Abstract
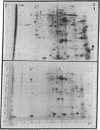
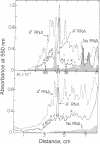
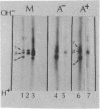
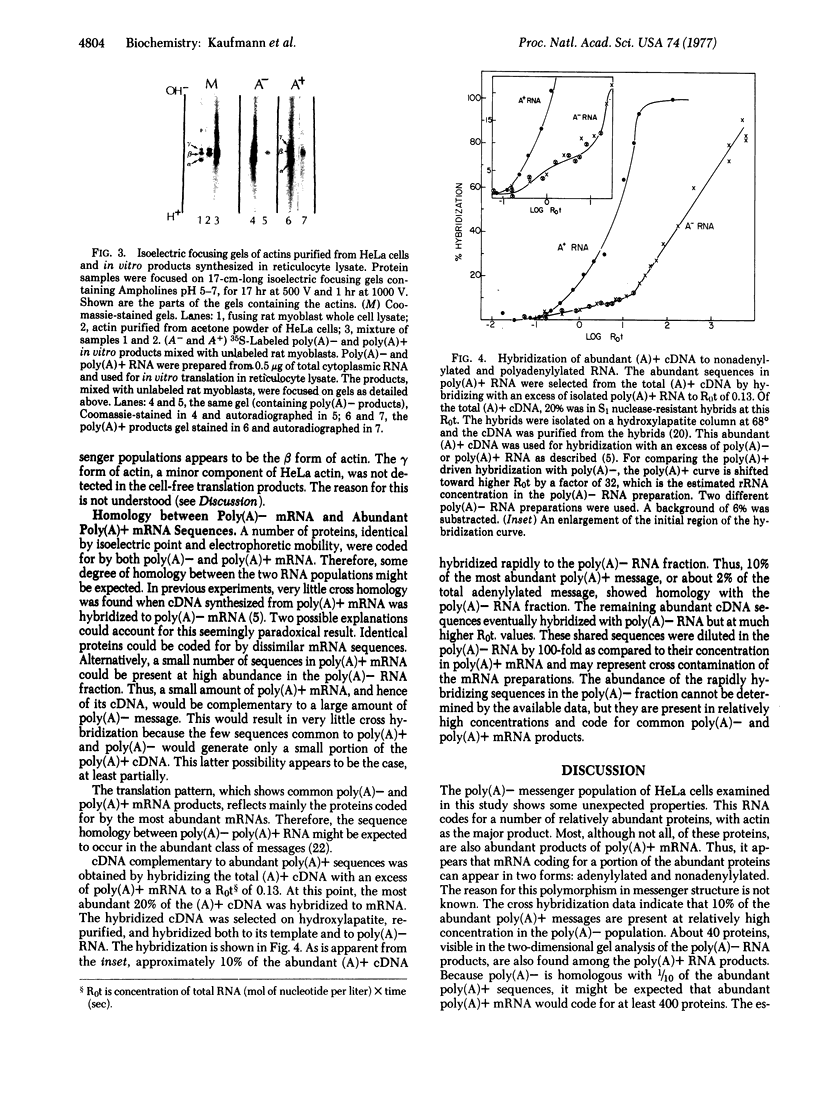
Poly(A)+ and poly(A)- mRNA from HeLa cells were separated and translated in heterologous messenger-dependent protein synthesizing systems. Two-dimensional electrophoretic analysis revealed three classes of polypeptides. At the level of detectability in the electropherograms, a small number (about 10) of proteins were detected only among the poly(A)- mRNA products, a larger number (about 40) were produced by both poly(A)- and poly(A)+ mRNA, and a large number of polypeptides were found exclusively in the poly(A)+ mRNA products. The major product of both poly(A)+ and poly(A)- mRNA was shown to be the β form of actin.
Previous cross hybridization measurements suggested little homology between poly(A)+ and poly(A)- mRNA populations. In view of the apparent identity of many poly(A)- products with those of poly(A)+, the homology between poly(A)+ and poly(A)- mRNA sequences was examined in greater detail. cDNA complementary to only the most abundant poly(A)+ message sequences was prepared. About 10% of this cDNA hybridized to abundant sequences in the poly(A)- fraction. This corresponded to only 2% of the total mass of poly(A)+ mRNA and accounted for the failure to detect cross hybridization in previous experiments. Thus, a small number of poly(A)+ sequences appear to be present in relatively high concentration in poly(A)- mRNA as evidenced by both the translation products and the cross hybridization results.
Keywords: mRNA·cDNA hybridization, cell-free translation, isoelectrofocusing, two-dimensional gel electrophoresis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromson D., Verma D. P. Translation of nonpolyadenylylated messenger RNA of sea urchin embryos. Proc Natl Acad Sci U S A. 1976 Jan;73(1):148–151. doi: 10.1073/pnas.73.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedamu L., Dixon G. H. Purification and properties of biologically active rainbow trout testis protamine mRNA. J Biol Chem. 1976 Mar 10;251(5):1455–1463. [PubMed] [Google Scholar]
- Gray R. E., Cashmore A. R. RNA synthesis in plant leaf tissue: the characterization of messenger RNA species lacking and containing polyadenylic acid. J Mol Biol. 1976 Dec 15;108(3):595–608. doi: 10.1016/s0022-2836(76)80139-8. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Isolation of L-cell messenger RNA which lacks poly(adenylate). Biochemistry. 1976 Aug 10;15(16):3516–3522. doi: 10.1021/bi00661a019. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Relative occurrence of polyadenylic acid sequences in messenger and heterogeneous nuclear RNA of L cells as determined by poly (U)-hydroxylapatite chromatography. J Mol Biol. 1972 Dec 14;72(1):91–98. doi: 10.1016/0022-2836(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Williams J. G., Penman S. Message and non-message sequences adjacent to poly(A) in steady state heterogeneous nuclear RNA of HeLa cells. Cell. 1976 Mar;7(3):429–437. doi: 10.1016/0092-8674(76)90173-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson R. G., Marcu K. B. On the existence of polyadenylated histone mRNA in Xenopus laevis oocytes. Cell. 1976 Oct;9(2):311–322. doi: 10.1016/0092-8674(76)90121-5. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Nemer M., Graham M., Dubroff L. M. Co-existence of non-histone messenger RNA species lacking and containing polyadenylic acid in sea urchin embryos. J Mol Biol. 1974 Nov 5;89(3):435–454. doi: 10.1016/0022-2836(74)90474-4. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Prives C. L., Aviv H., Paterson B. M., Roberts B. E., Rozenblatt S., Revel M., Winocour E. Cell-free translation of messenger RNA of simian virus 40: synthesis of the major capsid protein. Proc Natl Acad Sci U S A. 1974 Feb;71(2):302–306. doi: 10.1073/pnas.71.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragg H., Schröder J., Hahlbrock K. Translation of poly(A)-containing and poly(A)-free messenger RNA for phenylalanine ammonia-lyase, a plant-specific protein, in a reticulocyte lysate. Biochim Biophys Acta. 1977 Jan 20;474(2):226–233. doi: 10.1016/0005-2787(77)90197-6. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein P. A., Spudich J. A. Actin microheterogeneity in chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):120–123. doi: 10.1073/pnas.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein G. E., Geoghegan T. E., Brawerman G. A major species of mammalian messenger RNA lacking a polyadenylate segment. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3088–3092. doi: 10.1073/pnas.73.9.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Penman S. The messenger RNA sequences in growing and resting mouse fibroblasts. Cell. 1975 Oct;6(2):197–206. doi: 10.1016/0092-8674(75)90010-0. [DOI] [PubMed] [Google Scholar]