (3×25 mg/d) induced gastric mucosal damage were tested in a randomized, prospective study of 84 healthy human subjects. The possible role of desensitization of capsaicin-sensitive afferents was tested by repeated exposures and during a prolonged treatment.
RESULTS: Intragastric application of capsaicin decreased the BAO and enhanced “non-parietal” component (GTPD) in a dose-dependent manner. The decrease of GTPD evoked by ethanol was inhibited by the capsaicin application, which was reproducible. Gastric microbleeding induced by IND was inhibited by co-administration with capsaicin, but was not influenced by two weeks pretreatment with a daily capsaicin dose of 3×400 μg i.g.
CONCLUSION: Capsaicin in low concentration range protects against gastric injuries induced by ethanol or IND, which is attributed to stimulation of the sensory nerve endings.
Keywords: Capsaicin, Ethanol, Indomethacin, Gastric transmucosal potential difference, Gastric microbleeding, Gastroprotection, Healthy human subjects
INTRODUCTION
Spicy or hot foods are traditionally considered as dietary factors implicated in the causation of peptic ulcer[1,2]. Early and more recent studies[3-6] in human beings with pungent chilli powders and extracts have come to controversial conclusions about their injurious influence on gastric mucosa. Capsaicin is a hot topic in studies on gastro-protection[7-16]. In contrast, the effect of this pure ingredient of red peppers in defined concentrations on the human gastric mucosa seems not to be interesting for research. Capsaicin activates the TRPV1/VR1 capsaicin (vanilloid) receptor expressed by a subgroup of primary afferent nociceptive neurons. The TRPV1/VR1 receptor has been cloned[21] and turns out to be a cation channel. It is gated besides capsaicin and some vanilloids by low pH, noxious heat and various pain-producing endogenous and exogenous chemicals. Thus, these sensory nerve endings equipped with these ion channels are prone to be stimulated in gastric mucosa. The aim of the present study was to analyze the effect of capsaicin on mucosal injury induced by ethanol or the non-selective cyclooxygenase inhibitor, indomethacin (IND). Particular attention was paid to define whether the action of capsaicin was related to its acute stimulatory effect or to the sensory desensitization, a well known long lasting consequence of sensory stimulation in animal experiments[22,23].
MATERIALS AND METHODS
The observations were carried out in 84 healthy human subjects aged 25-65 years (40±10 years). The physical, laboratory, and iconographic examinations were normal and indicated normal.
The healthy persons were randomized into different groups to study the effect of intragastric application of capsaicin on their gastric mucosa and gastric acid secretion under different conditions.
The observations were carried out according to the good clinical practice. The studies were carried out from 1997 to 2002, which were permitted by the Regional Ethical Committee of Pécs, University of Pécs, Hungary. Written informed consent was obtained from all participants.
Identification of gastric basal acid output (BAO) without and with capsaicin
After an overnight fasting, a nasogastric tube was introduced at 8:00 a.m., and the total gastric content was completely suctioned.
Then the secreted gastric juice was suctioned every 15 min for 1 h (basal acid output, BAO). The healthy human subjects received intragastric capsaicin at the doses of 100, 200, 400, and 800 μg in 100 mL volume of saline solution. In the control group, 100 mL of saline solution was given through the nasogastric tube and the same doses of capsaicin. Gastric acid secretion was measured by titration of gastric juice with 0.1 N NaOH to pH 7 (pH titrimeter, Radelkis, Budapest, Hungary). Gastric acid outputs were calculated and expressed in mEq/h after capsaicin administration. The values effective inhibitory dose of capsaicin (ED50) was identified on the gastric BAO.
Chemical composition of gastric juice without and with capsaicin
The concentrations of Na+, K+, and Ca2+ in gastric juice were measured flamephotometrically. The concentration of Mg2+ was measured by atom absorption spectrometry, the chloride concentration by colorimetric method, the protein concentration by the method of biuret reaction.
The chloride linked to H+ and sodium was calculated for the determination of “parietal” (chloride linked to H+) and of “non-parietal” (liked to sodium) components of the gastric BAO[30].
Measurement of gastric transmucosal potential difference (GTPD)
GTPD was measured during endoscopy. The exploring mucosal electrode was passed through the biopsy force channel of gastroscope and the reference electrode was placed on the volar surface of the left forearm. The electrodes were connected to a digital voltmeter (Radelkis, Budapest, Hungary, OP 211/1). GTPD measurements were done at the greater curvature of the gastric body and the results were expressed in -mV (without and with intragastric application of different doses of capsaicin)[24-27]. Capsaicin in 5 mL saline solution was intragastrically applied and only saline solution was given to identify the baseline in GTPD.
The GTPD values were expressed in -mV, while -△PD max was calculated after intragastric application of capsaicin (n = 10).
Effect of capsaicin on ethanol-induced GTPD changes
The GTPD baseline was identified, and ethanol (5 mL 300 mL/L i.g.) was given intragastrically. The GTPD change was determined after the ethanol passed through the biopsy force channel of gastroscope without and with capsaicin (given in different doses in the same pathway after 1 min of ethanol administration) (n = 10).
Gastric microbleeding measurement during 1-d treatment with indomethacin (IND) and indomethacin plus capsaicin (n = 14)
Fourteen healthy human subjects were studied. They were randomly divided into different treatment groups.
Examinations were carried out before and after treatment after an overnight fasting. A plastic tube was inserted through his or her mouth until the intragastric end was 55 cm from the incisors. There were six openings in the intragastric part of the tube.
A test solution containing 100 mL of saline solution and 10 mL solution of concentrated phenol red (40 mg/100 mL), as a non-absorbable marker, was installed into the stomach without and with capsaicin (at doses of 200, 400, and 800 μg). The gastric content was recovered 10 min later[28-30]. The whole procedure was repeated thrice before and after the administration of IND (without and with application of capsaicin).
Gastric juice was aspirated. The strength of suction was adjusted to -50 mmHg.
The volume of each recovery sample was measured after homogenization for 10 min. Hemoglobin was determined as previously described[36,37]. The quantity of blood in the aspirated gastric samples was measured. Blue color developed (640 nm, pH 3.78, room temperature) and could be determined. Phenol red was measured spectroph-otometrically[29,30]. The values of gastric microbleeding were expressed as milliliter per day.
Chronic capsaicin (3×400 μg i.g./d) treatment for 2 wk
Ten healthy human subjects were treated with capsaicin (3×400 μg orally) for 2 wk. Capsaicin substance (400 μg) was put into a gelatin capsule containing 0.23 g lactose.
The extent of IND-induced gastric mucosal bleeding and gastric mucosal preventive effect of capsaicin (200, 400 μg) were tested before and after capsaicin treatment. Prospective randomized studies were done for three consecutive days before and after capsaicin treatment.
Chemicals
Capsaicin was from Sigma, Budapest, Hungary. IND was from Sanofi-Synthelabo, Budapest, Hungary. Capsaicin solution was made by dilution with distilled water.
Statistical analysis
The results were calculated as mean±SE. The unpaired or paired Student’s t-tests were used for the calculation of the results between the identical observations. P<0.05 was considered statistically significant.
RESULTS
The BAO decreased significantly and dose-dependently (Figure 1). The ED50 value of capsaicin was 400 μg.
Figure 1.
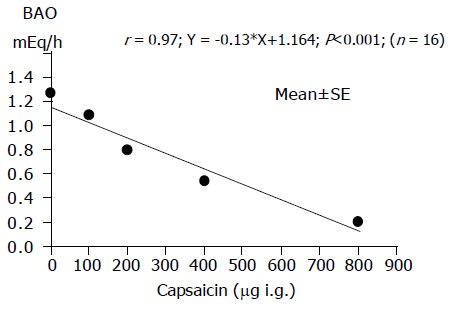
Inhibition of gastric acid output (BAO) by capsaicin in 16 healthy human subjects.
Electrolyte and albumin concentrations in gastric juice were measured in the healthy human subjects without and with capsaicin treatment. H+, K+, Ca2+, and Mg2+ significantly decreased (P<0.001), while Na+ and its protein content increased (P<0.001) after capsaicin treatment (Table 1).
Table 1.
Chemical composition of gastric juice without (A) and with (B) application of capsaicin in healthy human subjects (mEq/L or in g/L) (mean±SE)
| H+ | Na+ | K+ | Ca2+ | Mg2+ | “Parietal” | “Non-parietal” | Albumin (g/L) | ||||||||
| component | component | ||||||||||||||
|
A |
B |
A |
B |
A |
B |
A |
B |
A |
B |
A |
B |
A |
B |
A |
B |
| 43±3 | 25±1 | 73±4 | 89±2 | 13±1 | 8±0.6 | 0.98±0.02 | 0.88±0.01 | 0.49±0.01 | 0.38±0.01 | 43±3 | 25±2 | 126±4 | 145±4 | 1.24±0.001 | 1 630 002 |
| P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | ||||||||
| 100±7 | 58±2 | 100±5 | 122±3 | 100±8 | 62±5 | 100±2 | 90±1 | 100±2 | 78±2 | 100±7 | 58±5 | 100±3 | 115±3 | 100±1 | 131±2 |
The “parietal” and “non-parietal” components of BAO were calculated by taking the H+ output equivalent to Na+ and Cl- as gastric H+, Na+, and chloride in gastric juice without and with capsaicin treatment. The parietal component decreased (-△18 mmol/L) while the non-parietal component increased (+△19 mmol/L) in the BAO after capsaicin application (Figure 2).
Figure 2.
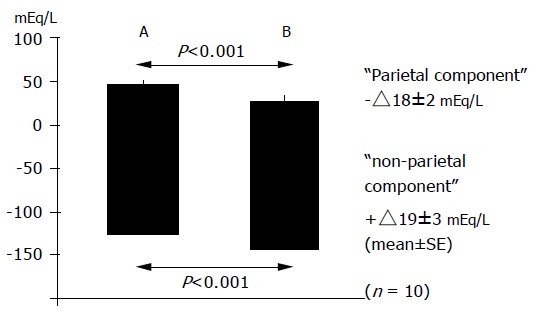
“Parietal” and “non-parietal” components before (A) and after (B) intragastric application of capsaicin in 10 healthy human subjects.
Capsaicin increased the GTPD in a dose-dependent manner. The peak values reached within 3-5 min after capsaicin application (Figure 3A).
Figure 3.
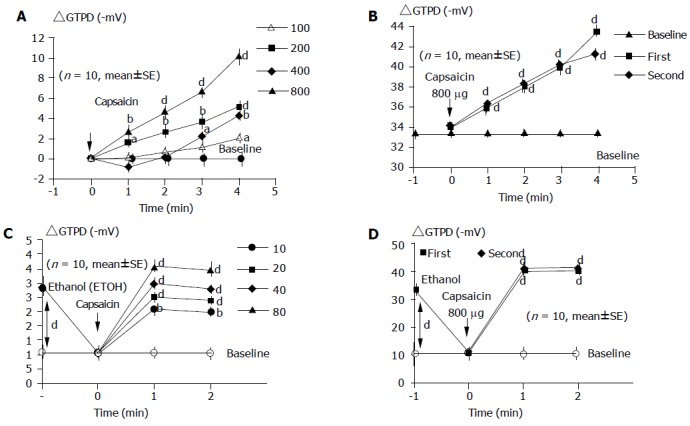
Dose-dependent gastric mucosal protective effect of capsaicin. A: Dose-response curve for capsaicin-induced changes in GTPD; B: effect of repeated capsaicin application on GTPD applied with 5-min time intervals; C: dose-response curves for capsaicin-induced gastric mucosal prevention on ethanol-produced decrease in GTPD; D: effects of repeated capsaicin application on ethanol-induced GTPD changes. aP<0.05, bP<0.01, dP<0.01 vs others.
After intragastric application of ethanol (300 mL/L), the GTPD dropped from -33.4±2.7 to -10.5±2.4 mV (P<0.001) within 3 min (Figure 3C).
Capsaicin application (at doses of 400 and 800 μg) significantly prevented the ethanol-induced decrease in GTPD (Figure 3C).
The protective effect of capsaicin was reproducible before (Figure 3B) and after (Figure 3D) ethanol administration.
The gastric microbleeding was measured before and after IND (3×25 mg orally) application alone and in combination with 200, 400, and 800 μg capsaicin in 14 healthy human volunteers. The gastric microbleeding induced by IND increased (8; 25±0.5 mL/d from the basic level of 2.1±0.1; P<0.001) which was dose-dependently prevented by capsaicin at the dose of 200-800 μg (Y = 0.0071X+7.78; r = -0.98; P<0.001, Figure 4A).
Figure 4.
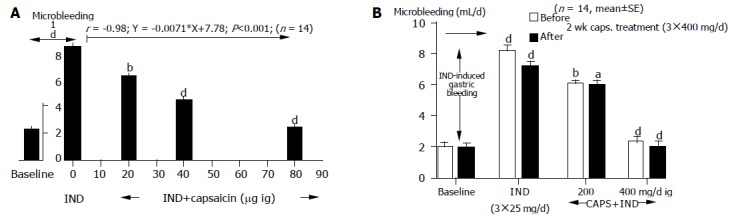
Gastric mucosal protection produced by capsaicin on IND-induced gastric mucosal damage before (A) and after (B) capsaicin treatment. aP<0.05, bP<0.01, dP<0.01 vs others.
In order to decide the potential role of desensitizing in the gastric protective effect of capsaicin, a daily dose of 3×400 μg capsaicin was applied for 2 wk in 14 healthy human subjects. Capsaicin protected gastric mucosal against IND-induced gastric microbleeding. There was no difference between the pretreated group and the central group (Figure 4B).
DISCUSSION
The present study provides evidence for the powerful gastroprotective potency of capsaicin. The threshold concentration of capsaicin in producing definite hot sensation is around 1-2 μg/mL and the capsaicinoid level in chilli sauce varies from 25 μg/mL to 0.5 mg/mL[22,24]. Therefore, the observed defensive responses using capsaicin have a clear dietary relevance.
Chronic peptic ulcer patients are warned to avoid spicy foods, although in the era of H2-inhibitors this praxis is far less restrictive[2]. Nevertheless contradictory observations cannot decide whether spicy foods are harmful to or beneficial for gastric injury. In healthy subjects mucosal microbleeding with exfoliation and aggravation of aspirin-induced gastric bleeding are observed in response to chilli powder or red pepper “preparations”[3,4]. On the other hand, ingestion of “highly spiced” meals or chilli by normal individuals does not cause endoscopically gastric or duodenal mucosal damage[25] although gastric acid and pepsin secretion increases[31-33]. In other studies, red pepper sauce induces prolongation of gastric emptying[7] and chilli powder evokes protective effect against aspirin-induced gastric mucosal injury[6]. Improvement in dyspeptic symptoms of patients with and without gastro-esophageal reflux disease and irritable bowel syndrome after intake of red pepper powder in gelatin capsules has been reported[8,9]. In the latter case, the improvement of functional dyspepsia is attributed to desensitization of gastric nociceptive C-fibers induced by capsaicin although this conclusion is not supported by experimental evidence. In our earlier and present studies, pure capsaicin solution was injected into the stomach (1-8 μg/mL in 100 mL) of healthy subjects which inhibits the H+ output and total secreted volume of gastric juice for about 1 h in a dose-dependent manner and increases its “non-parietal” component, gastric emptying[23].
The important role of capsaicin-sensitive peptidergic sensory fibers in maintaining of gastric mucosal integrity against injurious interventions has been well established in rats[10-13,15]. Capsaicin-sensitive primary afferent neurons and their nerve endings express the TRPV1/VR1 vanilloid receptor[14,21,23]. Stimulation of these chemoreceptive nerve terminals by H+ or bradykinin, etc. is accompanied with release of CGRP, tachykinins, somatostatin, and NO from them. The arterial wall in stomach receives dense supply of these peptidergic fibers and capsaicin elicits neurogenic vasodilatation with enhanced mucosal blood flow. Clear evidence indicates that hyperemia is induced by sensory neuropeptides of CGRP neurokinin A with NO as well as somatostatin are involved in the sensory neuron-mediated gastroprotection[17-20]. Opposite effect has been observed in rats desensitized by capsaicin. Functional blockade of gastric sensory nerve endings elicited by systemic or intragastric capsaicin application results in impaired mucosal protection against various ulcer-provoking agents[10-14]. Repeated topical or systemic capsaicin application elicits reproducible effects at low concentrations and induces decreasing responses (desensitization) at high concentrations. In the rat’s eye, 10 μg/mL concentration of capsaicin does not induce any desensitization[22]. On the human oral mucosa, this concentration of capsaicin induces some diminished sensation of irritation but only for a short time[28].
In the present study, the following evidence suggests that capsaicin-induced gastric mucosal protection against the injurious effects of ethanol or IND is due to the acute stimulatory effect of the compound. Microbleeding detected after IND administration does not differ from that in the controls. Enhanced GTPD evoked by the first and second capsaicin exposure in 1 h shows no sign of decrement. Counteraction of the ethanol-induced drop of gastric transmucosal potential is identical after the first and second capsaicin application.
The enhancement of gastric transmucosal potential is probably related to the mucosal hyperemia[32,34]. This response plays a significant role in capsaicin-induced gastroprotection. Furthermore, the present and earlier[27] findings reveal that intragastric administration of capsaicin in low concentration inhibits but does not increase[4,5,26] the acid output of stomach and enhances the gastric emptying rate in healthy human subjects[29]. All these responses can be attributed to CGRP and NO released from the activated capsaicin-sensitive, TRPV1/VR1 expressing sensory nerve terminals. The present findings suggest that patients taking anti-inflammatory-analgesic agents regularly can decrease the incidence and severity of gastric ulceration, if they have moderate spicy foods[35-37].
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Anonymus Diet and peptic ulcer. Lancet. 1987;2:80–81. [PubMed] [Google Scholar]
- 2.Marotta RB, Floch MH. Diet and nutrition in ulcer disease. Med Clin North Am. 1991;75:967–979. doi: 10.1016/s0025-7125(16)30424-2. [DOI] [PubMed] [Google Scholar]
- 3.Desai HG, Venugopalan K, Antia FP. Effect of red chilli powder on DNA content of gastric aspirates. Gut. 1973;14:974–976. doi: 10.1136/gut.14.12.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers BM, Smith JL, Graham DY. Effect of red pepper and black pepper on the stomach. Am J Gastroenterol. 1987;82:211–214. [PubMed] [Google Scholar]
- 5.Graham DY, Smith JL, Opekun AR. Spicy food and the stomach. Evaluation by videoendoscopy. JAMA. 1988;260:3473–3475. [PubMed] [Google Scholar]
- 6.Yeoh KG, Kang JY, Yap I, Guan R, Tan CC, Wee A, Teng CH. Chili protects against aspirin-induced gastroduodenal mucosal injury in humans. Dig Dis Sci. 1995;40:580–583. doi: 10.1007/BF02064374. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez R, Dunkel R, Koletzko B, Schusdziarra V, Allescher HD. Effect of capsaicin-containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers. Dig Dis Sci. 1998;43:1165–1171. doi: 10.1023/a:1018831018566. [DOI] [PubMed] [Google Scholar]
- 8.Bortolotti M, Coccia G, Grossi G. Red pepper and functional dyspepsia. N Engl J Med. 2002;346:947–948. doi: 10.1056/NEJM200203213461219. [DOI] [PubMed] [Google Scholar]
- 9.Bortolotti M, Coccia G, Grossi G, Miglioli M. The treatment of functional dyspepsia with red pepper. Aliment Pharmacol Ther. 2002;16:1075–1082. doi: 10.1046/j.1365-2036.2002.01280.x. [DOI] [PubMed] [Google Scholar]
- 10.Szolcsányi J, Barth L. Impaired defense mechanism to peptic ulcer in the capsaicin-desensitized rat. In: Mózsik Gy, Hanninen O, Jávor T, eds , editors. Advances in Physiological Science. Vol. 29. Gastrointestinal Defense Mechanisms; Oxford: Pergamon Press- Budapest Akadémiai Kiadó; 1981. pp. 39–51. [Google Scholar]
- 11.Szolcsányi J, Barthó L. Capsaicin-sensitive afferents and their role in gastroprotection: an update. J Physiol Paris. 2001;95:181–188. doi: 10.1016/s0928-4257(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 12.Mózsik GY, Abdel-Salam OME, Szolcsányi J. Capsaicin-sensitive afferent nerves in gastric mucosal damage and protection. Budapest Akadémiai Kiadó. 1997:1–125. [Google Scholar]
- 13.Mózsik GY, Vincze , Szolcsányi J. Four responses of capsaicin-sensitive primary afferent neurons to capsaicin and its analog. Gastric acid secretion, gastric mucosal damage and protection. J Gastroenterol Hepatol. 2001;16:1093–1097. doi: 10.1046/j.1440-1746.2001.02598.x. [DOI] [PubMed] [Google Scholar]
- 14.Szállási Á, Blumberg M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1991;51:159–211. [PubMed] [Google Scholar]
- 15.Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- 16.Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- 17.Inui T, Kinoshita Y, Yamaguchi A, Yamatani T, Chiba T. Linkage between capsaicin-stimulated calcitonin gene-related peptide and somatostatin release in rat stomach. Am J Physiol. 1991;261:G770–G774. doi: 10.1152/ajpgi.1991.261.5.G770. [DOI] [PubMed] [Google Scholar]
- 18.Li DS, Raybould HE, Quintero E, Guth PH. Role of calcitonin gene-related peptide in gastric hyperemic response to intragastric capsaicin. Am J Physiol. 1991;261:G657–G661. doi: 10.1152/ajpgi.1991.261.4.G657. [DOI] [PubMed] [Google Scholar]
- 19.Kang JY, Teng CH, Wee A, Chen FC. Effect of capsaicin and chilli on ethanol induced gastric mucosal injury in the rat. Gut. 1995;36:664–669. doi: 10.1136/gut.36.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen RY, Guth PH. Interaction of endogenous nitric oxide and CGRP in sensory neuron-induced gastric vasodilation. Am J Physiol. 1995;268:G791–G796. doi: 10.1152/ajpgi.1995.268.5.G791. [DOI] [PubMed] [Google Scholar]
- 21.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 22.Szolcsányi J. Capsaicin type purgent agents producing pyrexia In: Milton ed. Handbook of Experimental Pharmacology. Pyretics and Antipyretcs Vol. 60. Berlin Springer-Verlag. 1982:437–478. [Google Scholar]
- 23.Hollander F. The component of the gastric secretion. Am J Dig Dis Sci. 1934;1:319–329. [Google Scholar]
- 24.Andersson S, Grossman MI. Profile of pH, pressure, and potential difference at gastroduodenal junction in man. Gastroenterology. 1965;49:364–371. [PubMed] [Google Scholar]
- 25.Hossenbocus A, Fitzpatrick P, Colin-Jones DG. Proceedings: Measurement of gastric mucosal potential difference at endoscopy. Gut. 1975;16:410. [PubMed] [Google Scholar]
- 26.Rácz I. Transmucosal potential diference. In: Cheli R, ed , editors. Gastric protection. New York: Raven Press; 1988. pp. 65–86. [Google Scholar]
- 27.Rácz I. Transmucosal potential difernce. In: Parodi MC, Iaquinto G, Cheli R, eds , editors. Gastroprotection Today. Verona: Cortina International; 1995. pp. 97–108. [Google Scholar]
- 28.Hunt JN, Knox MT. The regulation of gastric emptying of meals containing citric acid and salts of citric acid. J Physiol. 1962;163:34–45. doi: 10.1113/jphysiol.1962.sp006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher MA, Hunt JN. A sensitive method for measuring haemoglobin in gastric contents. Digestion. 1976;14:409–414. doi: 10.1159/000197964. [DOI] [PubMed] [Google Scholar]
- 30.Nagy L, Mózsik G, Feledi E, Ruzsa C, Vezekényi Z, Jávor T. Gastric microbleeding measurements during one day treatment with indomethacin and indomethacin plus sodium salicylate (1 : 10) in patients. Acta Physiol Hung. 1984;64:373–377. [PubMed] [Google Scholar]
- 31.Edwards SJ, Colquhoun EQ, Clark MG. Levels of pungent principles in chilli sauces and capsicum fruit in Australia. Food Australia. 1990;42:432–435. [Google Scholar]
- 32.Kang JY, Yap I, Guan P, Lim TC. Chilli ingestion does not lead to macroscopic gastroduodenal mucosal damage in healthy subjects. J Gastroenterol Hepatol. 1988;3:573–576. [Google Scholar]
- 33.Solanke TF. The effect of red pepper (Capsicum frutescens) on gastric acid secretion. J Surg Res. 1973;15:385–390. doi: 10.1016/0022-4804(73)90108-x. [DOI] [PubMed] [Google Scholar]
- 34.Debreceni A, Abdel-Salam OM, Figler M, Juricskay I, Szolcsanyi J, Mozsik G. Capsaicin increase gastric emptying rate in healthy human subjects measured by 13C-labeled octanoid acid breath test. J Physiol Paris. 1999;93:455–460. doi: 10.1016/s0928-4257(99)00114-x. [DOI] [PubMed] [Google Scholar]
- 35.Szolcsányi J. Capsaicin receptors as target molecules on nociceptors for development of analgesic agents. In: Kéri Gy, Tóth I., editors. eds. Molecular Pathomechanisms and New Trends in Drug Research. London, New York: Taylor and Francis; 2002. pp. 319–333. [Google Scholar]
- 36.Green BG. Rapid recovery from capsaicin desensitization during recurrent stimulation. Pain. 1996;68:245–253. doi: 10.1016/s0304-3959(96)03211-3. [DOI] [PubMed] [Google Scholar]
- 37.Mózsik GY, Debreceni A, Abdel-Salam OME, Szab I, Figler M, Ludány A, Juricskay I, Szolcsányi J. Small doses of capsaicin given intragastrically inhibit gastric basal acid secretion in healthy human subjects. J Physiol Paris. 1999;93:433–436. doi: 10.1016/s0928-4257(99)00117-5. [DOI] [PubMed] [Google Scholar]


