Abstract
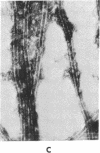
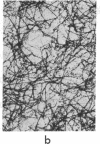
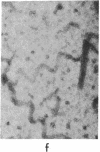
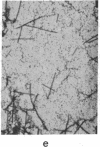
Incubation of 48,000 X g rat brain supernatants for 30 min at 37 degrees with 1-2 mM guanylyl 5'-methylenediphosphonate [Gmp(CH2)pp] results in polymerization of 95-98% of the tubulin present. This is considerably more than the 50% polymerization that can be achieved with the natural nucleotide, GTP, under optimal conditions. Gmp(CH2)pp is also much more effective than GTP in inducing polymerization of purified tubulin. Assembly of microtubules with Gmp(CH2)pp occurs at tubulin concentrations one-third of those possible with GTP. Furthermore, with Gmp(CH2)pp, microtubule assembly does not require the high molecular weight basic proteins needed with GTP. Polymerization of tubulin by Gmp(CH2)pp is neither prevented nor reversed by concentrations of calcium (2 mM) that can either prevent microtubule assembly or disrupt already formed microtubules if the nucleotide used is GTP or guanylyl imidodiphosphate. When Ca2+ is added before or after microtubule assembly, electron microscopy of the Gmp(CH2)pp preparations reveals normal microtubules turning into twisted ribbons. Low temperature (4 degrees) can both prevent and disrupt the tubulin assembled Gmp(CH2)pp although disruption proceeds much more slowly when GTP is used.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Kaziro Y. Effect of guanine nucleotides on the assembly of brain microtubles: ability of 5'-guanylyl imidodiphosphate to replace GTB in promoting the polymerization of microtubules in vitro. Biochem Biophys Res Commun. 1976 Mar 22;69(2):369–376. doi: 10.1016/0006-291x(76)90531-3. [DOI] [PubMed] [Google Scholar]
- Borisy G. G. A rapid method for quantitative determination of microtubule protein using DEAE-cellulose filters. Anal Biochem. 1972 Dec;50(2):373–385. doi: 10.1016/0003-2697(72)90046-2. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Smith H., Taylor E. W. Tublin: nucleotide binding and enzymic activity. J Mol Biol. 1974 Nov 5;89(3):455–468. doi: 10.1016/0022-2836(74)90475-6. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W., Honig L. S., Williams R. C. Quantitative electron microscopy of microtubule assembly in vitro. J Mol Biol. 1975 Dec 5;99(2):263–276. doi: 10.1016/s0022-2836(75)80144-6. [DOI] [PubMed] [Google Scholar]
- MacNeal R. K., Webb B. C., Purich D. L. Neurotubule assembly at substoichiometric nucleotide levels using a GTP regenerating system. Biochem Biophys Res Commun. 1977 Jan 24;74(2):440–447. doi: 10.1016/0006-291x(77)90323-0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975 Jul;14(13):2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J., Dickinson P. J. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976 Sep 21;15(19):4248–4254. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Zeeberg B., Hassid A., Caplow M. Microtubular protein catalytic interactions with nucleotides. J Biol Chem. 1977 Mar 25;252(6):2101–2105. [PubMed] [Google Scholar]