Abstract
Background
In the clinical practice of neoadjuvant chemotherapy, response markers are very important. We aimed o investigate whether tumor markers CEA(carcino-embryonic antigen), CA19-9(carbohydrate antigen 19–9), CA72-4(carbohydrate antigen 72–4), and CA125(carbohydrate antigen 125) can be used to evaluate the response to neoadjuvant chemotherapy, and to evaluate the diagnosis and prognosis value of four tumor markers in the patients of gastric cancer.
Methods
A retrospective review was performed of 184 gastric cancer patients who underwent a 5-Fu, leucovorin, and oxaliplatin (FOLFOX) neoadjuvant chemotherapy regimen, followed by surgical treatment. Blood samples for CEA, CA19-9, CA72-4, and CA125 levels were taken from patients upon admission to the hospital and after neoadjuvant chemotherapy. Statistical analysis was performed to identify the clinical value of these tumor markers in predicting the survival and the response to neoadjuvant chemotherapy.
Results
Median overall survival times of pretreatment CA19-9-positive and CA72-4-positive patients (14.0 +/−2.8 months and 14.8 +/−4.0 months, respectively) were significantly less than negative patients (32.5 +/−8.9 months and 34.0 +/−10.1 months, respectively) (P = 0.000 and P = 0.002, respectively). Pretreatment status of CA19-9 and CA72-4 were independent prognostic factors in gastric cancer patients (P = 0.029 and P = 0.008, respectively). Pretreatment CEA >50 ng/ml had a positive prediction value for clinical disease progression after neoadjuvant chemotherapy according to the ROC curve (AUC: 0.694, 95% CI: 0.517 to 0.871, P = 0.017). The decrease of tumor markers CEA, CA72-4, and CA125 was significant after neoadjuvant chemotherapy (P = 0.030, P = 0.010, and P = 0.009, respectively), especially in patients with disease control (including complete, partial clinical response, and stable disease) (P = 0.012, P = 0.020, and P = 0.025, respectively). A decrease in CA72-4 by more than 70% had a positive prediction value for pathologic response to neoadjuvant chemotherapy according to the ROC curve (AUC: 0.764, 95% CI: 0.584 to 0.945, P = 0.020).
Conclusions
Our results suggest that high preoperative serum levels of CA72-4 and CA19-9 are associated with higher risk of death, high pretreatment CEA levels (>50 ng/ml) may predict clinical disease progression after neoadjuvant chemotherapy, and a decrease (>70%) of CA72-4 may predict pathologic response to neoadjuvant chemotherapy.
Keywords: Gastric cancer, Neoadjuvant chemotherapy, CEA, CA19-9, CA72-4, CA125
Background
Gastric cancer is the fourth most common cancer worldwide and the second most frequent cause of cancer death, affecting about one million people per year [1]; surgery remains the only cure for this disease. However, in a recent study, the disease was too far advanced to receive curative resection in more than 30% of surgical patients [2]. Despite efforts for early detection, most patients with gastric cancer in Europe and the United States continue to present with advanced stages of the disease, therefore the current efforts to improve survival in patients with advanced gastric carcinoma aim to increase the rate of complete tumor removal. Preoperative neoadjuvant chemotherapy is an available option to achieve these goals [3].
Neoadjuvant chemotherapy is cancer treatment in which a drug is given to the patient prior to cancer surgery or radical radiotherapy, and it is mostly used in cases where surgery is planned in the future. The surgical interest in neoadjuvant therapy for gastric cancer is driven by a long-standing frustration with the poor outcomes achieved with surgery alone, as well as when surgery is followed by intense adjuvant chemotherapy and radiotherapy [4]. There are several studies which indicate that neoadjuvant chemotherapy could increase the rate of complete tumor resections, combat systemic metastasis, and prolong survival with tolerable and manageable toxicity, without increasing the mortality or morbidity of surgery in gastric cancer patients [3, 5, 6].
If particular patients not benefiting from preoperative treatment, who are suggested to have a prognosis inferior to surgery alone, could be identified, alternative therapies may offered at an early stage [7]. Response markers are needed to monitor treatment, and to improve quality of life of non-responders, reduce time until surgery in non-responders, and reduce costs [8].
Recently, tumor markers CEA, CA19-9, CA72-4, and CA125 are widely used in gastric cancer patients, although reports have shown the value of tumor markers as prognostic factors, clinical studies evaluating the roles of tumor markers in monitoring of chemotherapeutic efficacy are limited [9], especially in neoadjuvant chemotherapy. In this study, we analyzed the relationship between tumor markers CEA, CA19-9, CA72-4, and CA125 and the response to neoadjuvant chemotherapy in patients with gastric cancer. The aim of the study was to determine the clinical value of tumor markers in monitoring the response to neoadjuvant chemotherapy and in predicting the prognosis of gastric cancer patients.
Methods
The research was funded by Beijing Municipal Administration of Hospitals Clinical Medicne Development of Special Funding Support,code:XMLX201309,which was approved by the ethics committee on human research of the Beijing Shijitan Hospital in accordance with the Declaration of Helsinki.
Patients
The medical records of 184 patients who underwent neoadjuvant chemotherapy between 2009 and 2013 at Beijing Shijitan hospital, Capital Medical University, were retrospectively reviewed. All patients had histologically confirmed gastric cancer and received surgical treatment after neoadjuvant chemotherapy; patients were excluded from the study if they demonstrated other organs insufficiencies, if they had experienced an acute event within the last threemonths (cerebral, coronary, and so forth), acute infection, or major trauma. The clinicopathologic features of these patients including age, sex, and differentiation, tumor location, depth of wall invasion, lymph node metastasis, and vascular invasion, pTNM(Pathologic Tumor-Node-Metastasis) stage and responses to neoadjuvant chemotherapy were reviewed. pTNM stages were according to histological examination of a post-operation, and were assigned in accordance with the criteria established by American Joint Committee on Cancer in 2010 [10–12]. The mean follow-up time (+/− SD) for the entire patient population was 31.1 +/−23.2 months (median: 24.6 months).
Neoadjuvant chemotherapy
The decision to treat patients with neoadjuvant chemotherapy and the neoadjuvant regimen administered was determined by multidisciplinary tumor board discussions, tumor histology and stage, patient ability to tolerate therapy, and in accordance with National Comprehensive Cancer Network (NCCN) guidelines.
All 184 patients received neoadjuvant chemotherapy; 5-Fu, and Leucovorin, and Oxaliplatin (FOLFOX) regimen. The median number of cycles administered was three (range: one to ninecycles). In all cases, surgery was performed after neoadjuvant chemotherapy.
Evaluation of clinical and pathologic response
In this study, both clinical and pathologic responses are used to evaluate the response to neoadjuvant chemotherapy. Clinical responses to neoadjuvant chemotherapy were determined by RECIST 1.1 response evaluation criteria in solid tumors version 1.1 [13], and according to radiological examination, including ultrasonography, computed tomographic scan, upper gastrointestinal endoscopic assessment, and upper gastrointestinal tract radiography.
Pathologic responses to neoadjuvant chemotherapy were determined by the criteria defined by the MD Anderson center [14–16], and according to the histological examination of post-operation, board-certified pathologists who specialize in gastrointestinal malignancies reviewed all specimens.
Serum assays for CEA, CA19-9, CA72-4, and CA125
Serum samples for CEA, CA19-9, CA72-4, and CA125 levels were measured at baseline and after neoadjuvant chemotherapy respectively. Serum levels of CEA, CA19-9, and CA72-4 were assayed with electrochemiluminescence (ECL) method (E170, Roche Diagnostics, Rotkreuz,Switzerland), and CA125 was measured with enzyme-linked immunosorbent assay (ELISA, CA125-ELISA-Kit, CanAg, Gothenburg,Sweden).
The cut-off levels were 5.0 ng/ml, 37.0 U/ml, 6.7 U/ml, and 20.0 U/ml for CEA, CA19-9, CA72-4, and CA125, as recommended by the manufacturer. A result was considered positive when the marker serum level was higher than the cut-off value. Positive combined detection for four tumor markers was defined as one or more tumor markers above the cut-off levels.
Statistical analysis
Analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago,United States). Chi-square analysis was applied to determine the association between tumor markers and clinicopathologic features. Survival rates were defined in univariate analysis according to the Kaplan-Meier method, and log-rank test was used to assess statistical differences. The independent prognostic values of each tumor marker and clinicopathologic aspects which significantly affect survival were assessed by Cox multivariate analysis. T-test was applied to determine the differences between mean levels of tumor markers before and after neoadjuvant chemotherapy. Correlations were assessed using the Spearman rank order correlations. Receiver-operating characteristics (ROC) curve was used to evaluate the ability of tumor markers to predict the response to neoadjuvant chemotherapy. Differences were considered statistically significant when the P value was <0.05.
Results
The diagnosis and prognosis value of pretreatment levels of four tumor markers
The pretreatment levels of CEA, CA19-9, CA72-4, and CA125 were above the cut-off levels in 37.9, 28.7, 36.4, and 26.4% of cases, respectively. A total of 108 patients showed positivity for one or more tumor markers (overall sensitivity: 63.9%).
Table 1 shows the correlation between pretreatment status of tumor markers and different clinicopathological parameters. CEA was more frequently positive in the patients with lymph node involvement, well differentiation, and cardiac carcinoma (P = 0.005, P = 0.001, and P = 0.049, respectively), CA72-4 was more frequently positive in the patients with advanced tumor stage and vascular invasion (P = 0.007 and P = 0.006, respectively), and positive levels of combined detection were related to deeper tumor invasion, lymph node metastasis, and advanced tumor stage (P = 0.041, P = 0.014, and P = 0.040, respectively).
Table 1.
Association of pre-therapy status of tumor markers with clinicopathological parameters
| Variable | Cases (N) | CEA (+) N (%) | Pvalue | CA199 (+) N (%) | Pvalue | CA72-4 (+) N (%) | Pvalue | CA125 (+) N (%) | Pvalue | Combined detection (+) N (%) | Pvalue |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender: | |||||||||||
| Male | 127 | 47(39.8) | 32(28.1) | 37(35.6) | 14(23.3) | 78(66.1) | |||||
| Female | 57 | 17(33.3) | 0.424 | 15(30.0) | 0.801 | 18(38.3) | 0.748 | 9(33.3) | 0.328 | 30(58.8) | 0.366 |
| Age(years): | |||||||||||
| ≤60 | 92 | 29(33.3) | 25(29.1) | 31(40.8) | 9(22.0) | 52(59.8) | |||||
| >60 | 92 | 35(42.7) | 0.210 | 22(28.2) | 0.903 | 24(32.0) | 0.262 | 14(30.4) | 0.370 | 56(68.3) | 0.249 |
| Differentiation: | |||||||||||
| Moderately | 30 | 18(62.1) | 10(38.5) | 5(20.0) | 4(28.6) | 21(72.4) | |||||
| Poorly | 75 | 20(27.4) | 0.001 | 15(20.8) | 0.077 | 24(36.9) | 0.124 | 8(22.2) | 0.918 | 39(53.4) | 0.079 |
| Other | 29 | ||||||||||
| Missing value: | 50 | ||||||||||
| Tumorlocation | |||||||||||
| Cardiac carcinoma | 47 | 22(47.8) | 11(24.4) | 11(29.7) | 1(6.2) | 32(69.6) | |||||
| Other locations | 114 | 34(31.2) | 0.049 | 28(26.7) | 0.776 | 39(38.6) | 0.336 | 15(24.6) | 0.207 | 64(58.7) | 0.204 |
| Missing value | 23 | ||||||||||
| Invasion depth(pathological): | |||||||||||
| T1 + T2 | 29 | 8(30.8) | 5(20.8) | 5(21.7) | 4(28.6) | 12(46.2) | |||||
| T3 + T4 | 146 | 53(39.6) | 0.399 | 39(29.8) | 0.372 | 47(39.5) | 0.106 | 17(25.0) | 1.000 | 90(67.2) | 0.041 |
| Missing value | 9 | ||||||||||
| Lymph node metastasis (pathological): | |||||||||||
| Negative | 44 | 8(20.0) | 7(18.4) | 9(25.7) | 5(26.3) | 19(47.5) | |||||
| Positive | 131 | 54(45.0) | 0.005 | 37(31.6) | 0.117 | 42(39.3) | 0.147 | 16(25.4) | 1.000 | 83(69.2) | 0.014 |
| Missing value | 9 | ||||||||||
| Vascular invasion (pathological) | |||||||||||
| Negative | 101 | 32(35.6) | 24(27.6) | 21(26.2) | 14(29.8) | 54(60.0) | |||||
| Positive | 66 | 26(41.9) | 0.426 | 19(30.6) | 0.685 | 27(49.1) | 0.006 | 7(21.9) | 0.435 | 43(69.4) | 0.238 |
| Missing value | 17 | ||||||||||
| pTNM stage: | |||||||||||
| I + II | 53 | 13(27.1) | 10(21.3) | 9(20.5) | 7(26.9) | 25(52.1) | |||||
| III + IV | 126 | 50(43.1) | 0.055 | 36(32.1) | 0.168 | 45(44.1) | 0.007 | 14(24.1) | 0.785 | 80(69.0) | 0.040 |
| Missing value | 5 | ||||||||||
| Clinical response: | |||||||||||
| PR + SD | 137 | 48(36.9) | 35(27.6) | 40(34.5) | 16(24.6) | 82(63.1) | |||||
| PD | 14 | 10(71.4) | 0.012 | 4(28.6) | 1.000 | 4(30.8) | 1.000 | 3(50.0) | 0.389 | 11(78.6) | 0.391 |
| Missing value | 33 | ||||||||||
| Pathologicresponse: | |||||||||||
| Complete and partial response | 84 | 7(24.1) | 6(21.4) | 9(32.1) | 4(25.0) | 14(48.3) | |||||
| Noresponse | 33 | 31(39.2) | 0.145 | 21(26.6) | 0.590 | 24(32.9) | 0.944 | 12(26.1) | 1.000 | 52(65.8) | 0.097 |
| Missing value | 67 | ||||||||||
Positive of combined detection for four tumor markers was defined as one or more tumor markers were above the cut-off levels.
Survival curves of patients according to pretreatment levels of four tumor markers are shown in Figure 1. Significant differences in survival rates were observed for CA19-9, CA72-4 (log-rank test: P = 0.000 and P = 0.002, respectively), a worse prognosis was observed in positive cases. According to the survival curve for CEA and CA125, positive cases also showed poor prognosis, but the difference did not reached statistical significance (P = 0.085 and P = 0.409, respectively).
Figure 1.
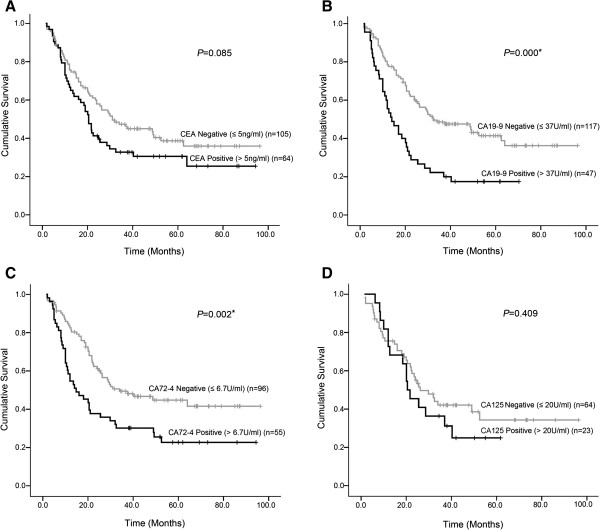
Survival curves of patients according to CEA, CA199, CA724, and CA242 pretreatment serum positivity. A. The difference between CEA-negative patients and CEA-positive patients was not statistically significant (P = 0.085). B. The difference between CA19-9-negative patients and CA19-9-positive patients was statistically significant (P = 0.000). C. The difference between CA72-4-negative patients and CA72-4-positive patients was statistically significant (P = 0.002). D. The difference between CA242-negative patients and CA242-positive patients wasnot statistically significant (P = 0.409).
Furthermore, to evaluate the potential of four tumor markers as independent predictors for overall survival of gastric cancer, univariate and multivariate analyses were performed (Table 2). Univariate Cox regression analysis was used to identify the factors which were significantly associated with overall survival. Selected these factors into multivariate Cox regression analysis, CA19-9, CA72-4, and pTNM stage were found as independent predictors (P = 0.029, P = 0.008, and P = 0.001, respectively). An increased pretreatment level of CA19-9 was associated with a 2.183-fold (95% CI: 1.085 to 4.392) higher risk of death (P = 0.029) and an increased pretreatment level of CA72-4 was associated with a 2.500-fold (95% CI: 1.269 to 4.926) higher risk of death (P = 0.008).
Table 2.
Univariate and multivariate analyses of factors associated with survival in gastric cancer patients with neoadjuvant chemotherapy
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | Pvalue | Hazard ratio (95% CI) | Pvalue | |
| CA19-9 | ||||
| Positive versusnegative | 2.198 (1.459-3.311) | 0.000 | 2.183 (1.085-4.392) | 0.029 |
| CA72-4 | ||||
| Positive versusnegative | 1.887 (1.241-2.869) | 0.003 | 2.500 (1.269-4.926) | 0.008 |
| Vascular invasion | ||||
| Positive versus negative | 2.401 (1.616-3.567) | 0.000 | 1.564 (0.817-2.995) | 0.177 |
| TNM (pathological) | ||||
| III + IV versusI + II | 6.041 (3.295-11.074) | 0.000 | 5.066 (1.887-13.599) | 0.001 |
| Recurrence | ||||
| Positive versusnegative | 1.745 (1.161-2.625) | 0.007 | 0.755 (0.337-1.693) | 0.495 |
| Clinical response to NCT | ||||
| PD versus DC | 2.474 (1.274-4.802) | 0.007 | 1.103 (0.404-3.013) | 0.849 |
| Pathologic response to NCT | ||||
| pCR + pPR versus pNR | 0.419 (0.218-0.805) | 0.009 | 0.739 (0.322-1.696) | 0.475 |
Changes of tumor markers and correlation with response to neoadjuvant chemotherapy
All 184 patients were treated with neoadjuvant chemotherapy and clinical response to neoadjuvant chemotherapy was noted in 151 patients; complete and partial clinical response (CR and PR) was achieved in 65 (43.0%), stable disease (SD) in 72 (47.7%), and progression disease (PD) in 14 (9.3%) of patients. Pathologic response to neoadjuvant chemotherapy was noted in 117 patients; complete response (pCR) was achieved in 12 (10.3%), partial response (pPR) in 21 (17.9%), and nonresponse (pNR) in 84 (71.8%) of patients. The major toxic effects were nausea, anorexia, fatigue, anemia, thrombocytopenia, and sensory neuropathy, the major gradethree or four adverse events include anemia, and there were no treatment-related deaths.
The levels of four tumor markers were recorded at baseline and after neoadjuvant chemotherapy, respectively. The mean levels of four tumor markers decreased after neoadjuvant chemotherapy, in particular, CEA, CA72-4, and CA125 decreased significantly (P = 0.030, P = 0.010, and P = 0.009, respectively) (Figure 2), and these three tumor markers decreased more significantly in the disease control (CR + PR + SD) group than the disease progression (PD) group (P = 0.012, P = 0.020, and P = 0.025, respectively) (Figure 3).
Figure 2.
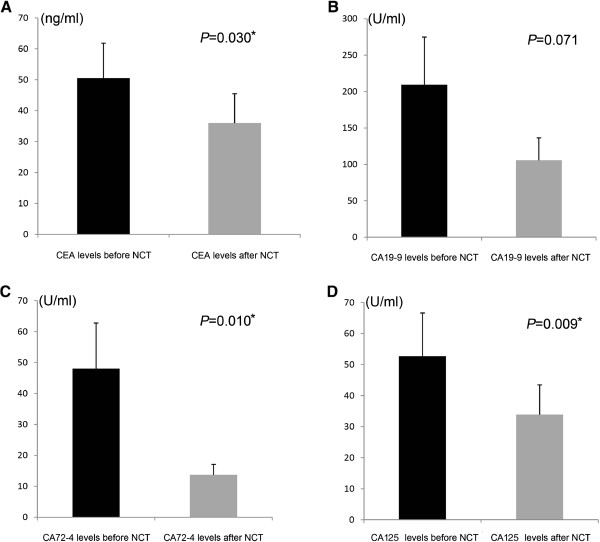
Mean levels of four tumor markers before and after neoadjuvant chemotherapy. A. Mean level of CEA was decreased significantly after neoadjuvant chemotherapy (P = 0.030). B. Mean level of CA19-9 was decreased after neoadjuvant chemotherapy, the difference was not statistically significant (P = 0.251). C. Mean level of CA72-4 was decreased significantly after neoadjuvant chemotherapy (P = 0.010). D. Mean level of CA242 was decreased significantly after neoadjuvant chemotherapy (P = 0.009).
Figure 3.
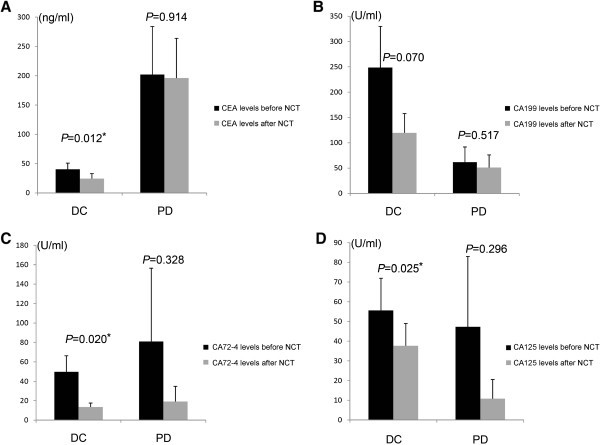
Mean levels of four tumor markers before and after neoadjuvant chemotherapy in disease control (CR + PR + SD) and PD (disease progression) group respectively. A. C. D shows CEA, CA724, and CA125 decreased more significantly in the disease control (CR + PR + SD) group than the disease progression (PD) group (P = 0.012, P = 0.020, and P = 0.025, respectively). B. show the decreased levels of CA199 was not statistically significant (P values was 0.849).
The changes of tumor markers were observed in this study, and patients were excluded if baseline serum concentration was below the upper limit of normal (ULM).
To assess the ability of the changes of tumor markers to predict the pathologic complete and partial response to neoadjuvant chemotherapy, ROC curve was carried out (Figure 4). The area under the ROC curve of CA72-4 change values was 0.764 (95% CI: 0.584 to 0.945, P = 0.020); the optimal cutoff which simultaneously maximized both the sensitivity (77.8%) and specificity (80%) of the test was decreased by 71.1%. The area under the ROC curve of CA125 change values was 0.800 (95% CI: 0.537 to 1.063, P = 0.128); the optimal cutoff which simultaneously maximized both the sensitivity (100%) and specificity (60%) of the test was decreased by 40.0%.
Figure 4.
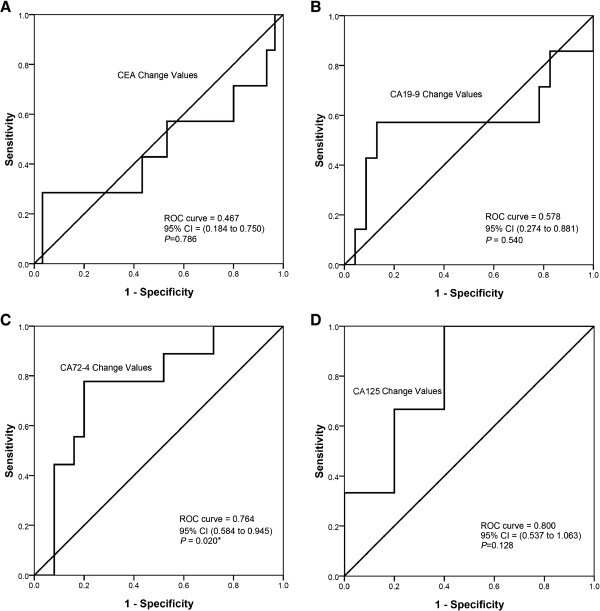
ROC curves for the decrease of the four tumor markers to predict the disease control after neoadjuvant chemotherapy. A, B. shows the decrease of CEA, CA199 was not statistically significant (P values were 0.785, and 0.540, respectively). C. The area under the ROC curve of CA72-4 change values was 0.764 (95% CI: 0.584 to 0.945, P = 0.020); the optimal cutoff which simultaneously maximized both the sensitivity (77.8%) and specificity (80%) of the test was decreased by 71.1%. D. The area under the ROC curve of CA125 change values was 0.800 (95% CI: 0.537 to 1.063, P = 0.128); the optimal cutoff which simultaneously maximized both the sensitivity (100%) and specificity (60%) of the test was decreased by 40.0%.
To confirm the relationship between tumor marker decrease and pathologic response, Spearman correlation was performed;the correlation coefficient between CA72-4 decreased and pathologic response was 0.404, and the P value was 0.018. The correlation coefficient between CA125 decreased and pathologic response was 0.439, and the P value was 0.133.
Association of pretreatment CEA levels with response to neoadjuvant chemotherapy
According to Table 1, the positivity rate of pretreatment CEA was higher when clinical disease progression and no pathologic response were present (P = 0.012 and P = 0.145). Figure 3 shows that serum levels of pretreatment CEA were higher in patients with clinical disease progression after neoadjuvant chemotherapy.
To evaluate whether pretreatment levels of tumor markers can be used as marker of clinical disease progression after neoadjuvant chemotherapy, an ROC curve was carried out (Figure 5). The area under the ROC curve of CEA pretreatment levels was 0.694 (95% CI: 0.517 to 0.871, P = 0.017); the cutoff that maximized both sensitivity (57.1%) and specificity (85.4%) of this test was 50 ng/ml.
Figure 5.
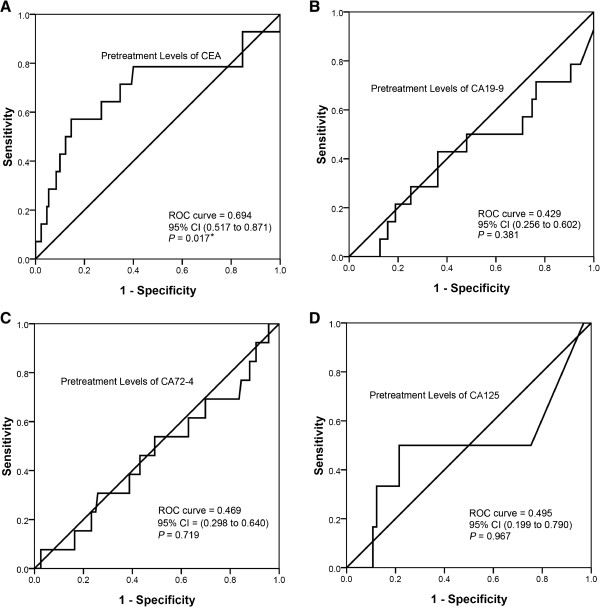
ROC curves for pretreatment levels of tumor markers to predict the disease progression after neoadjuvant chemotherapy. A. The area under the ROC curve of CEA pretreatment levels was 0.694 (95% CI: 0.517 to 0.871, P = 0.017); the cutoff that maximized both sensitivity (57.1%) and specificity (85.4%) of this test was 50 ng/ml. The correlation coefficient between CEA pretreatment levels and pathologic response was 0.199, and the P value was 0.017. B.C.D show the pretreatment levels of CA72-4, CA199, and CA125 were not statistically significant (P values were 0.381, 0.719, and 0.967 respectively).
The correlation between pretreatment levels of tumor markers and pathologic response was also analyzed in our study. The correlation coefficient between CEA pretreatment levels and pathologic response was 0.199, and the P value was 0.017.
Discussion
Gastric cancer is still a major health problem worldwide due to its frequency, poor prognosis, and limited treatment options. Despite efforts for early detection, most patients with gastric cancer continue to present with an advanced of the disease. To improve survival in these patients, preoperative neoadjuvant chemotherapy became an available option, and several studies have certified that neoadjuvant chemotherapy could down-stage patients, improve curative respectability, and improve survival rates [3, 5, 6, 17]. Despite these advantages, some patients still have with no response to neoadjuvant chemotherapy, to improve the quality of life of non-responders, reduce time until surgery, and reduce costs, the markers which can monitor the response to neoadjuvant chemotherapy are needed. Our study focuses on clinical utility of tumor markers CEA, CA19-9, CA72-4, and CA125 in gastric cancer patients with neoadjuvant chemotherapy, and the aim of the study is to measure whether these tumor markers might be useful in monitoring response and in predicting the prognosis of patients.
It is well known that the serum levels of various tumor markers such as CEA, CA19-9, CA72-4, and CA125 are elevated in patients with gastric cancer; however, there are still controversies in the clinical use of these tumor markers. The pretreatment positivity rates of tumor markers are different in previous studies., CEA positivity is normally reported at between 15.8 and 57.6%, CA19-9 at between 23.1 and 50%, and CA72-4 at between 18.6 and 58% [14, 18–22], and results of our study on the percentage of marker positivity are within these ranges (CEA: 37.7%; CA19-9: 23.1%; CA72-4: 42.1%; and CA125: 23.2%). As previously reported [19, 20, 22, 23], we also demonstrated that the combined evaluation of the markers in the serum showed a significant increase in the diagnostic sensitivity documented by the percentage of cases where the level of at least one marker was higher than its cut-off value.
To find out the correlation between pretreatment status of tumor markers and different clinicopathologic parameters, we performed a chi-square test, and found that CEA positivity was associated with lymph node involvement; our result was in line with a previous study [22]. In the literature, CA72-4 appeared to be the most sensitive and specific marker in gastric cancer patients, and its positivity was associated with advanced tumor stage, lymph node metastasis, and distant metastasis [18, 19, 21, 24–27]. Our results confirm that it was associated with advanced tumor stage and vascular invasion. In our study, we also found that the positivity of combined detection of four tumor markers was related to deeper tumor invasion, lymph node metastasis, and advance tumor stage. Through these results, we confirmed that the pretreatment levels of tumor markers such as CEA and CA72-4 can predict the biological behavior of gastric cancer, to some extent.
According to univariate and multivariate analyses, we found that patients with pretreatment positive values for tumor markers showed worse prognosis. Survival curves (Kaplan-Meier curves) according to pretreatment levels of CA19-9 and CA72-4 showed significant differences between positive and negative patients, and the two markers were also confirmed to be independent prognostic factors in multivariate analysis; our experience was consistent with previous reports [18–22, 28, 29]. Cox proportional hazards regression analysis indicated that patients with elevated levels of CA19-9 and CA72-4 had a 2.183-fold and 2.500-fold higher risk of death than patients with low levels, respectively. The reason preoperative levels of tumor markers influence long-term survival is not clear; a number of biological factors are probably involved [18, 21, 22].
Previous studies have confirmed that tumor markers can be predictors of the response to chemotherapy in patients with pancreatic cancer, breast cancer, epithelial ovarian cancer, and colorectal cancer [30, 34]; however, clinical studies evaluating the roles of tumor markers in the monitoring of chemotherapeutic efficacy in patients with gastric cancer are limited [9]. In the present study, we pay attention to the utility of tumor markers in monitoring the response to neoadjuvant chemotherapy in patients with gastric cancer. We found that mean levels of tumor markers were decreased after neoadjuvant chemotherapy, especially in the disease control group, which included patients with complete response (CR), partial response (PR), and stable disease (SD); mean levels decreased more significantly than disease progression group. The decreases in CEA, CA72-4, and CA125 levels achieved statistical significance; we saw the same tendency in CA19-9 levels but the difference did not achieve statistical significance. To explain this phenomenon, we reviewed the medical records and found that pretreatment CA19-9 levels in a small number of patients were extremely elevated; all these patients appeared to be in the disease control after neoadjuvant chemotherapy group, and this may be the reason why the decrease of CA19-9 levels did not achieve statistical significance.
Based on the result that tumor markers decreased more significantly in patients with disease control, we did an ROC curve to assess the ability of changes in tumor markers to predict response to neoadjuvant chemotherapy. We found that the decrease of CA72-4 after neoadjuvant chemotherapy could predict pathologic response, and the cutoff value decreased by 71.1%. This means that if CA72-4 levels decrease by more than 71.1%, the patient has more chance to achieve pCR and pPR. We also found that the area under the ROC curve of CA24-2 change values was 0.800; the cutoff value was decreased 40.0% but the difference did not achieve statistical significance. Our inability to demonstrate that the decrease of CA125 can also predict pathologic response to neoadjuvant chemotherapy may be due to the relatively small number of patients analyzed.
Using chi-square analysis, we found positive pretreatment levels of CEA were associated with clinical disease progression after neoadjuvant chemotherapy; and using t-test, we found CEA levels in the disease progression group were higher than the disease control group. Therefore, we speculated that high pretreatment level of CEA may predict clinical disease progression. In order to confirm this, we carried out an ROC curve and found pretreatment level of CEA can predict clinical disease progression after neoadjuvant chemotherapy; the cutoff value was 50 ng/ml. Therefore, if the pretreatment level of a patient is more than 50 ng/ml, he has a higher of being a non-responder.
It is well known that elevated marker levels decrease after curative resection of the tumor, only to elevate again on recurrence [9, 35, 36]. Therefore some researchers have postulated that the change in these tumor markers would reflect the relative tumor burden in individual patients after chemotherapy, when the marker levels had been elevated prior to chemotherapy [9]. We are agree with this speculation as tumor markers can be produced directly by the tumor or non-tumor cells as a response to the presence of a tumor, so a high level of tumor marker may mean a large tumor burden. This may be the reason why high pretreatment levels of tumor markers can predict poor prognosis, and why high CEA levels can predict poor response to neoadjuvant chemotherapy. Tumor burden will be reduced by effective treatment such as neoadjuvant chemotherapy, and tumor shrinkage always accompanies a drop in marker levels, therefore we can see a decrease in tumor markers after neoadjuvant chemotherapy, especially in patients with disease control.
The limitations of our study include that the number of patients analyzed here is very small and missing values still exist. Due to the low sensitivity and specificity, these tumor markers cannot replace other detection methods, such as pathological examination and imaging studies.
Conclusions
In conclusion, the measurement of tumor markers might be useful in the monitoring of response, and in the prediction of prognosis in patients treated with neoadjuvant chemotherapy. This is especially true in the cases in which disease is difficult to evaluate by imaging studies. Further studies are required to confirm these findings.
Acknowledgements
The research was funded by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support, code:XMLX201309. We thank the foundation and all the patients studied in this project.
Abbreviations
- 5-FU
5-Flourouracil
- AJCC
American Joint Committee on Cancer
- AUC
Area Under the Curve
- CA19-9
Carbohydrate Antigen 19–9
- CA242
Carbohydrate Antigen 242
- CA72-4
Carbohydrate Antigen 72–4
- CEA
Carcinoembryonic Antigen
- CR
Complete Response
- CT
Computed Tomography
- CTCs
Circulating Tumor Cells
- DC
Disease Control
- EUS
Endoscopic Ultrasonography
- LV
Leucovorin
- MDT
Multidisciplinary Team
- NAC
Neoadjuvant Chemotherapy
- NCCN
National Comprehensive Cancer Network
- NR
No Response
- OXA
Oxaliplatin
- PD
Progressive Disease
- PR
Partial Response
- ROC Curve
Receiver Operating Characteristic Curve
- SD
Stable Disease
- UICC
International Union Against Cancer
- WHO
World Health Organization.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZS performed design, material and data collecting, the statistical analysis, and drafted the manuscript. NZ participated in its design and coordination, and helped to draft the manuscript. Both authors read and approved the final manuscript.
Contributor Information
Zhipeng Sun, Email: sunzhipeng56084@163.com.
Nengwei Zhang, Email: zhangnw1@sohu.com.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Wang CS, Chao TC, Jan YY. Benefits of palliative surgery for far-advanced gastric cancer. Chang Gung Med J. 2002;25:792–802. [PubMed] [Google Scholar]
- 3.Fink U, Stein HJ, Schuhmacher C, Wilke HJ. Neoadjuvant chemotherapy for gastric cancer: update. World J Surg. 1995;19:509–516. doi: 10.1007/BF00294711. [DOI] [PubMed] [Google Scholar]
- 4.Niederhuber JE. Neoadjuvant therapy. Ann Surg. 1999;229:309–312. doi: 10.1097/00000658-199903000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto J. Neoadjuvant chemotherapy: a standard treatment for locally advanced gastric cancer in the near future? Gastric Cancer. 2003;6:131–133. doi: 10.1007/s10120-003-0254-3. [DOI] [PubMed] [Google Scholar]
- 6.Mezhir JJ, Tang LH, Coit DG. Neoadjuvant therapy of locally advanced gastric cancer. J Surg Oncol. 2010;101:305–314. doi: 10.1002/jso.21483. [DOI] [PubMed] [Google Scholar]
- 7.Vallbohmer D, Holscher AH, Schneider PM. 18 F-fluorodeoxyglucose- positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemotherapy in gastric cancer. J Surg Oncol. 2010;102:135–140. doi: 10.1002/jso.21592. [DOI] [PubMed] [Google Scholar]
- 8.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamao T, Kai S, Kazami A. Tumor markers CEA, CA19-9 and CA125 in monitoring of response to systemic chemotherapy in patients with advanced gastric cancer. Jpn J Clin Oncol. 1999;29:550–555. doi: 10.1093/jjco/29.11.550. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 11.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 12.Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti A. (Eds): AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Ajani JA, Ota DM, Jessup JM. Resectable gastric carcinoma. An evaluation of preoperative and postoperative chemotherapy. Cancer. 1991;68:1501–1506. doi: 10.1002/1097-0142(19911001)68:7<1501::AID-CNCR2820680706>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Becker K, Mueller JD, Schulmacher C. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–1530. doi: 10.1002/cncr.11660. [DOI] [PubMed] [Google Scholar]
- 16.Ninomiya Y, Yanagisawa A, Kato Y. Histological indications of a favorable prognosis with far-advanced gastric carcinomas after preoperative chemotherapy. J Cancer Res Clin Oncol. 1999;125:699–706. doi: 10.1007/s004320050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moehler M, Schimanski CC, Gockel I. (Neo)adjuvant strategies of advanced gastric carcinoma: time for a change? Dig Dis. 2004;22:345–350. doi: 10.1159/000083597. [DOI] [PubMed] [Google Scholar]
- 18.Gaspar MJ, Arribas I, Coca MC, Diez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19–9 and CA 72–4 in gastric carcinoma. Tumour Biol. 2001;22:318–322. doi: 10.1159/000050633. [DOI] [PubMed] [Google Scholar]
- 19.Tocchi A, Costa G, Lepre L. The role of serum and gastric juice levels of carcinoembryonic antigen, CA19.9 and CA72.4 in patients with gastric cancer. J Cancer Res Clin Oncol. 1998;124:450–455. doi: 10.1007/s004320050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ychou M, Duffour J, Kramar A. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers. 2000;16:105–110. doi: 10.1155/2000/595492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ucar E, Semerci E, Ustun H. Prognostic value of preoperative CEA, CA 19–9, CA 72–4, and AFP levels in gastric cancer. Adv Ther. 2008;25:1075–1084. doi: 10.1007/s12325-008-0100-4. [DOI] [PubMed] [Google Scholar]
- 22.Marrelli D, Roviello F, De Stefano A. Prognostic significance of CEA, CA 19–9 and CA 72–4 preoperative serum levels in gastric carcinoma. Oncology. 1999;57:55–62. doi: 10.1159/000012001. [DOI] [PubMed] [Google Scholar]
- 23.Marrelli D, Pinto E, De Stefano A. Clinical utility of CEA, CA 19–9, and CA 72–4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001;181:16–19. doi: 10.1016/S0002-9610(00)00549-3. [DOI] [PubMed] [Google Scholar]
- 24.Byrne DJ, Browning MC, Cuschieri A. CA72-4: a new tumour marker for gastric cancer. Br J Surg. 1990;77:1010–1013. doi: 10.1002/bjs.1800770918. [DOI] [PubMed] [Google Scholar]
- 25.Spila A, Roselli M, Cosimelli M. Clinical utility of CA 72–4 serum marker in the staging and immediate post-surgical management of gastric cancer patients. Anticancer Res. 1996;16:2241–2247. [PubMed] [Google Scholar]
- 26.Carpelan-Holmstrom M, Louhimo J, Stenman UH. CEA, CA 19–9 and CA 72–4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002;22:2311–2316. [PubMed] [Google Scholar]
- 27.Hamazoe R, Maeta M, Matsui T. Ca72-4 compared with carcinoembryonic antigen as a tumor-marker for gastric-cancer. Eur J Cancer. 1992;28A:1351–1354. doi: 10.1016/0959-8049(92)90517-6. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez A, Vizoso F, Allende MT. Preoperative CEA and TAG-72 serum levels as prognostic indicators in resectable gastric carcinoma. Int J Biol Markers. 1996;11:165–171. doi: 10.1177/172460089601100305. [DOI] [PubMed] [Google Scholar]
- 29.Ikeguchi M, Katano K, Saitou H. Pre-operative serum levels of CA72-4 in patients with gastric adenocarcinoma. Hepato-Gastroenterology. 1997;44:866–871. [PubMed] [Google Scholar]
- 30.Iwanicki-Caron I, Di Fiore F, Roque I. Usefulness of the serum carcinoembryonic antigen kinetic for chemotherapy monitoring in patients with unresectable metastasis of colorectal cancer. J Clin Oncol. 2008;26:3681–3686. doi: 10.1200/JCO.2007.15.0904. [DOI] [PubMed] [Google Scholar]
- 31.Hess V, Glimelius B, Grawe P. CA 19–9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132–138. doi: 10.1016/S1470-2045(08)70001-9. [DOI] [PubMed] [Google Scholar]
- 32.Katz MH, Varadhachary GR, Fleming JB. Serum CA 19–9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann Surg Oncol. 2010;17:1794–1801. doi: 10.1245/s10434-010-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Precht LM, Lowe KA, Atwood M, Beatty JD. Neoadjuvant chemotherapy of breast cancer: tumor markers as predictors of pathologic response, recurrence, and survival. Breast J. 2010;16:362–368. doi: 10.1111/j.1524-4741.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 34.Kang S, Kim TJ, Seo SS. Interaction between preoperative CA-125 level and survival benefit of neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol Oncol. 2010;120:18–22. doi: 10.1016/j.ygyno.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Wanebo JH, Stearns M, Schwartz MK. Use of CEA as an indicator of early recurrence and as a guide to a selected second-look procedure in patients with colorectal cancer. Ann Surg. 1978;188:481–493. doi: 10.1097/00000658-197810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attiyeh FF, Stearns MW., Jr Second-look laparotomy based on CEA elevations in colorectal cancer. Cancer. 1981;47:2119–2125. doi: 10.1002/1097-0142(19810501)47:9<2119::AID-CNCR2820470903>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]


