Abstract
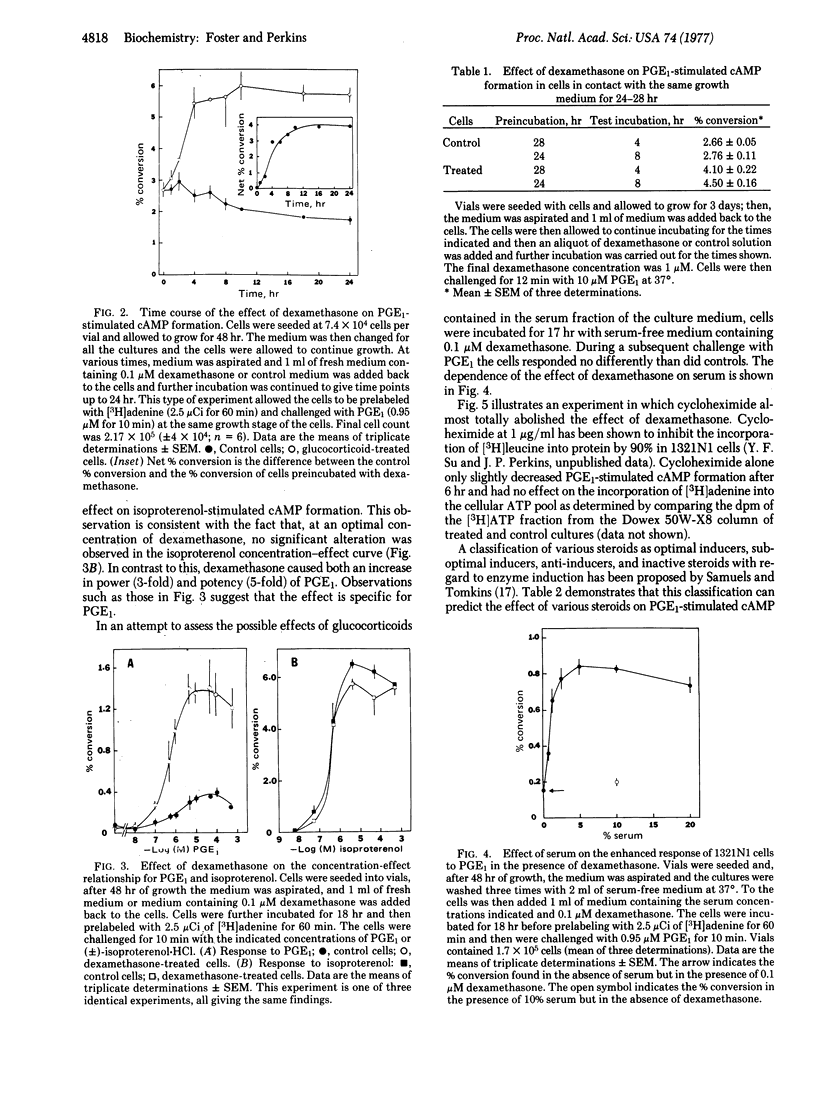
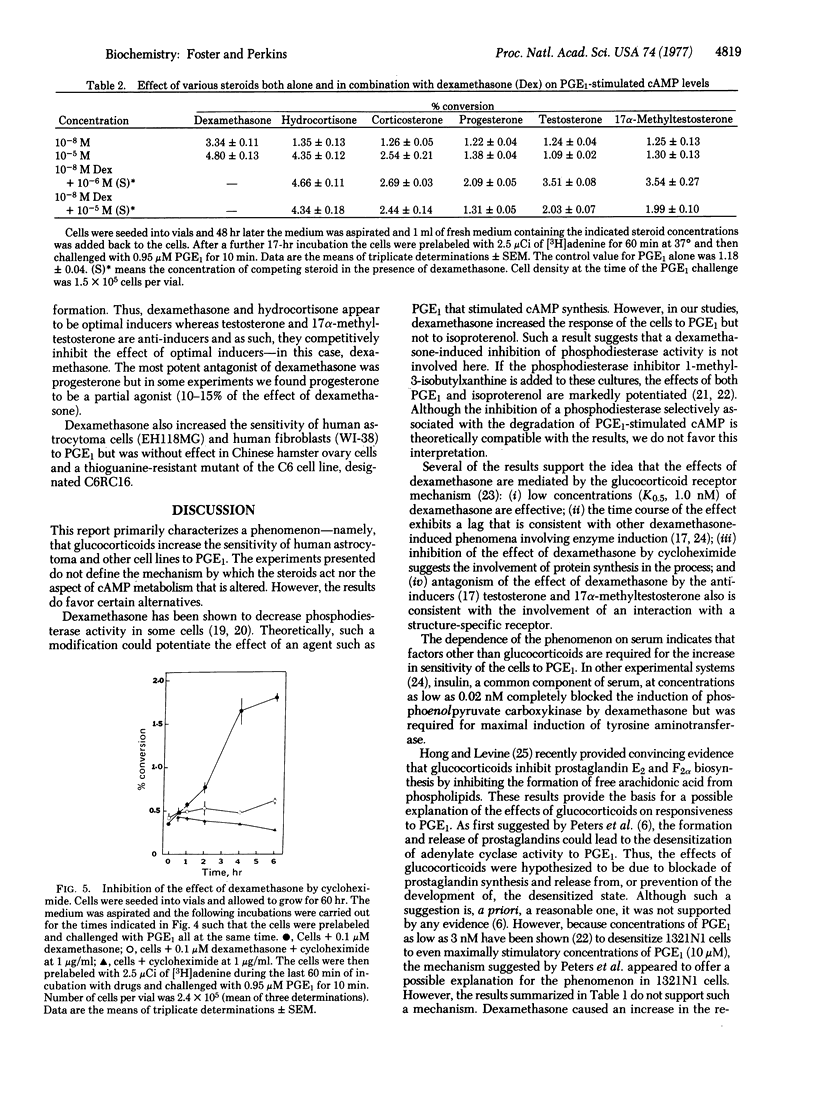
The influence of steroid hormones on the response of human astrocytoma cells (1321N1) to prostaglandin E1 (PGE1) has been investigated. Responsiveness to PGE1 was determined by measuring the conversion of [3H]ATP to cyclic [3H]AMP in cells prelabeled with [3H]adenine. After incubation of the cells with dexamethasone, a marked increase in both the maximal effect (2- to 3-fold) and the potency (5-fold) of PGE1 was observed. The effect was specific for the action of PGE1 in that no change in the response of the cells to isoproterenol was observed. The EC50 for dexamethasone was 0.001 μM and the effect was dependent on the presence of serum. The effect of dexamethasone was first observed after a 30- to 60-min lag and was maximal by 6-8 hr. Preconfluent cultures (3 days after seeding) exhibited optimal responsiveness to glucocorticoids. Both hydrocortisone and corticosterone mimicked the effect of dexamethasone but both were less potent. The action of dexamethasone was blocked by progesterone, testosterone, and 17α-methyltestosterone. Cycloheximide, at a concentration (1.0 μg/ml) that blocked protein synthesis (>90%) in 1321N1 cells, totally prevented the effect of dexamethasone on the response of the cells to PGE1. Upon removal of dexamethasone from cells treated for 16 hr, responsiveness to PGE1 returned to control levels with a half-time of 4 hr. Dexamethasone also was found to increase the response to PGE1 of a Rous sarcoma virus-transformed human astrocytoma cell line and the WI-38 human fibroblast line. The most obvious interpretation of our findings is that glucocorticoids induce the synthesis of a protein that selectively modifies the sensitivity of adenylate cyclase to PGE1.
Keywords: cyclic AMP, human astrocytoma cells, dexamethasone, cycloheximide, serum dependence
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett C. A., Wicks W. D. Regulation of phosphoenolpyruvate carboxykinase and tyrosine transaminase in hepatoma cell cultures. I. Effects of glucocorticoids, N 6 ,O 2' -dibutyryl cyclic adenosine 3',5'-monophosphate and insulin in Reuber H35 cells. J Biol Chem. 1971 Dec 10;246(23):7201–7206. [PubMed] [Google Scholar]
- Chang J., Lewis G. P., Piper P. J. Inhibition by glucocorticoids of prostaglandin release from adipose tissue in vitro. Br J Pharmacol. 1977 Mar;59(3):425–432. doi: 10.1111/j.1476-5381.1977.tb08396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Gross R., Su Y. F., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content in human astrocytoma cells by adenosine and the adenine nucleotides. J Biol Chem. 1974 Aug 25;249(16):5296–5303. [PubMed] [Google Scholar]
- Clark R. B., Su Y. F., Ortmann R., Cubeddu L., Johnson G. L., Perkins J. P. Factors influencing the effect of hormones on the accumulation of cyclic AMP in cultured human astrocytoma cells. Metabolism. 1975 Mar;24(3):343–358. doi: 10.1016/0026-0495(75)90115-8. [DOI] [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Panczenko B., Korbut R., Grodzinska L., Ocetkiewicz A. Corticosteroids inhibit prostaglandin release from perfused mesenteric blood vessels of rabbit and from perfused lungs of sensitized guinea pig. Prostaglandins. 1975 Aug;10(2):343–355. doi: 10.1016/0090-6980(75)90053-2. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Goldstein S. Adenosine 3': 5'-cyclic monophosphate in young and senescent human fibroblasts during growth and stationary phase in vitro. Effects of prostaglandine E1 and of adrenaline. Biochem J. 1974 Nov;144(2):253–263. doi: 10.1042/bj1440253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leichtling B. H., Drotar A. M., Ortmann R., Perkins J. P. Growth of astrocytoma cells in the presence of prostaglandin E1: effect on the regulation of cyclic AMP metabolism. J Cyclic Nucleotide Res. 1976;2(2):89–97. [PubMed] [Google Scholar]
- Lewis G. P., Piper P. J. Inhibition of release of prostaglandins as an explanation of some of the actions of anti-inflammatory corticosteroids. Nature. 1975 Mar 27;254(5498):308–311. doi: 10.1038/254308a0. [DOI] [PubMed] [Google Scholar]
- Macintyre E. H., Pontén J., Vatter A. E. The ultrastructure of human and murine astrocytes and of human fibroblasts in culture. Acta Pathol Microbiol Scand A. 1972;80(2):267–283. doi: 10.1111/j.1699-0463.1972.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Manganiello V. C., Breslow J. Effects of prostaglandin E1 and isoproterenol on cyclic AMP content of human fibroblasts modified by time and cell density in subculture. Biochim Biophys Acta. 1974 Oct 8;362(3):509–520. doi: 10.1016/0304-4165(74)90146-9. [DOI] [PubMed] [Google Scholar]
- Peters H. D., Dinnendahl V., Schönhöfer P. S. Mode of action of antirheumatic drugs on the cyclic 3',5'-AMP regulated glycosaminoglycan secretion in fibroblasts. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(1):29–40. doi: 10.1007/BF00498027. [DOI] [PubMed] [Google Scholar]
- Peters H. D., Peskar B. A., Schönhöfer P. S. Glucocorticoids: effects on prostaglandin release, cyclic AMP levels and glycosaminoglycan synthesis in fibroblast tissue cultures. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jan;296(2):131–137. doi: 10.1007/BF00508464. [DOI] [PubMed] [Google Scholar]
- Pontén J., Macintyre E. H. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Ross P. S., Manganiello V. C., Vaughan M. Regulation of cyclic nucleotide phosphodiesterases in cultured hepatoma cells by dexamethasone and N6,O2'-dibutyryl adenosine 3':k'-monophosphate. J Biol Chem. 1977 Feb 25;252(4):1448–1452. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tomkins G. M. Relation of steroid structure to enzyme induction in hepatoma tissue culture cells. J Mol Biol. 1970 Aug 28;52(1):57–74. doi: 10.1016/0022-2836(70)90177-4. [DOI] [PubMed] [Google Scholar]
- Schönhöfer P. S., Peters H. D., Karzel K., Dinnendahl V., Westhofen P. Influence of antiphlogistic drugs on prostaglandin E1 stimulated cyclic 3',5'-AMP levels and glycosaminoglycan synthesis in fibroblast tissue cultures. Pol J Pharmacol Pharm. 1974 Jan-Apr;26(1):51–60. [PubMed] [Google Scholar]
- Shimizu H., Daly J. W., Creveling C. R. A radioisotopic method for measuring the formation of adenosine 3',5'-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969 Dec;16(12):1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- Su Y. F., Cubeddu L., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content of human astrocytoma cells: desensitization to catecholamines and prostaglandins. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):257–270. [PubMed] [Google Scholar]
- Su Y. F., Johnson G. L., Cubeddu L., Leichtling B. H., Ortmann R., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content of human astrocytoma cells: mechanism of agonist-specific desensitization. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):271–285. [PubMed] [Google Scholar]
- Wicks W. D., Barnett C. A., McKibbin J. B. Interaction between hormones and cyclic AMP in regulating specific hepatic enzyme synthesis. Fed Proc. 1974 Apr;33(4):1105–1111. [PubMed] [Google Scholar]


