Abstract
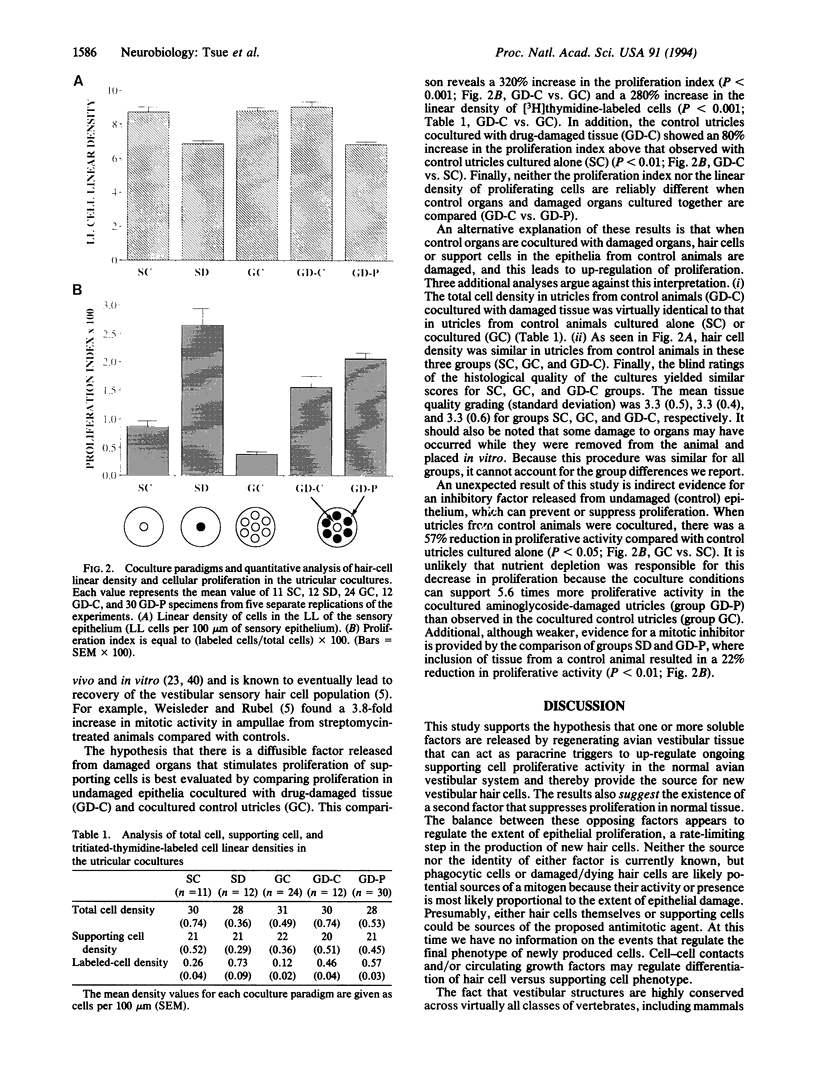
Damage to the avian inner ear results in up-regulation of mitotic activity resulting in regeneration of hair cells. The objective of this investigation was to determine whether the damaged inner ear epithelium releases a soluble mitogen that is responsible for the up-regulation of proliferation. The sensory epithelium from normal and drug-damaged avian inner ears was cultured alone or in the presence of other cultures. As previously shown in vivo and in vitro, damaged organs displayed increased supporting cell proliferation compared with undamaged organs, leading to eventual morphologic and functional recovery. When damaged organs were cocultured with an undamaged organ, proliferation was increased in the undamaged tissue. When undamaged organs were cultured together, proliferation was decreased. These results indicate that a soluble factor released from the damaged inner ear epithelium stimulates proliferation and suggest the release of a factor from normal tissue that suppressed mitotic activity. Thus, reparative hair cell regeneration in the inner ear appears to be regulated by a balance between proliferative and antiproliferative paracrine factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corwin J. T., Cotanche D. A. Regeneration of sensory hair cells after acoustic trauma. Science. 1988 Jun 24;240(4860):1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Corwin J. T., Jones J. E., Katayama A., Kelley M. W., Warchol M. E. Hair cell regeneration: the identities of progenitor cells, potential triggers and instructive cues. Ciba Found Symp. 1991;160:103–130. doi: 10.1002/9780470514122.ch6. [DOI] [PubMed] [Google Scholar]
- Corwin J. T., Warchol M. E. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci. 1991;14:301–333. doi: 10.1146/annurev.ne.14.030191.001505. [DOI] [PubMed] [Google Scholar]
- Cotanche D. A., Corwin J. T. Stereociliary bundles reorient during hair cell development and regeneration in the chick cochlea. Hear Res. 1991 Apr;52(2):379–402. doi: 10.1016/0378-5955(91)90027-7. [DOI] [PubMed] [Google Scholar]
- Cotanche D. A. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30(2-3):181–195. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cruz R. M., Lambert P. R., Rubel E. W. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987 Oct;113(10):1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Duckert L. G., Rubel E. W. Morphological correlates of functional recovery in the chicken inner ear after gentamycin treatment. J Comp Neurol. 1993 May 1;331(1):75–96. doi: 10.1002/cne.903310105. [DOI] [PubMed] [Google Scholar]
- Duckert L. G., Rubel E. W. Ultrastructural observations on regenerating hair cells in the chick basilar papilla. Hear Res. 1990 Sep;48(1-2):161–182. doi: 10.1016/0378-5955(90)90206-5. [DOI] [PubMed] [Google Scholar]
- Forge A., Li L., Corwin J. T., Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993 Mar 12;259(5101):1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Girod D. A., Duckert L. G., Rubel E. W. Possible precursors of regenerated hair cells in the avian cochlea following acoustic trauma. Hear Res. 1989 Nov;42(2-3):175–194. doi: 10.1016/0378-5955(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Girod D. A., Tucci D. L., Rubel E. W. Anatomical correlates of functional recovery in the avian inner ear following aminoglycoside ototoxicity. Laryngoscope. 1991 Nov;101(11):1139–1149. doi: 10.1288/00005537-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Hashino E., Sokabe M. Kanamycin induced low-frequency hearing loss in the budgerigar (Melopsittacus undulatus). J Acoust Soc Am. 1989 Jan;85(1):289–294. doi: 10.1121/1.397736. [DOI] [PubMed] [Google Scholar]
- Hashino E., Tanaka Y., Salvi R. J., Sokabe M. Hair cell regeneration in the adult budgerigar after kanamycin ototoxicity. Hear Res. 1992 Apr;59(1):46–58. doi: 10.1016/0378-5955(92)90101-r. [DOI] [PubMed] [Google Scholar]
- Hashino E., Tanaka Y., Sokabe M. Hair cell damage and recovery following chronic application of kanamycin in the chick cochlea. Hear Res. 1991 Apr;52(2):356–368. doi: 10.1016/0378-5955(91)90025-5. [DOI] [PubMed] [Google Scholar]
- Henry W. J., Makaretz M., Saunders J. C., Schneider M. E., Vrettakos P. Hair cell loss and regeneration after exposure to intense sound in neonatal chicks. Otolaryngol Head Neck Surg. 1988 Jun;98(6):607–611. doi: 10.1177/019459988809800613. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Nelson R. C. Recovery of vestibular function following hair cell destruction by streptomycin. Hear Res. 1992 Oct;62(2):181–186. doi: 10.1016/0378-5955(92)90184-o. [DOI] [PubMed] [Google Scholar]
- Jørgensen J. M., Mathiesen C. The avian inner ear. Continuous production of hair cells in vestibular sensory organs, but not in the auditory papilla. Naturwissenschaften. 1988 Jun;75(6):319–320. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., MEYER H., HAMBURGER V. In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res. 1954 Jan;14(1):49–57. [PubMed] [Google Scholar]
- Lippe W. R., Westbrook E. W., Ryals B. M. Hair cell regeneration in the chicken cochlea following aminoglycoside toxicity. Hear Res. 1991 Nov;56(1-2):203–210. doi: 10.1016/0378-5955(91)90171-5. [DOI] [PubMed] [Google Scholar]
- Marsh R. R., Xu L. R., Moy J. P., Saunders J. C. Recovery of the basilar papilla following intense sound exposure in the chick. Hear Res. 1990 Jul;46(3):229–237. doi: 10.1016/0378-5955(90)90004-9. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nawa H., Patterson P. H. Separation and partial characterization of neuropeptide-inducing factors in heart cell conditioned medium. Neuron. 1990 Feb;4(2):269–277. doi: 10.1016/0896-6273(90)90101-k. [DOI] [PubMed] [Google Scholar]
- O'Malley E. K., Sieber B. A., Black I. B., Dreyfus C. F. Mesencephalic type I astrocytes mediate the survival of substantia nigra dopaminergic neurons in culture. Brain Res. 1992 Jun 5;582(1):65–70. doi: 10.1016/0006-8993(92)90317-3. [DOI] [PubMed] [Google Scholar]
- Oesterle E. C., Tsue T. T., Reh T. A., Rubel E. W. Hair-cell regeneration in organ cultures of the postnatal chicken inner ear. Hear Res. 1993 Oct;70(1):85–108. doi: 10.1016/0378-5955(93)90054-5. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. I. Effects of conditioned medium. Dev Biol. 1977 Apr;56(2):263–280. doi: 10.1016/0012-1606(77)90269-x. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The influence of non-neuronal cells on catecholamine and acetylcholine synthesis and accumulation in cultures of dissociated sympathetic neurons. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3607–3610. doi: 10.1073/pnas.71.9.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Ramprashad F., Landolt J. P., Money K. E., Laufer J. Comparative morphometric study of the vestibular system of the vertebrata: reptilia, aves, amphibia, and pisces. Acta Otolaryngol Suppl. 1986;427:1–42. [PubMed] [Google Scholar]
- Raphael Y. Evidence for supporting cell mitosis in response to acoustic trauma in the avian inner ear. J Neurocytol. 1992 Sep;21(9):663–671. doi: 10.1007/BF01191727. [DOI] [PubMed] [Google Scholar]
- Represa J. J., Miner C., Barbosa E., Giraldez F. Bombesin and other growth factors activate cell proliferation in chick embryo otic vesicles in culture. Development. 1988 May;103(1):87–96. doi: 10.1242/dev.103.1.87. [DOI] [PubMed] [Google Scholar]
- Represa J., Bernd P. Nerve growth factor and serum differentially regulate development of the embryonic otic vesicle and cochleovestibular ganglion in vitro. Dev Biol. 1989 Jul;134(1):21–29. doi: 10.1016/0012-1606(89)90074-2. [DOI] [PubMed] [Google Scholar]
- Represa J., León Y., Miner C., Giraldez F. The int-2 proto-oncogene is responsible for induction of the inner ear. Nature. 1991 Oct 10;353(6344):561–563. doi: 10.1038/353561a0. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals B. M., Rubel E. W. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988 Jun 24;240(4860):1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Ryals B. M., Westbrook E. W., Stoots S., Spencer R. F. Changes in the acoustic nerve after hair cell regeneration. Exp Neurol. 1992 Jan;115(1):18–22. doi: 10.1016/0014-4886(92)90214-b. [DOI] [PubMed] [Google Scholar]
- Tucci D. L., Rubel E. W. Physiologic status of regenerated hair cells in the avian inner ear following aminoglycoside ototoxicity. Otolaryngol Head Neck Surg. 1990 Sep;103(3):443–450. doi: 10.1177/019459989010300317. [DOI] [PubMed] [Google Scholar]
- Varela-Nieto I., Represa J., Avila M. A., Miner C., Mato J. M., Giraldez F. Inositol phospho-oligosaccharide stimulates cell proliferation in the early developing inner ear. Dev Biol. 1991 Feb;143(2):432–435. doi: 10.1016/0012-1606(91)90095-k. [DOI] [PubMed] [Google Scholar]
- Warchol M. E., Lambert P. R., Goldstein B. J., Forge A., Corwin J. T. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993 Mar 12;259(5101):1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Weisleder P., Rubel E. W. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol. 1993 May 1;331(1):97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]