Abstract
A total of 36 consecutive patients with AML in CR underwent reduced-intensity allogeneic hematopoietic SCT (RISCT) with fludarabine and melphalan conditioning. All patients were ineligible for myeloablative transplantation because of age or comorbidity. In total, 30 patients were in first CR and six patients were in second CR. Donors were siblings in 21 (58%) patients and were unrelated in 15 (42%) patients. Hematopoietic cell transplant specific comorbidity scores ≥3 were present in 26 (72%) patients. With a median follow-up of 52 months (range, 34–103 months), OS and PFS rates at 4 years were 71% (s.e., 8%) and 68% (s.e., 8%), respectively. At 4 years, the cumulative incidence of non-relapse mortality was 20% (s.e., 7%) and of relapse mortality was 8% (s.e., 5%). Neither OS nor PFS was affected by older age (>60 years), unrelated donor, melphalan dose, or comorbidity score. At last follow up, of the 24 surviving patients, 21 (88%) had performance status (ECOG) of 0 without any active chronic GVHD requiring steroids. Hence, RISCT with fludarabine and melphalan conditioning produces durable long-term remission in older patients with AML.
Keywords: reduced intensity allogeneic transplantation, AML, hematopoietic SCT
Introduction
Despite advances in our understanding of the molecular mechanisms of disease process and the availability of novel agents for treatment, the outcome of AML in older patients (>60 years) is dismal, with 5-year survival rate ranging from 10 to 15%.1–3 Most patients who achieve remission after induction therapy eventually relapse. Allogeneic transplant reduces the risk of relapse,4,5 but conventional myeloablative allogeneic transplant and conventional conditioning entail a high risk of treatment-related mortality for patients older than 60 years and/or who have comorbidities.4,6,7 Therefore, conventional myeloablative allogeneic transplant is not suitable for most patients with AML.
An alternative for these AML patients is reduced-intensity allogeneic hematopoietic SCT (RISCT), which is based on the premise that the antileukemic activity of an allogeneic transplant is mainly because of graft-vs-leukemia immune effect of allogeneic cells and that conditioning chemotherapy and radiotherapy dose can be decreased to reduce treatment-related mortality, but still allow engraftment of allogeneic cells. Several studies have reported encouraging early results, but long-term outcome data are limited.8–14 To augment these limited data, we analyzed long-term results of RISCT in patients with AML in CR.
Patients and methods
Eligibility
Patients with AML in CR undergoing reduced intensity allogeneic transplant with fludarabine and melphalan conditioning over a 8-year period between April 1998 and Sept 2006 are included in this retrospective analysis. To avoid a selection bias, all patients who had received a transplant, whether on or off protocol, were included in this analysis. Patients included in this analysis had been treated under protocols approved by the institutional review board or with institutional review board approval under the compassionate IND mechanism. All patients had provided written informed consent. The institutional review board approved this analysis. CR was defined as <5% BM blasts, neutrophils >1 × 109/L and platelets >100 × 109/L.15
Conditioning regimen and supportive care
The conditioning regimen had consisted of fludarabine 25–30 mg/m2 for 4–5 days (transplant days −6 or −5 to −2) with melphalan 100 mg/m2 (n=21; 58%) or 140 mg/m2 (n=15; 42%) given on day −2. Anti-thymocyte globulin (horse 40–60 mg/kg or rabbit 4–7.5 mg/kg in divided doses) had been given to 17 patients who had received either an unrelated donor graft (n=15; 42%) or a mismatched-related donor graft (n=2; 6%).16–18 GVHD prophylaxis had consisted of tacrolimus and mini MTX. Standard antimicrobial prophylaxis had been given. Donor BM or G-CSF primed PBPCs had been procured using standard mobilization protocols and apheresis techniques. All donors had provided written informed consent. BM procured from unrelated donors had been obtained through the National Marrow Donor Program according to applicable guidelines. As required by the National Marrow Donor Program, donors had provided informed consent at the donor center.
Statistical analysis
Primary outcomes were OS, PFS, relapse mortality and non-relapse mortality (NRM). We calculated actuarial estimates of OS and PFS using the Kaplan–Meier method. We calculated all outcomes since the date of transplant. PFS was defined as time to disease progression or death. The cumulative incidence of NRM and of relapse mortality was estimated accounting for the competing risks of progression of malignancy and NRM, respectively. Cumulative incidence of chronic GVHD was estimated considering relapse or death in the absence of GVHD as competing events. Predictors of OS and PFS were evaluated using Cox proportional hazards regression model. Statistical significance was defined at the 0.05 level. Analysis was performed using STATA 9.0. (StataCorp. 2005. Stata Statisitcal Software: Release 9. College Station, TX, USA: StataCorp LP.).
Results
Patient characteristics
Detailed characteristics of the 36 consecutive patients are shown in Table 1. The 24 men and 12 women had a median age of 57 years (range, 21–71 years) and had high-risk disease as indicated by antecedent myelodysplastic syndrome (n=9; 25%), therapy-related AML (n=9; 25%) and second remission (n=6). The majority of patients had intermediate (n=22) or poor-risk karyotype (n=10). For 18 patients, the stem cell source had been BM; for the remaining 18 patients, mobilized PBSCs had been used. As would be expected in a clinical trial of a reduced-intensity regimen that included patients who had not qualified for a myeloablative transplant, the majority of the patients had significant comorbidities. A total of 26 (72%) patients had a high score ≥3, and of those, 10 (28%) had a score ≥6, indicating a significant coexisting medical illness.6
Table 1.
Patient characteristics
| Characteristic | N | % (n=36) |
|---|---|---|
| Age | ||
| Median (range) | 57 (21–71) | |
| Sex | ||
| Male | 24 | 67 |
| Female | 12 | 33 |
| Diagnosis | ||
| AML, secondary to MDS | 9 | 25 |
| AML, therapy-related | 9 | 25 |
| AML, de novo | 18 | 50 |
| CMV status (patient/donor) | ||
| Positive | 29 | 81 |
| Negative/negative | 3 | 8 |
| Negative/positive | 4 | 11 |
| FAB | ||
| M0/M1/M2 | 2/3/8 | |
| M4/M5/M6 | 5/6/1 | |
| M7/unclassified | 1/10 | |
| Cytogenetics | ||
| Good | 2 | 5 |
| Intermediate | 22 | 61 |
| Poor | 10 | 28 |
| Unknown | 2 | 5 |
| Stem cell source | ||
| BM | 18 | 50 |
| Blood | 18 | 50 |
| Conditioning regimen | ||
| Melphalan 100 | 21 | 58 |
| Melphalan 140 | 15 | 42 |
| Donor type | ||
| Unrelated, 6/6 matched | 15 | 42 |
| Related, 6/6 matched | 19 | 53 |
| Related, 1 Ag mismatched | 2 | 5 |
| Disease status | ||
| CR1 | 30 | 83 |
| CR2 | 6 | 17 |
| HCT score | ||
| 0–2 | 10 | 28 |
| 3–5 | 16 | 44 |
| ≥6 | 10 | 28 |
Abbreviations: HCT=Hematopoietic cell transplantation; MDS=myelodysplastic syndrome.
Engraftment and chimerism
All patients had engrafted with a median time to neutrophils >0.5×109/L of 12.5 days (range, 8–19 days) and platelets >20×109/L of 16 days (range, 9–39 days). Chimerism studies on day 30 had showed 100% donor hematopoiesis in T and myeloid lineage in 35 (97%) of 36 patients. One patient had 98% donor cells at day 30, but had converted to 100% donor hematopoiesis a month later.
GVHD
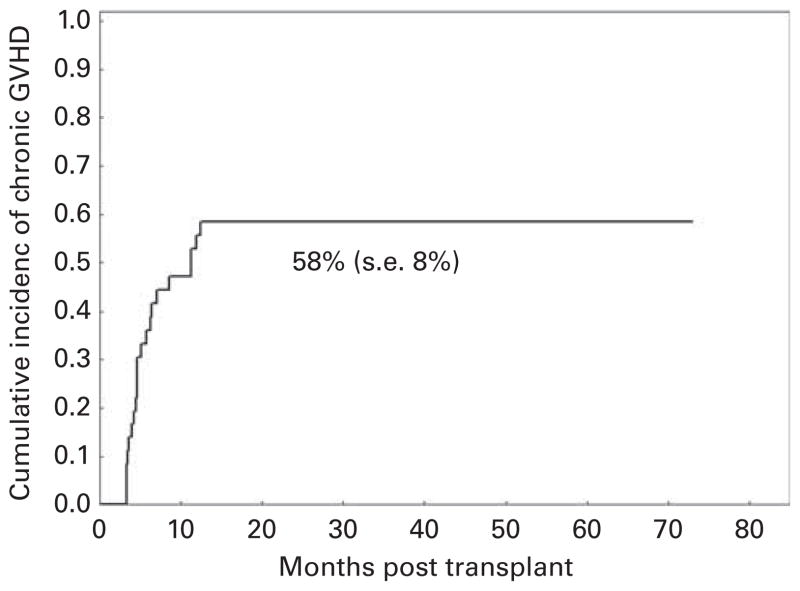
Cumulative incidence of grades II–IV acute GVHD was 25% (s.e., 7%) with 9 of 36 patients developing acute GVHD, which was severe (grades III–IV) in 4 (11%) patients. Cumulative incidence of chronic GVHD (Figure 1) was 58% (s.e., 8%), occurring in 20 patients: 10 (29%) with limited disease and 10 (29%) with extensive disease.
Figure 1.
Incidence of chronic GVHD.
Survival data
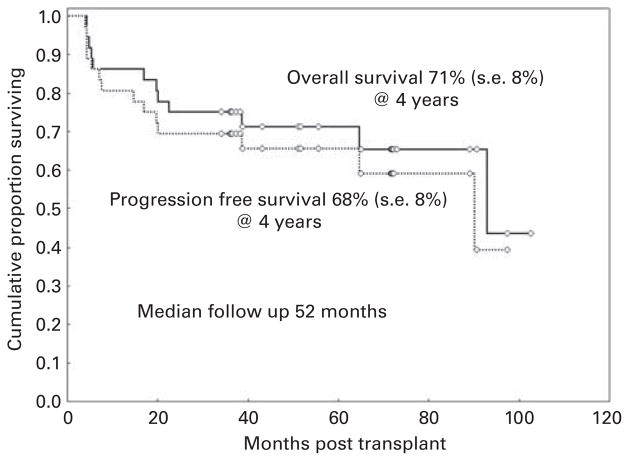
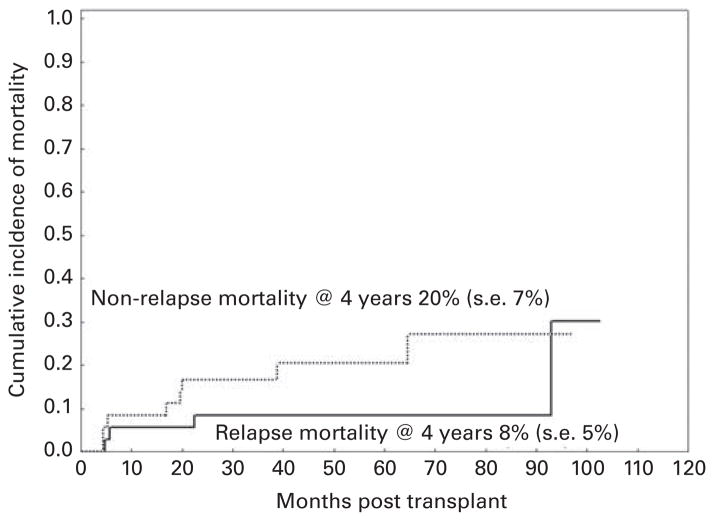
With a median follow-up of 52 months (range, 34–103 months), OS and PFS rates at 4 years were 71% (s.e., 8%) and 68% (s.e., 8%), respectively (Figure 2). At 4 years, the cumulative incidence of NRM was 20% (s.e., 7%) and relapse mortality was 8% (s.e., 5%) (Figure 3). Neither OS nor PFS was significantly affected by older age (>60 years), unrelated donor, dose of melphalan, or comorbidity score in an univariate analysis (Table 2). Overall, 12 patients had died: four of recurrent disease and eight of other causes. Chronic GVHD had been the main cause of death in five of these eight patients who had been in remission at the time of their death. Of the remaining patients, two patients had died of pneumonia and multiorgan failure, and one had died of acute myocardial infarction.
Figure 2.
OS and PFS.
Figure 3.
Cumulative incidence of non-relapse mortality (NRM) and relapse mortality (RM).
Table 2.
Risk factors affecting progression-free and OS
| Progression-free survival
|
OS
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=36 | N events at 4 years | HR | 95% CI | P | N=36 | N died at 4 years | HR | 95% CI | P | |
| Age | ||||||||||
| ≤60 | 23 | 7 | ref | 23 | 5 | ref | ||||
| >60 | 13 | 5 | 1.4 | 0.4–4.4 | 0.6 | 13 | 5 | 2 | 0.6–7.0 | 0.3 |
| Donor type | ||||||||||
| Matched sibling | 19 | 5 | 0.6 | 0.2–1.8 | 0.4 | 19 | 4 | 0.6 | 0.2–2.0 | 0.4 |
| Matched Unrelated | 15 | 6 | ref | 15 | 5 | ref | ||||
| 1 Ag MM related | 2 | 1 | ref | 2 | 1 | ref | ||||
| Melphalan dose | ||||||||||
| 100 | 21 | 7 | ref | 21 | 5 | ref | ||||
| 140 | 15 | 5 | 1 | 0.3–3.2 | 0.9 | 15 | 5 | 1.5 | 0.4–5.1 | 0.5 |
| Disease status | ||||||||||
| CR1 | 30 | 9 | ref | 30 | 7 | ref | ||||
| CR2 | 6 | 3 | 1.9 | 0.5–7.0 | 0.3 | 6 | 3 | 2.3 | 0.6–9.0 | 0.2 |
| Comorbidity score | ||||||||||
| <3 | 11 | 4 | ref | 11 | 2 | ref. | ||||
| 3–5 | 15 | 5 | 0.8 | 0.2–3.1 | 0.8 | 15 | 5 | 1.7 | 0.3–9.0 | 0.5 |
| >5 | 10 | 3 | 0.7 | 0.2–3.2 | 0.7 | 10 | 3 | 1.7 | 0.3–10.2 | 0.6 |
Abbreviations: CI=confidence interval; HR=hazards ratio.
Long-term data for survivors
Of the 24 patients who were still alive at last follow-up, 22 (92%) had been in remission after the transplant, and two (8%) remaining patients had been in remission after a second allograft for recurrent disease. Of these 24 patients, 21 (88%) had a performance status of 0 without any active chronic GVHD. These patients were off all immunosuppressive agents (n=15) or had been on tapering doses of tacrolimus (n=6), but not steroids. Three patients had some sequelae from the transplant process: one patient had post-herpetic neuralgia, one patient had extremity weakness because of polyneuropathy and required long-term hemodialysis for end-stage renal disease, and one patient, who had been non-compliant with immunosuppressive medications, had active, extensive, chronic GVHD.
Discussion
In this retrospective study, we report long-term results of RISCT for older patients (median age, 57) with AML in CR: with a median follow-up of 52 months at 4 years, OS, PFS and NRM rates were 71, 68 and 20%, respectively, with the majority (88%) of surviving patients having normal performance status without any need for significant immunosuppression. These findings are particularly important, as all these patients were ineligible for myeloablative transplant because of older age or comorbidities and because their anticipated prognosis with standard chemotherapy was guarded. That two-thirds of patients had remained in long-term remission certainly indicates that RISCT should be a treatment option for most patients with high-risk AML in CR and who are unable to receive a conventional myeloablative allogeneic transplant, regardless of age or comorbidities. Because of a small sample size and limited number of events, we could not identify any factors predictive of OS or PFS.
Consistent with our results, several research groups have reported outcomes after RISCT in older patients with AML in remission that appear better than those reported with standard therapy. OS rates of 34–79% at 2 years after transplant has been reported in these studies with RISCT in patients with AML in remission.8–14 Our results confirm those findings with a longer follow-up period and demonstrate that the majority of patients wean off immunosuppressants and achieve normal functional status.
Although different reduced-intensity conditioning regimens were used in those studies,8–14 the best reduced-intensity regimen for patients with AML in remission remains to be identified. In an attempt to further reduce the toxicity without affecting the relapse rate, the dose of melphalan in our study patients had been reduced from 140 mg/m2 to 100 mg/m2. In all, 58% of our patients had 100 mg/m2 of melphalan and had outcomes similar to those of patients who had received melphalan 140 mg/m2 (Table 2).
A much larger randomized study will be needed to identify the agent, which will yield better results when added to fludarabine as a part of a conditioning regimen; melphalan 100 mg/m2, melphalan 140 mg/m2, BU and low-dose total-body radiation are all suitable alternatives. Although PFS and OS in this study are comparable with those obtained with myeloablative regimens, RISCT cannot be recommended for younger patients who are able to undergo conventional myeloablative transplant in the absence of a comparative phase III trial, despite several reports of similar outcomes between two regimens in retrospective studies.10,19
What is the role of comorbidities or donor type? As measured by HCT index,6 NRM does not correlate with higher comorbidity score in our study, which is possibly because of a smaller number of deaths caused by something other than recurrent disease. However, for patients in CR with comorbidities, the reduced-intensity regimen may be more tolerable. Likewise, our results were similar for unrelated and related donors as previously reported.10,20,21 Thus, age, donor type and comorbidities should not deter patients with AML in remission from undergoing RISCT.
Although a limitation of our study is inclusion of a small number of patients and a single-center selection bias, our study did include all consecutive patients with AML in CR undergoing RISCT with this regimen at our center, whether they were treated on or off protocol. Small sample size reflects the knowledge that fewer older patients achieve remission than do younger patients,2 and a suitable donor can only be found in a timely manner in a small fraction of these patients.22 Therefore, every attempt should be made to improve induction chemotherapy and remission rates in these older patients, and a donor search should begin when the disease is diagnosed. Despite these limitations, if a suitable donor can be identified for older and infirm patients who have AML in remission, RISCT should be a treatment consideration, as it can lead to long-term remission and a possible cure.
Footnotes
Conflict of interest
Richard Champlin has received consultant/advisory fees from Genzyme; and research funding from Otsuka and Genzyme. The remaining authors declare no competing financial interests.
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 4.Hamadani M, Awan FT, Copelan EA. Hematopoietic stem cell transplantation in adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2008;14:556–567. doi: 10.1016/j.bbmt.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)- specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 8.Baron F, Storb R. Hematopoietic cell transplantation after reduced-intensity conditioning for older adults with acute myeloid leukemia in complete remission. Curr Opin Hematol. 2007;14:145–151. doi: 10.1097/MOH.0b013e3280168462. [DOI] [PubMed] [Google Scholar]
- 9.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 11.Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24:444–453. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 12.van Besien K, Artz A, Smith S, Cao D, Rich S, Godley L, et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5728–5738. doi: 10.1200/JCO.2005.15.602. [DOI] [PubMed] [Google Scholar]
- 13.Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23:9387–9393. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 14.Grigg AP, Gibson J, Bardy PG, Reynolds J, Shuttleworth P, Koelmeyer RL, et al. A prospective multicenter trial of peripheral blood stem cell sibling allografts for acute myeloid leukemia in first complete remission using fludarabine-cyclophosphamide reduced intensity conditioning. Biol Blood Marrow Transplant. 2007;13:560–567. doi: 10.1016/j.bbmt.2006.12.449. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 17.Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 18.Oran B, Giralt S, Saliba R, Hosing C, Popat U, Khouri I, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant. 2007;13:454–462. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 20.Schetelig J, Bornhauser M, Schmid C, Hertenstein B, Schwerdtfeger R, Martin H, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26:5183–5191. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- 21.Walter RB, Pagel JM, Gooley TA, Petersdorf EW, Sorror ML, Woolfrey AE, et al. Comparison of matched unrelated and matched related donor myeloablative hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Leukemia. 2010;24:1276–1282. doi: 10.1038/leu.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]