Abstract
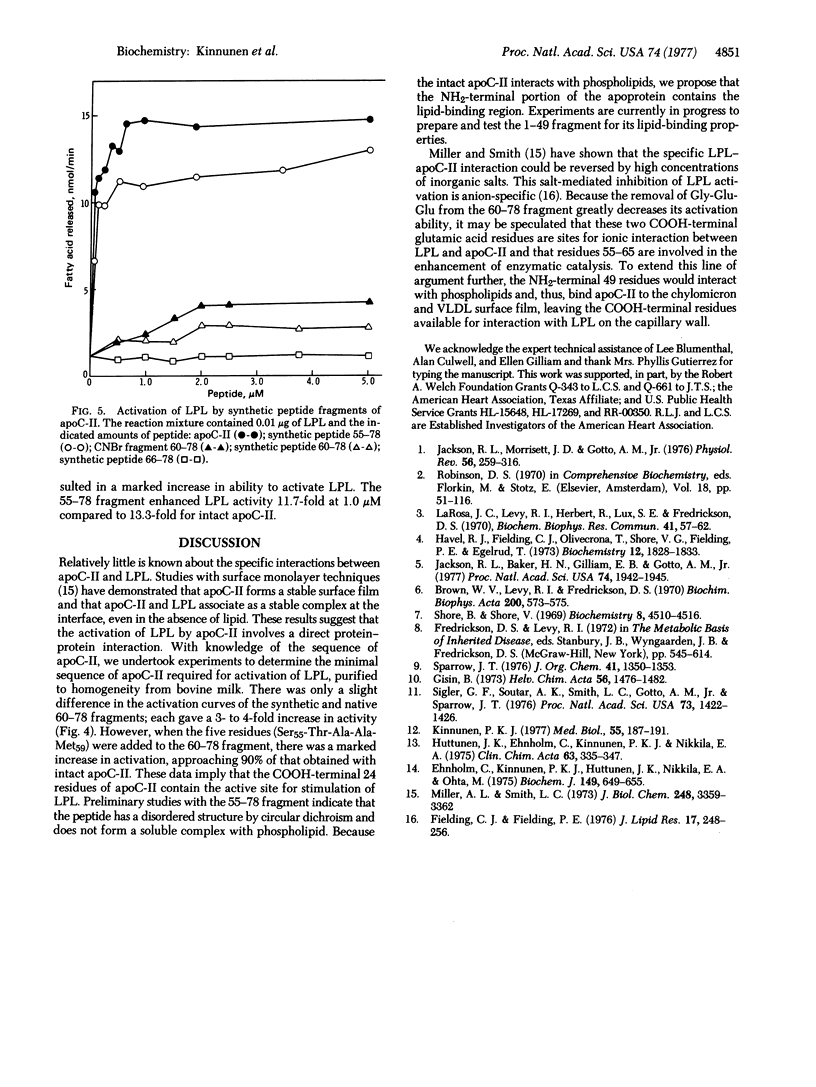
Apolipoprotein C-II (apoC-II), a protein constituent of human very low density lipoproteins, is the activator for lipoprotein lipase (LPL; triacylglycerol acyl-hydrolase, EC 3.1.1.3). The amino acid sequence of the 78 residues of apoC-II has recently been established in this laboratory. To determine the minimal sequence requirements for activation, we have prepared both native and synthetic fragments of apoC-II and tested them for their ability to activate LPL. Cyanogen bromide fragments of apoC-II corresponding to residues 1--9 and 10--59 had little ability to activate LPL. However, the COOH-terminal cyanogen bromide fragment corresponding to residues 60--78 increased hydrolysis 4-fold compared to an average of 9-fold activation for the same concentration of apoC-II. The synthetic peptide containing residues 60--78 prepared by solid-phase techniques enhanced the lipolysis 3-fold. Addition of five residues produced a synthetic fragment 55--78 that enhanced the release of fatty acid 12-fold compared to 13-fold for intact apoC-II. By contrast, the synthetic peptide containing residues 66--78 did not activate. Removal of the three COOH-terminal residues, Gly-Glu-Glu, from fragment 60--78 decreased the ability to activate LPL by greater than 95%. These studies suggest that the maximal activation of LPL by apoC-II requires a minimal sequence contained within residues 55--78.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. V., Levy R. I., Fredrickson D. S. Further separation of the apoproteins of the human plasma very low density lipoproteins. Biochim Biophys Acta. 1970 Mar 31;200(3):573–575. doi: 10.1016/0005-2795(70)90115-7. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Kinnunen P. K., Huttunen J. K., Nikkilä E. A., Ota M. Purification and characterization of lipoprotein lipase from pig myocardium. Biochem J. 1975 Sep;149(3):649–655. doi: 10.1042/bj1490649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Mechanism of salt-mediated inhibition of lipoprotein lipase. J Lipid Res. 1976 May;17(3):248–256. [PubMed] [Google Scholar]
- Havel R. J., Fielding C. J., Olivecrona T., Shore V. G., Fielding P. E., Egelrud T. Cofactor activity of protein components of human very low density lipoproteins in the hydrolysis of triglycerides by lipoproteins lipase from different sources. Biochemistry. 1973 Apr 24;12(9):1828–1833. doi: 10.1021/bi00733a026. [DOI] [PubMed] [Google Scholar]
- Huttunen J. K., Ehnholm C., Kinnunen P. K., Nikkilä E. A. An immunochemical method for the selective measurement of two triglyceride lipases in human postheparin plasma. Clin Chim Acta. 1975 Sep 16;63(3):335–347. doi: 10.1016/0009-8981(75)90055-8. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Baker H. N., Gilliam E. B., Gotto A. M., Jr Primary structure of very low density apolipoprotein C-II of human plasma. Proc Natl Acad Sci U S A. 1977 May;74(5):1942–1945. doi: 10.1073/pnas.74.5.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Morrisett J. D., Gotto A. M., Jr Lipoprotein structure and metabolism. Physiol Rev. 1976 Apr;56(2):259–316. doi: 10.1152/physrev.1976.56.2.259. [DOI] [PubMed] [Google Scholar]
- Kinnunen P. K. Purification of bovine milk lipoprotein lipase with the aid of detergent. Med Biol. 1977 Jun;55(3):187–191. [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Herbert P., Lux S. E., Fredrickson D. S. A specific apoprotein activator for lipoprotein lipase. Biochem Biophys Res Commun. 1970 Oct 9;41(1):57–62. doi: 10.1016/0006-291x(70)90468-7. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Smith L. C. Activation of lipoprotein lipase by apolipoprotein glutamic acid. Formation of a stable surface film. J Biol Chem. 1973 May 10;248(9):3359–3362. [PubMed] [Google Scholar]
- Shore B., Shore V. Isolation and characterization of polypeptides of human serum lipoproteins. Biochemistry. 1969 Nov;8(11):4510–4516. doi: 10.1021/bi00839a043. [DOI] [PubMed] [Google Scholar]
- Sigler G. F., Soutar A. K., Smith L. C., Gotto A. M., Jr, Sparrow J. T. The solid phase synthesis of a protein activator for lecithin-cholesterol acyltransferase corresponding to human plasma apoC-I. Proc Natl Acad Sci U S A. 1976 May;73(5):1422–1426. doi: 10.1073/pnas.73.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow J. T. An improved polystyrene support for solid phase peptide synthesis. J Org Chem. 1976 Apr 16;41(8):1350–1353. doi: 10.1021/jo00870a013. [DOI] [PubMed] [Google Scholar]



