Abstract
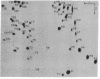
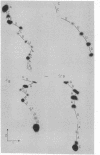
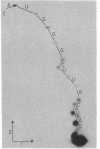
A 3'-terminal oligonucleotide fragment, 161 bases long, can be obtained from each of the four brome mosaic virus RNAs by means of nuclease digestion. Like the four intact brome mosaic virus RNAs, each fragment accepts tyrosine in a reaction catalyzed by wheat germ aminoacyl-tRNA synthetase. The complete nucleotide sequence of the RNA 4 fragment has been determined by use of standard radiochemical methods. Comparative data for the fragments from RNAs 1, 2, and 3 show that they have nearly the same sequence as the RNA 4 fragment. The eight bases adjacent to the 3' terminus of the RNA 4 fragment are identical in sequence to the eight terminal bases of tyrosine tRNA from Torula utilis and eleven interior bases are identical in sequence to eleven bases encompassing the anticodon region of tyrosine tRNA from Saccharomyces cerevisiae, T. utilis, and Escherichia coli. Nevertheless, reasonable base-pairing schemes yield, at best, a distorted cloverleaf secondary structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastin M., Dasgupta R., Hall T. C., Kaesberg P. Similarity in structure and function of the 3'-terminal region of the four brome mosaic viral RNAs. J Mol Biol. 1976 Jun 5;103(4):737–745. doi: 10.1016/0022-2836(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Bastin M., Kaesberg P. Radioactive labelling of borme mosaic virus. J Gen Virol. 1975 Mar;26(3):321–325. doi: 10.1099/0022-1317-26-3-321. [DOI] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand J. P., Richards K. E., Bouley J. P., Witz J., Hirth L. Stucture of the amino-acid accepting 3'-end of high-molecular-weight eggplant mosaic virus RNA. Proc Natl Acad Sci U S A. 1976 Mar;73(3):737–741. doi: 10.1073/pnas.73.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. W. On the recognition of tRNA by its aminoacyl-tRNA ligase. Prog Nucleic Acid Res Mol Biol. 1971;11:489–525. doi: 10.1016/s0079-6603(08)60336-0. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Shih D. S., Saris C., Kaesberg P. Nucleotide sequence of a viral RNA fragment that binds to eukaryotic ribosomes. Nature. 1975 Aug 21;256(5519):624–628. doi: 10.1038/256624a0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Dahlberg J. E., Sawyer R. C., Harada F., Taylor J. M., Levinson W. E., Bishop J. M., Goodman H. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. II. Structure of a 4S RNA primer. J Virol. 1974 May;13(5):1134–1142. doi: 10.1128/jvi.13.5.1134-1142.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Hirth L. Sequence of 71 nucleotides at the 3'-end of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1975 Mar;72(3):864–868. doi: 10.1073/pnas.72.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni A. L., Prochiantz A., Bernard O., Chapeville F. TYMV valyl-RNA as an amino-acid donor in protein biosynthesis. Nat New Biol. 1973 Feb 7;241(110):166–168. doi: 10.1038/newbio241166a0. [DOI] [PubMed] [Google Scholar]
- Hall T. C., Shih D. S., Kaesberg P. Enzyme-mediated binding of tyrosine to brome-mosaic-virus ribonucleic acid. Biochem J. 1972 Oct;129(4):969–976. doi: 10.1042/bj1290969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Dahlberg J. E. Specific cleavage of tRNA by nuclease S1. Nucleic Acids Res. 1975 Jun;2(6):865–871. doi: 10.1093/nar/2.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl R. J., Hall T. C. Loss of infectivity of brome mosaic virus RNA after chemical modification of the 3' or 5' terminus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2682–2686. doi: 10.1073/pnas.74.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers T. A., Blumenthal T., Weber K. Function and structure in ribonucleic acid phage Q beta ribonucleic acid replicase. The roles of the different subunits in transcription of synthetic templates. J Biol Chem. 1974 Sep 25;249(18):5801–5808. [PubMed] [Google Scholar]
- Lindley I. G., Stebbing N. Aminoacylation of encephalomyocarditis virus RNA. J Gen Virol. 1977 Jan;34(1):177–182. doi: 10.1099/0022-1317-34-1-177. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Oberg B., Philipson L. Binding of histidine to tobacco mosaic virus RNA. Biochem Biophys Res Commun. 1972 Aug 21;48(4):927–932. doi: 10.1016/0006-291x(72)90697-3. [DOI] [PubMed] [Google Scholar]
- Pinck M., Genevaux M., Duranton H. Studies on the amino acid acceptor activities of the Eggplant Mosaic Viral RNA and its satellite RNA. Biochimie. 1974;56(3):423–428. doi: 10.1016/s0300-9084(74)80150-1. [DOI] [PubMed] [Google Scholar]
- Pinck M., Yot P., Chapeville F., Duranton H. M. Enzymatic binding of valine to the 3' end of TYMV-RNA. Nature. 1970 Jun 6;226(5249):954–956. doi: 10.1038/226954a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Lodish H. F. Translation in vitro of vesicular stomatitis virus mRNA lacking 5'-terminal 7-methylguanosine. Nature. 1976 Jul 1;262(5563):32–37. doi: 10.1038/262032a0. [DOI] [PubMed] [Google Scholar]
- Rushizky G. W., Mozejko J. H. Optimization of conditions for cleavage of tRNA at the anticodon loop by S1 nucleases. Anal Biochem. 1977 Feb;77(2):562–566. doi: 10.1016/0003-2697(77)90274-3. [DOI] [PubMed] [Google Scholar]
- Salomon R., Littauer U. Z. Enzymatic acylation of histidine to mengovirus RNA. Nature. 1974 May 3;249(452):32–34. doi: 10.1038/249032a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Dasgupta R., Kaesberg P. 7-Methyl-guanosine and efficiency of RNA translation. J Virol. 1976 Aug;19(2):637–642. doi: 10.1128/jvi.19.2.637-642.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P., Hall T. C. Messenger and aminoacylation functions of brome mosaic virus RNA after chemical modification of 3' terminus. Nature. 1974 May 24;249(455):353–355. doi: 10.1038/249353a0. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Lane L. C., Kaesberg P. Origin of the small component of brome mosaic virus RNA. J Mol Biol. 1972 Mar 14;64(2):353–362. doi: 10.1016/0022-2836(72)90503-7. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J. D., Kaesberg P. Amino acid incorporation in an Escherichia coli cell-free system directed by bromegrass mosaic virus ribonucleic acid. Virology. 1967 Nov;33(3):385–397. doi: 10.1016/0042-6822(67)90114-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]