Abstract
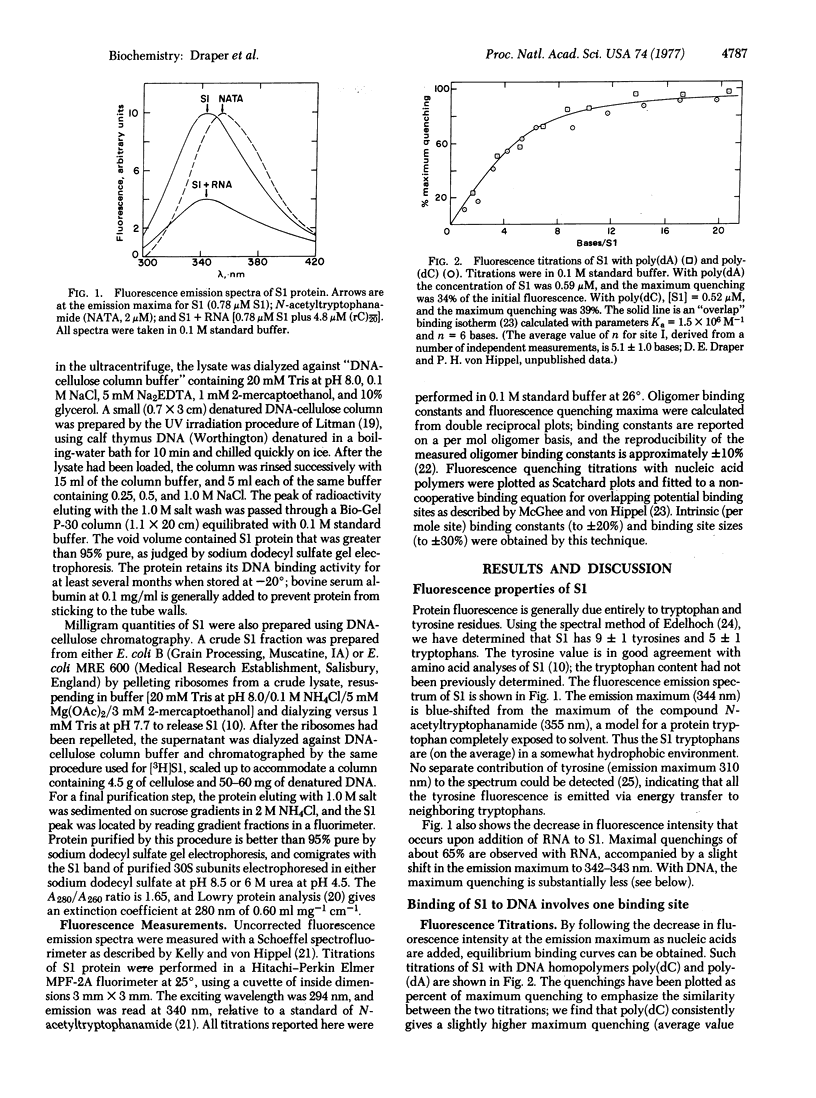
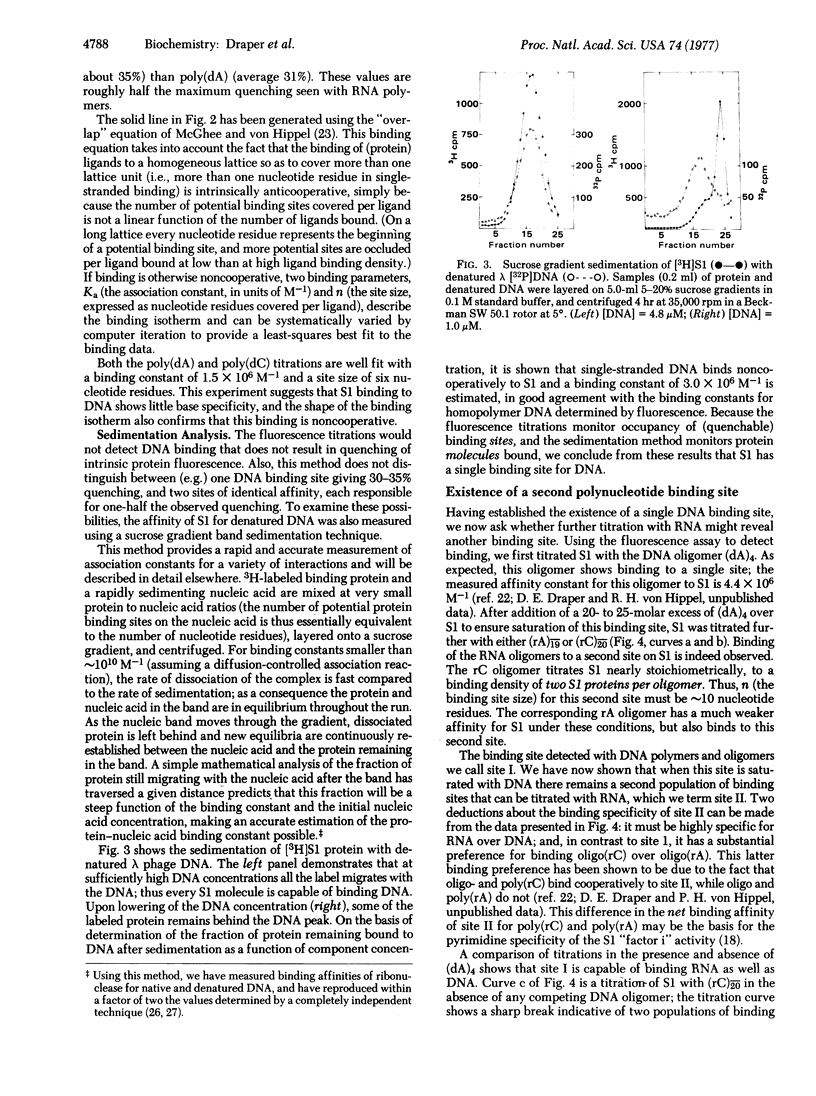
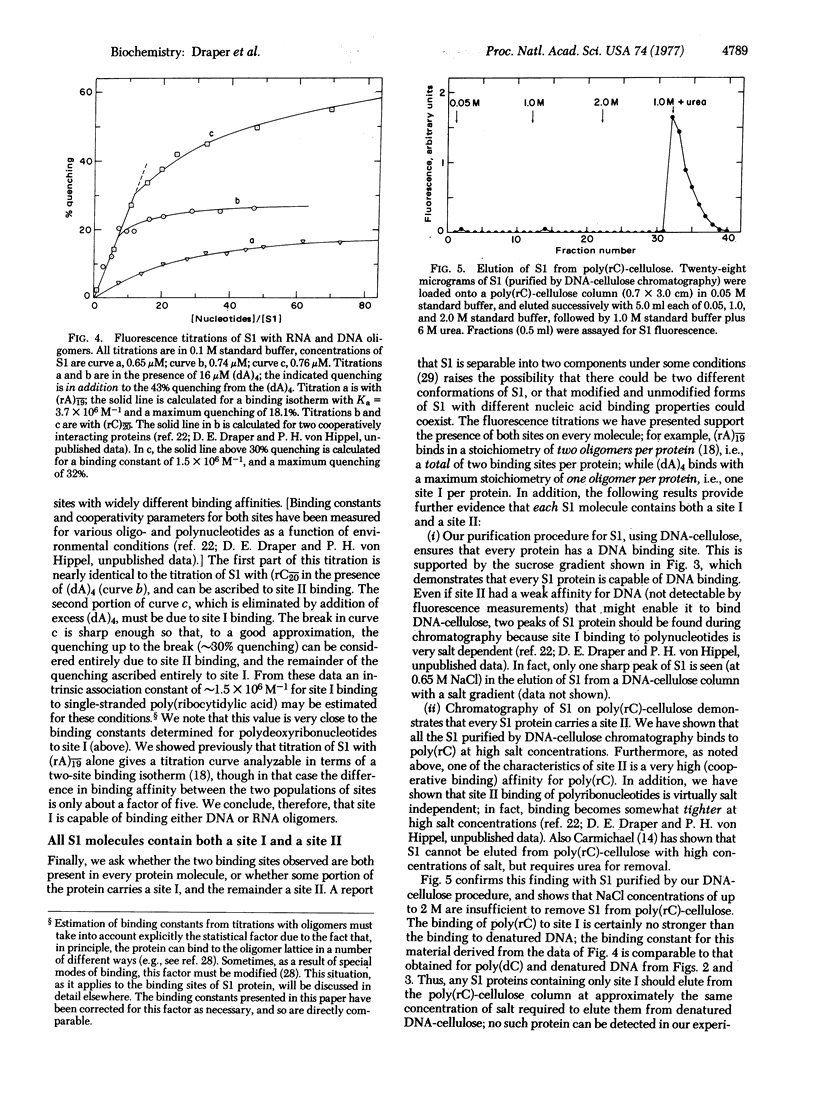
The interaction of Escherichia coli ribosomal protein S1 with a variety of RNA and DNA oligomers and polymers has been studied, using both a sedimentation technique and the quenching of intrinsic protein fluorescence upon nucleic acid binding to obtain equilibrium binding parameters. Two polynucleotide binding sites have been detected on S1: site I binds either single-stranded DNA or RNA and does not discriminate between adenine- and cytidine-containing polynucleotides, while the II binding is highly specific for RNA over DNA and shows a marked preference for cytidine polynucleotides over the corresponding adenine-containing species. On the basis of the binding properties of S1 to denatured DNA cellulose and poly(rC)-cellulose, it is demonstrated that every S1 molecule carries both a site I and a site II. Some possible implications of these results for mechanisms of protein synthesis and phage Qbeta replication are briefly considered.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear D. G., Ng R., Van Derveer D., Johnson N. P., Thomas G., Schleich T., Noller H. F. Alteration of polynucleotide secondary structure by ribosomal protein S1. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1824–1828. doi: 10.1073/pnas.73.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G. Isolation of bacterial and phage proteins by homopolymer RNA-cellulose chromatography. J Biol Chem. 1975 Aug 10;250(15):6160–6167. [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E. Two forms of the 30 S ribosomal subunit of Escherichia coli. J Biol Chem. 1974 Dec 10;249(23):7673–7678. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Eisinger J. Intramolecular energy transfer in adrenocorticotropin. Biochemistry. 1969 Oct;8(10):3902–3908. doi: 10.1021/bi00838a004. [DOI] [PubMed] [Google Scholar]
- Groner Y., Scheps R., Kamen R., Kolakofsky D., Revel M. Host subunit of Q replicase is translation control factor i. Nat New Biol. 1972 Sep 6;239(88):19–20. doi: 10.1038/newbio239019a0. [DOI] [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Host interference with viral gene expression: mode of action of bacterial factor i. J Mol Biol. 1974 Jan 15;82(2):193–212. doi: 10.1016/0022-2836(74)90341-6. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., von Hippel P. H. A boundary sedimentation velocity method for determining nonspecific nucleic acid-protein interaction binding parameters. Anal Biochem. 1977 May 15;80(1):267–281. doi: 10.1016/0003-2697(77)90645-5. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., von Hippel P. H. DNA "melting" proteins. I. Effects of bovine pancreatic ribonuclease binding on the conformation and stability of DNA. J Biol Chem. 1976 Nov 25;251(22):7198–7214. [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Kelly R. C., Jensen D. E., von Hippel P. H. DNA "melting" proteins. IV. Fluorescence measurements of binding parameters for bacteriophage T4 gene 32-protein to mono-, oligo-, and polynucleotides. J Biol Chem. 1976 Nov 25;251(22):7240–7250. [PubMed] [Google Scholar]
- Kelly R. C., von Hippel P. H. DNA "melting" proteins. III. Fluorescence "mapping" of the nucleic acid binding site of bacteriophage T4 gene 32-protein. J Biol Chem. 1976 Nov 25;251(22):7229–7239. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee-Huang S., Ochoa S. Specific inhibitors of MS2 and late T4 RNA translation in E. coli. Biochem Biophys Res Commun. 1972 Oct 17;49(2):371–376. doi: 10.1016/0006-291x(72)90420-2. [DOI] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Wahba A. J. Inhibition of synthetic and natural messenger translation. II. Specificity and mechanism of action of a protein isolated from Escherichia coli MRE 600 ribosomes. J Biol Chem. 1974 Jun 25;249(12):3808–3813. [PubMed] [Google Scholar]
- Revel M., Pollack Y., Groner Y., Scheps R., Inouye H., Berissi H., Zeller H. IF3-interference factors: protein factors in Escherichia coli controlling initiation of mRNA translation. Biochimie. 1973;55(1):41–51. doi: 10.1016/s0300-9084(73)80235-4. [DOI] [PubMed] [Google Scholar]
- Senear A. W., Steitz J. A. Site-specific interaction of Qbeta host factor and ribosomal protein S1 with Qbeta and R17 bacteriophage RNAs. J Biol Chem. 1976 Apr 10;251(7):1902–1912. [PubMed] [Google Scholar]
- Steitz J. A., Wahba A. J., Laughrea M., Moore P. B. Differential requirements for polypeptide chain initiation complex formation at the three bacteriophage R17 initiator regions. Nucleic Acids Res. 1977 Jan;4(1):1–15. doi: 10.1093/nar/4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Boublik M. Destabilization of the secondary structure of RNA by ribosomal protein S1 from Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):957–964. doi: 10.1016/0006-291x(76)90685-9. [DOI] [PubMed] [Google Scholar]
- Tal M., Aviram M., Kanarek A., Weiss A. Polyuridylic acid binding and translating by Escherichia coli ribosomes: stimulation by protein I, inhibition by aurintricarboxylic acid. Biochim Biophys Acta. 1972 Oct 27;281(3):381–392. doi: 10.1016/0005-2787(72)90452-2. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Koller T. Physical mapping of Qbeta replicase binding sites on Qbeta RNA. J Mol Biol. 1976 Mar 5;101(3):367–377. doi: 10.1016/0022-2836(76)90153-4. [DOI] [PubMed] [Google Scholar]
- Voynow P., Kurland C. G. Stoichiometry of the 30S ribosomal proteins of Escherichia coli. Biochemistry. 1971 Feb 2;10(3):517–524. doi: 10.1021/bi00779a026. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]
- van Dieijen G., van Knippenberg P. H., van Duin J. The specific role of ribosomal protein S1 in the recognition of native phage RNA. Eur J Biochem. 1976 May 1;64(2):511–518. doi: 10.1111/j.1432-1033.1976.tb10330.x. [DOI] [PubMed] [Google Scholar]
- van Duin J., van Knippenberg P. H. Functional heterogeneity of the 30 S ribosomal subunit of Escherichia coli. 3. Requirement of protein S1 for translation. J Mol Biol. 1974 Mar 25;84(1):185–195. doi: 10.1016/0022-2836(74)90221-6. [DOI] [PubMed] [Google Scholar]



