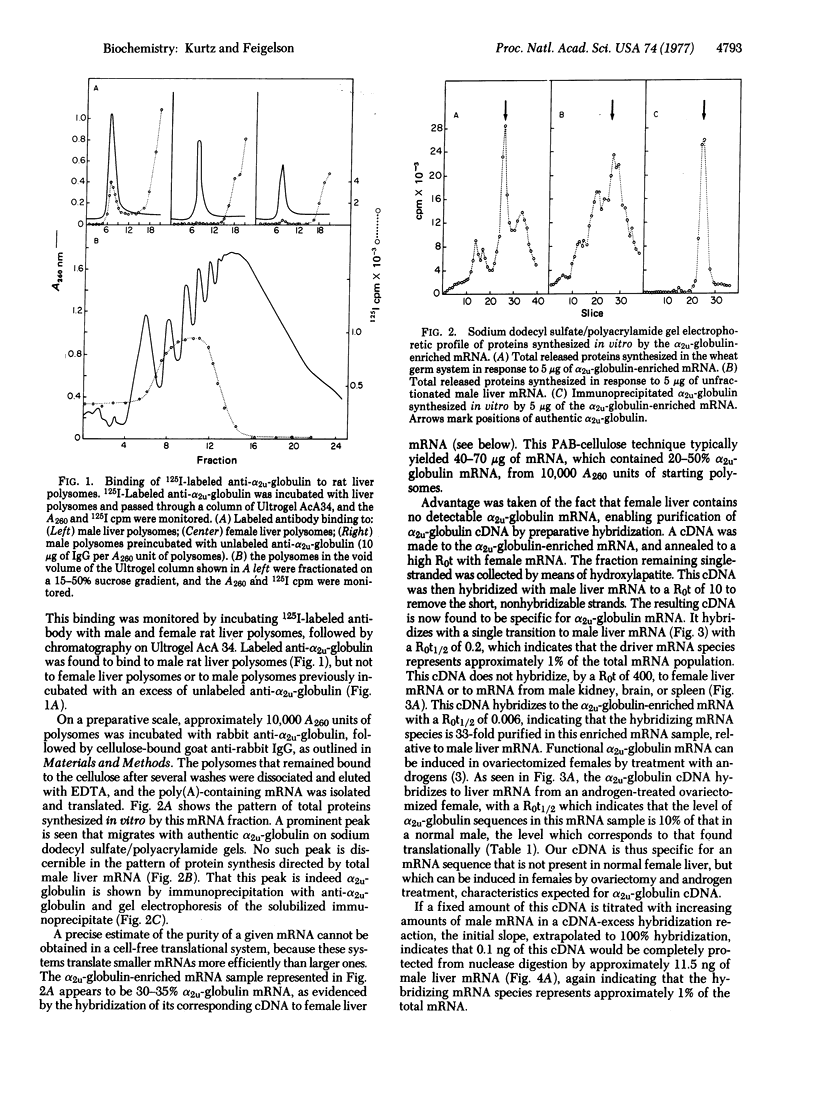
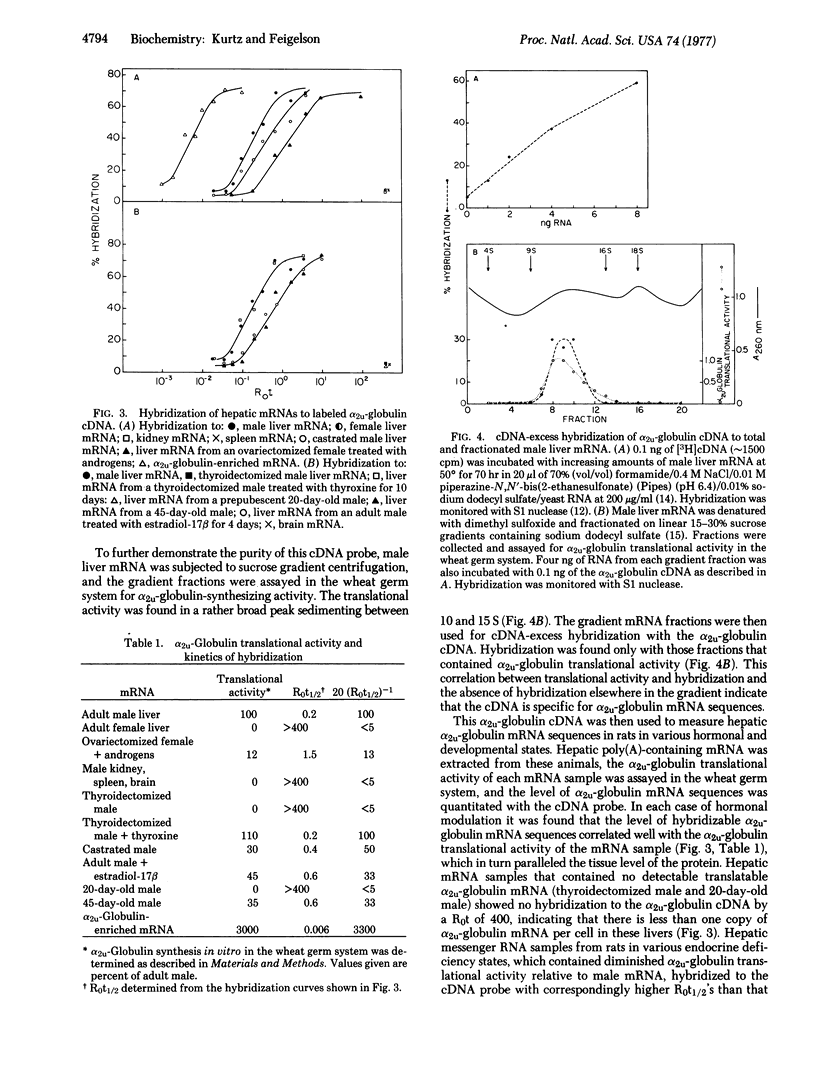
Abstract
A procedure is presented for the preparation of a 3H-labeled complementary DNA (cDNA) specific for the mRNA coding for α2u-globulin, a male rat liver protein under multihormonal control that represents approximately 1% of hepatic protein synthesis. Rat liver polysomes are incubated with monospecific rabbit antiserum to α2u-globulin, which binds to the nascent α2u-globulin chains on the polysomes. These antibody-polysome complexes are then adsorbed to goat antiserum to rabbit IgG that is covalently linked to p-aminobenzylcellulose. mRNA preparations are thus obtained that contain 30-40% α2u-globulin mRNA. A labeled cDNA is made to this α2u-globulin-enriched mRNA preparation by using RNA-dependent DNA polymerase (reverse transcriptase). To remove the non-α2u-globulin sequences, this cDNA preparation is hybridized to an RNA concentration × incubation time (R0t) of 1000 mol of ribonucleotide per liter × sec with female rat liver mRNA, which, though it shares the vast majority of mRNA sequences with male liver, contains no α2u-globulin mRNA sequences. The cDNA remaining single-stranded is isolated by hydroxylapatite chromatography and is shown to be specific for α2u-globulin mRNA by several criteria.
Good correlation was found in all endocrine states studied between the hepatic level of α2u-globulin, the level of functional α2u-globulin mRNA as assayed in a wheat germ cell-free translational system, and the level of α2u-globulin mRNA sequences as measured by hybridization to the α2u-globulin cDNA. Thus, the hormonal control of hepatic α2u-globulin synthesis by sex steroids and thyroid hormone occurs through modulation of the cellular level of α2u-globulin mRNA sequences, presumably by hormonal control of transcriptive synthesis.
Keywords: mRNA purification, preparative hybridization, transcriptional control
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Feigelson P., Schutz G. Analysis of the complexity and diversity of mRNA from chicken liver and oviduct. Cell. 1976 Feb;7(2):247–254. doi: 10.1016/0092-8674(76)90024-6. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigelson P., Beato M., Colman P., Kalimi M., Killewich L. A., Schutz G. Studies on the hepatic glucocorticoid receptor and on the hormonal modulation of specific mRNA levels during enzyme induction. Recent Prog Horm Res. 1975;31:213–242. doi: 10.1016/b978-0-12-571131-9.50010-4. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Sippel A. E., Jeep S., Chi-Nguyen-Huu M., Schütz G. Immunoadsorption of specific chicken oviduct polysomes. Isolation of ovalbumin, ovomucoid, and lysozyme messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6666–6674. [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Jones R. E., Pulkrabek P., Grunberger D. Mouse pituitary tumor mRNA directed cell-free synthesis of polypeptides that are cross-reactive with adrenocorticotropic hormone antiserum. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1490–1495. doi: 10.1016/0006-291x(77)90610-6. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Ansah-Yiadom R., Feigelson P. Effects of sex hormones on the level of the messenger RNA for the rat hepatic protein alpha 2u globulin. J Biol Chem. 1976 Jun 25;251(12):3594–3598. [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Feigelson P. Effect of thyroid hormones on the level of the hepatic mRNA for alpha2u globulin. Biochemistry. 1976 Mar 9;15(5):1031–1036. doi: 10.1021/bi00650a013. [DOI] [PubMed] [Google Scholar]
- Levenson R. G., Marcu K. B. On the existence of polyadenylated histone mRNA in Xenopus laevis oocytes. Cell. 1976 Oct;9(2):311–322. doi: 10.1016/0092-8674(76)90121-5. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. A procedure for the quantitative recovery of homogeneous populations of undegraded free and bound polysomes from rat liver. Biochemistry. 1976 Apr 20;15(8):1704–1712. doi: 10.1021/bi00653a018. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Barker S. W. Quantitation of casein messenger ribonucleic acid sequences using a specific complementary DNA hybridization probe. Biochemistry. 1976 Nov 30;15(24):5272–5280. doi: 10.1021/bi00669a012. [DOI] [PubMed] [Google Scholar]
- Roy A. K., Neuhaus O. W. Identification of rat urinary proteins by zone and immunoelectrophoresis. Proc Soc Exp Biol Med. 1966 Mar;121(3):894–899. doi: 10.3181/00379727-121-30917. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Wahli W., Weber R. Quantitation of vitellogenin messenger RNA in the liver of male Xenopus toads during primary and secondary stimulation by estrogen. Cell. 1977 May;11(1):213–221. doi: 10.1016/0092-8674(77)90332-4. [DOI] [PubMed] [Google Scholar]
- Schutz G., Kieval S., Groner B., Sippel A. E., Kurtz D., Feigelson P. Isolation of specific messenger RNA by adsorption of polysomes to matrix-bound antibody. Nucleic Acids Res. 1977 Jan;4(1):71–84. doi: 10.1093/nar/4.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E., Feigelson P., Roy A. K. Hormonal regulation of the hepatic messenger RNA levels for alpha2u globulin. Biochemistry. 1975 Feb 25;14(4):825–829. doi: 10.1021/bi00675a028. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Kurtz D. T., Morris H. P., Feigelson P. Comparison of in vivo translation rates and messenger RNA levels of alpha2U-globulin in rat liver and Morris hepatoma 5123D. Cancer Res. 1976 Oct;36(10):3588–3593. [PubMed] [Google Scholar]


