Abstract
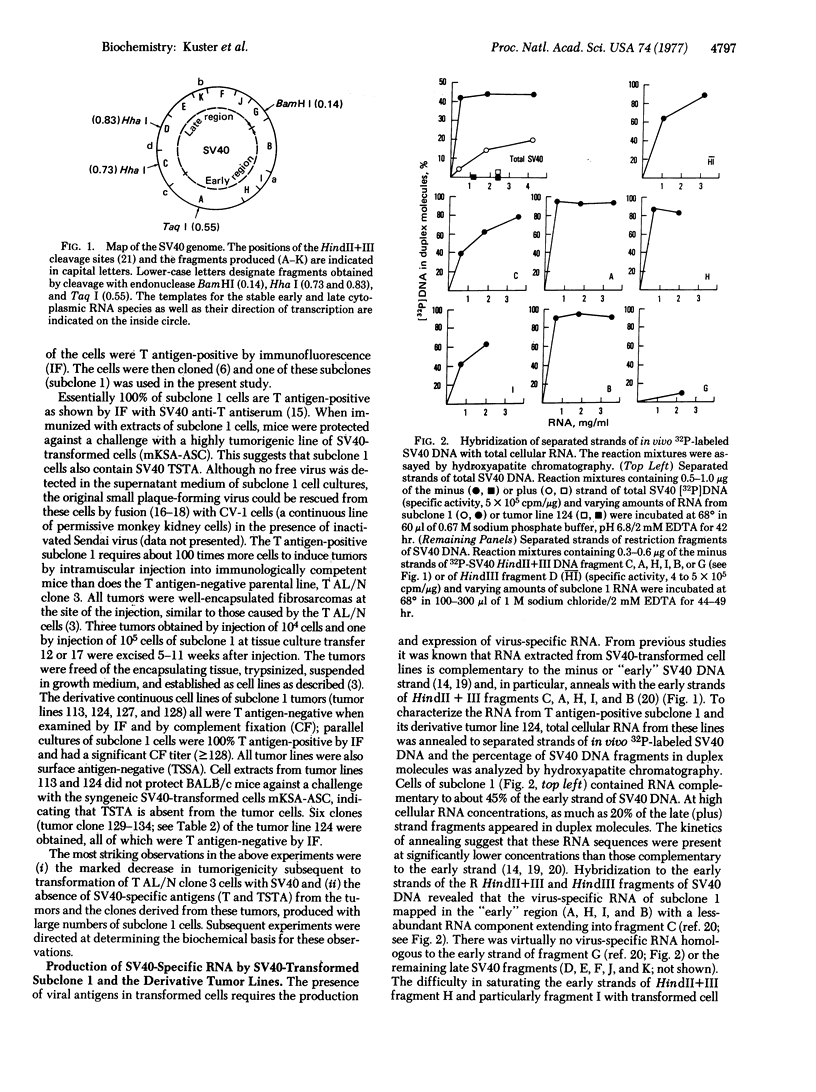
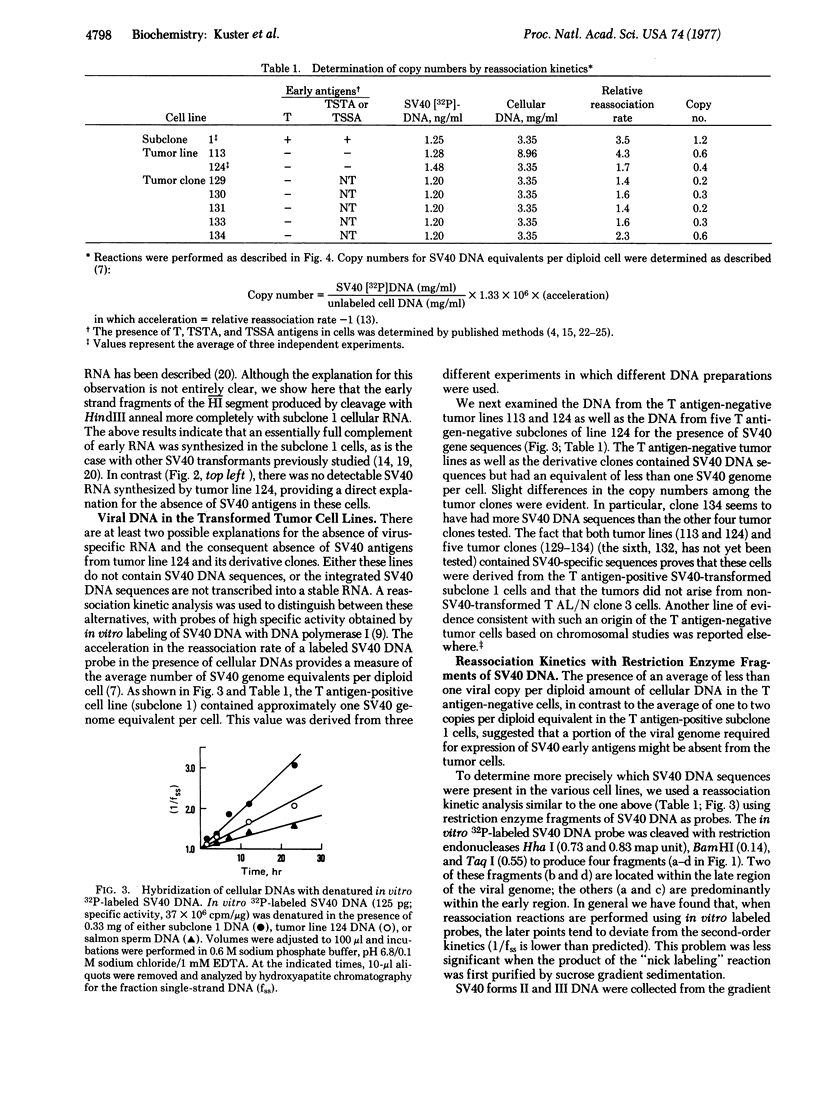
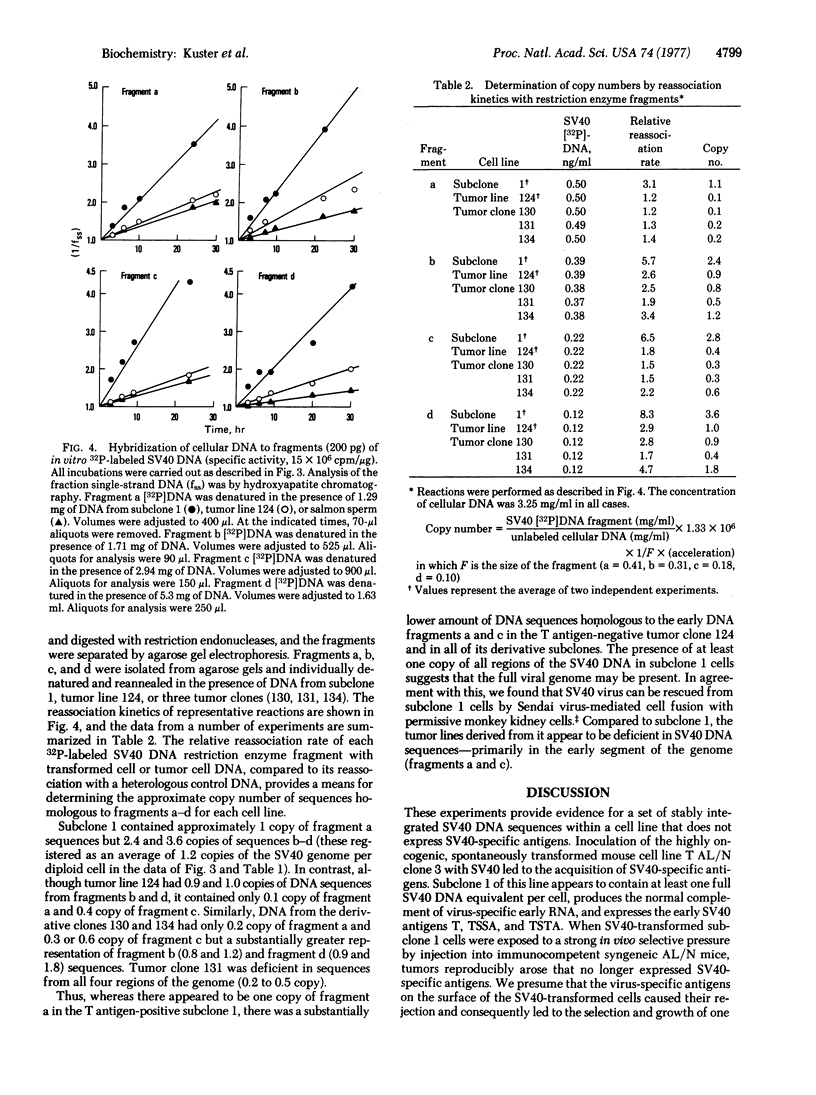
A clonal line of highly oncogenic “spontaneously transformed” mouse cells (T AL/N clone 3) was transformed in tissue culture by simian virus 40 (SV40) and subsequently recloned. The clone of SV40-transformed cells (subclone 1) expressed SV40-specific T (nuclear) and transplantation antigens but was 100 times less tumorigenic than the parent T AL/N clone 3 cells. When large numbers of subclone 1 cells (104-105) were injected into syngeneic AL/N mice, tumors were produced. From the tumors, cell lines were established in culture, all of which were consistently negative for T antigen. Tumor lines tested were found not to contain SV40-specific transplantation antigen and had again become highly tumorigenic. The original subclone 1 cells contained about one copy of SV40 DNA per diploid amount of cell DNA, as well as RNA complementary to the early region of the SV40 genome. The T antigen-negative cells from tumor line 124 contained approximately 0.5 copy of SV40 DNA per diploid equivalent and did not synthesize any detectable virus-specific RNA. Reassociation kinetic analysis with restriction enzyme fragments of viral DNA demonstrated that the cells from tumor line 124 (and also the clones of this line) had lost DNA sequences predominantly from the early region of the SV40 genome. The results indicate that a set of stably integrated SV40 DNA sequences can be present in a cell without the expression of viral antigens.
Keywords: viral integration, reassociation kinetics, genome mapping, tumorigenesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. L., Martin R. G., Chang C., Mora P. T., Livingston D. M. Nuclear preparations of SV40-transformed cells contain tumor-specific transplantation antigen activity. Virology. 1977 Jan;76(1):420–425. doi: 10.1016/0042-6822(77)90314-2. [DOI] [PubMed] [Google Scholar]
- Botchan M., Ozanne B., Sugden B., Sharp P. A., Sambrook J. Viral DNA in transformed cells. III. The amounts of different regions of the SV40 genome present in a line of transformed mouse cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4183–4187. doi: 10.1073/pnas.71.10.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Chang C., Anderson J. L., Martin R. G., Mora P. T. Expression of tumor-specific transplantation antigen in cell lines transformed by wild-type of tsA mutant simian virus 40. J Virol. 1977 May;22(2):281–289. doi: 10.1128/jvi.22.2.281-289.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Pancake S. J., Luborsky S. W., Mora P. T. Detergent solubilization and partial purification of tumor specific surface and transplantation antigens from SV40-virus-transformed mouse cells. Int J Cancer. 1977 Feb 15;19(2):258–266. doi: 10.1002/ijc.2910190216. [DOI] [PubMed] [Google Scholar]
- Coll J. M., Luborsky S. W., Mora P. T. Metabolically labeled cell membrane proteins in spontaneously and in SV40 virus transformed mouse fibroblasts. Biochemistry. 1977 Jul 12;16(14):3169–3177. doi: 10.1021/bi00633a020. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walter G. Simian virus to (SV40) tumor-specific proteins in nucleus and plasma membrane of HeLa cells infected by adenovirus 2-SV40 hybrid virus Ad2+ND2. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2505–2509. doi: 10.1073/pnas.73.7.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Patterns of simian virus 40 deoxyribonucleic acid transcription. II. In transformed cells. J Virol. 1973 Jan;11(1):54–60. doi: 10.1128/jvi.11.1.54-60.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969 Jul 15;4(4):384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. N., Nathans D. A transcriptional map of the SV40 genome in transformed cell lines. Virology. 1975 Jan;63(1):263–272. doi: 10.1016/0042-6822(75)90390-6. [DOI] [PubMed] [Google Scholar]
- Luborsky S. W., Chang C., Pancake S. J., Mora P. T. Detergent solubilized and molecular weight estimation of tumor specific surface antigen from SV40 virus transformed cells. Biochem Biophys Res Commun. 1976 Aug 23;71(4):990–996. doi: 10.1016/0006-291x(76)90752-x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland V. W., Mora P. T., Schultz A., Pancake S. Cell properties after repeated transplantation of spontaneously and of SV40 virus transformed mouse cell lines. I. Growth in culture. J Cell Physiol. 1975 Feb;85(1):101–111. doi: 10.1002/jcp.1040850111. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Brady R. O., Bradley R. M., McFarland V. W. Gangliosides in DNA virus-transformed and spontaneously transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1290–1296. doi: 10.1073/pnas.63.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancake S. J., Mora P. T. Limitations and utility of a cytolytic assay for measuring simian virus 40-induced cell surface antigens. Cancer Res. 1976 Jan;36(1):88–94. [PubMed] [Google Scholar]
- Pontén J. The relationship between in vitro transformation and tumor formation in vivo. Biochim Biophys Acta. 1976 Dec 23;458(4):397–422. doi: 10.1016/0304-419x(76)90009-3. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Smith R. W., Morganroth J., Mora P. T. SV40 virus-induced tumour specific transplantation antigen in cultured mouse cells. Nature. 1970 Jul 11;227(5254):141–145. doi: 10.1038/227141a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Todaro G. J., Habel K. Recovery of SV40 virus with genetic markers of original inducing virus from SV40-transformed mouse cells. Virology. 1968 May;35(1):1–8. doi: 10.1016/0042-6822(68)90299-7. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]


