Deficiency in 25-hydroxyvitamin D (25[OH]D) is a treatable condition that has been associated with coronary artery disease and many of its risk factors. A practical time to assess for 25(OH)D deficiency, and to initiate treatment, is at the time of an acute myocardial infarction. The prevalence of 25(OH)D deficiency and the characteristics associated with it in patients with acute myocardial infarction are unknown. In this study, 25(OH)D was assessed in 239 subjects enrolled in a 20-hospital prospective myocardial infarction registry. Patients enrolled from June 1 to December 31, 2008, had serum samples sent to a centralized laboratory for analysis using the DiaSorin 25(OH)D assay. Normal 25(OH)D levels are >30 ng/ml, and patients with levels <30 and >20 ng/ml were classified as insufficient and those with levels <20 ng/ml as deficient. Vitamin D levels and other baseline characteristics were analyzed with the linear or Mantel-Haenszel trend test. Of the 239 enrolled patients, 179 (75%) were 25(OH)D deficient and 50 (21%) were insufficient, for a total of 96% of patients with abnormally low 25(OH)D levels. No significant heterogeneity was observed among age or gender subgroups, but 25(OH)D deficiency was more commonly seen in non-Caucasian patients and those with lower social support, no insurance, diabetes, and lower activity levels. Higher parathyroid hormone levels (45.3 vs 32.7 pg/ml, p _ 0.029) and body mass indexes (31.2 vs 29.0 kg/m2, p _ 0.025) were also observed in 25(OH)D-deficient subjects. In conclusion, vitamin D deficiency is present in almost all patients with acute myocardial infarction in a multicenter United States cohort.
From the current body of evidence, it is clear that there is an association between cardiovascular (CV) disease and vitamin D deficiency.1–5 However, the prevalence of vitamin D deficiency as well the characteristics associated with it in patients presenting with acute myocardial infarction (AMI) is unknown. This is an important gap in knowledge, because vitamin D deficiency is readily treatable and, if common in patients with AMI, can identify an opportunity to recognize, initiate treatment, and potentially improve the outcomes of deficient patients. This was a substudy of the Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) registry to describe the prevalence of vitamin D deficiency at the time of AMI care.
Methods
We studied subjects enrolled in TRIUMPH. This registry collected information on patients admitted for AMI through chart abstraction, detailed patient interviews, as well as serum samples at 24 United States hospitals from April 11, 2005, to December 31, 2008. Inclusion criteria were very similar to TRIUMPH's predecessor, the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER),6 and included patients aged >18 years with biomarker evidence of myocardial injury (elevated troponins or creatinine kinase-MB), supporting evidence of AMI (e.g., prolonged ischemic signs or symptoms, electrocardiographic ST-segment changes) who presented <24 hours after symptom onset.6
In the last 239 patients consenting to baseline blood work in the enrollment period of June 1 to December 31, 2008, patients also had 25-hydroxyvitamin D (25[OH]D) levels quantified from the centralized laboratory that was performing all other analysis. Data in TRIUMPH were collected through chart abstraction, standardized in-depth interviews by trained hospital research staff members 24 to 72 hours after AMI admission, and serum sample collection during admission. Patient data included demographics including age, gender, and race as well as marital status, education, access to health insurance, and employment status, region of country, as well as season at time of admission. Collected clinical variables included hypercholesterolemia, hypertension, peripheral arterial disease, diabetes mellitus, previous AMI, previous angina, previous percutaneous coronary intervention or coronary artery bypass surgery, previous stroke, chronic lung disease, chronic renal failure, chronic heart failure, nonskin cancer, smoking, body mass index, family history of coronary artery disease, and history of depression. Other collected data included blood pressure, apolipoprotein A1, glycosylated hemoglobin, insulin, troaponin, pro– brain natriuretic peptide, calcium, phosphate, a lipid panel, and 25(OH)D and parathyroid hormone.
Enrolled patients’ serum samples were sent to a centralized laboratory (CRL, Lenexa, Kansas) for analysis using the DiaSorin (Saluggia, Italy) 25(OH)D assay. Normal 25(OH)D levels are >30 ng/ml, and we classified patients with levels <30 and >20 ng/ml as insufficient and those with levels <20 ng/ml as deficient (Table 1).4,7,8
Table 1.
Vitamin D status
| Serum 25(OH)D (ng/mL) | Vitamin D Status |
|---|---|
| ≤ 20 | Deficient |
| 21–29 | Insufficient |
| ≥30 | Sufficient |
The DiaSorin 25(OH)D assay uses an in vitro radioimmunoassay for quantitative determination of 25(OH)D and other hydroxylated vitamin D metabolites in human serum or plasma.9 This assay consists of a 2-step procedure in which (1) a rapid extraction of 25(OH)D and other hydroxylated metabolites from serum or plasma with acetonitrile is performed, at which time (2) the sample is assayed using an equilibrium radioimmunoassay procedure based on an antibody with specificity to 25(OH)D. The sample, antibody, and tracer are incubated for 90 minutes at 20°C to 25°C. Phase separation is accomplished after a 20-minute incubation at 20°C to 25°C with a second antibody precipitating complex. A nonspecific binding/addition buffer is added after this incubation before centrifugation to aid in reducing nonspecific binding.9 Assay precision was evaluated at Dia-Sorin by testing 4 control levels spanning the curve over 23 operating days in 40 assays. Each assay included the 4 controls X 2 extractions (4 replications).9 Levels of 25(OH)D and other baseline characteristics were analyzed using linear or Mantel-Haenszel trend tests.
Results
During the enrollment period of June 1 to December 31, 2008, 239 patients from 20 of the 24 TRIUMPH sites consented to baseline blood work and had 25(OH)D levels assessed via the DiaSorin radioimmunoassay method. The total population mean age was 57.6 ± 11.4 years, and 73.2% of the enrolled subjects were men (Table 2).
Table 2.
Univariate association with vitamin D deficiency and insufficiency or Sufficiency
| Variable | 25(OH)D (ng/ml) |
p Value | |
|---|---|---|---|
| ≤20 (n = 179) | >20 (n = 60) | ||
| Age (yrs) | 57.0 ± 11.5 | 59.4 ± 10.9 | 0.151 |
| Men | 129 (72%) | 46 (77%) | 0.487 |
| Women | 50 (28%) | 14 (23%) | |
| Caucasian | 127 (71%) | 51 (85%) | 0.031 |
| BMI (kg/m2) | 31.2 ± 6.6 29. | 0 ± 5.5 | 0.025 |
| Obese (BMI ≥30 kg/m2) | 83 (49%) | 27 (47%) | 0.791 |
| Marital status | |||
| Married | 98 (55%) | 41 (68%) | 0.073 |
| Divorced/separated | 46 (26%) | 12 (20%) | |
| Widowed | 11 (6%) | 3 (5%) | |
| Single | 23 (12%) | 4 (7%) | |
| Avoid service | 61 (34%) | 13 (22%) | 0.092 |
| Education | |||
| Did not finish high school | 33 (19%) | 5 (8%) | 0.129 |
| Completed high school | 42 (24%) | 15 (25%) | |
| Some college/vocational school | 60 (34%) | 22 (37%) | |
| Graduated from college | 28 (16%) | 12 (20%) | |
| Postgraduate degree | 15 (8%) | 6 (10%) | |
| High school | 145 (82%) | 55 (92%) | 0.063 |
| Low social support | 31 (18%) | 3 (5%) | 0.015 |
| No health insurance | 42 (24%) | 6 (10%) | 0.026 |
| Medical costs burden | 0.307 | ||
| Severe burden | 23 (13%) | 4 (7%) | |
| Moderate burden | 12 (7%) | 5 (9%) | |
| Somewhat of a burden | 25 (14%) | 7 (11%) | |
| A little burden | 12 (7%) | 6 (10%) | |
| No burden at all | 103 (59%) | 37 (63%) | |
| Live alone | 42 (24%) | 9 (15%) | 0.161 |
| Region | 0.093 | ||
| Midwest | 72 (48%) | 20 (39%) | |
| Northeast | 19 (13%) | 5 (10%) | |
| Southeast | 28 (19%) | 10 (19%) | |
| Southwest | 25 (17%) | 14 (27%) | |
| West | 6 (4%) | 3 (6%) | |
| Season 0.124 | |||
| April to June | 2 (1%) | 0 (0%) | |
| July to September | 67 (38%) | 31 (52%) | |
| October to December | 109 (61%) | 29 (48%) | |
Data are expressed as mean ± SD or as number (percentage). Continuous variables were compared using linear trend tests. Categorical variables were compared using Mantel-Haenszel trend tests.
BMI = body mass index.
No significant heterogeneity was observed among age or gender subgroups (Table 2), but vitamin D deficiency was more commonly seen in non-Caucasian patients and those with lower social support, no insurance, diabetes mellitus, and lower activity levels. Higher parathyroid hormone levels (45.3 vs 32.7 pg/ml, p = 0.029) and body mass indexes (31.2 vs 29.0 kg/m2, p = 0.025) were also observed in 25(OH)D-deficient subjects.
No significant differences were observed among regions and seasons of enrollment. However, 48% of the subjects with 25(OH)D <20 ng/ml and 38.5% of the subjects with 25(OH)D >20 ng/ml were enrolled from the Midwest. Additionally, 61.2% of the subjects with 25(OH)D <20 ng/ml and 48.3% of the subjects with 25(OH)D >20 ng/ml were enrolled between October and December, with only 1.1% and 0%, respectively, being enrolled from April to June.
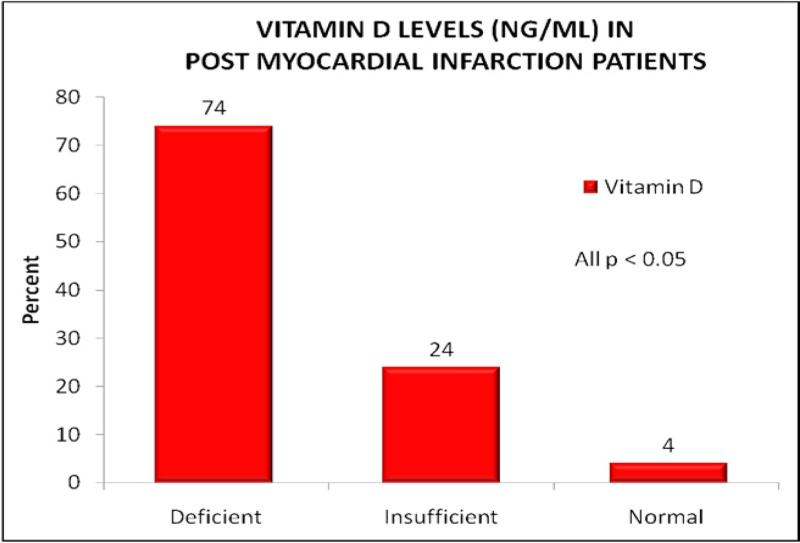
At baseline, 179 subjects (75%) were found to have 25(OH)D levels <20 ng/ml, which is in the deficient range. Another 50 subjects were in the insufficient range, with vitamin D levels of 20 to 30 ng/ml. This placed a total of 229 of 239 subjects (96%) in the suboptimal range of 25(OH)D (normal range >30 ng/ml; Figure 1).
Figure 1.
Vitamin D levels in post-AMI patients.
Discussion
This study describes the vitamin D status in post-AMI patients from a diverse population enrolled from across the United States from 20 separate sites, including academic and private institutions. Our findings of an extraordinarily high prevalence of vitamin D deficiency or insufficiency (96%) in the patients admitted for AMI are consistent with data associating CV disease and many of its risk factors with 25(OH)D deficiency.4 In addition to the findings of a high prevalence of 25(OH)D deficiency in patients with AMI, we also confirmed previously described associations between demographic traits and vitamin D deficiency, including subjects with darker skin,10 diabetes mellitus,11,12 and higher body mass indexes.5
Research has demonstrated that skin pigmentation plays an important role in the ability of human beings to produce vitamin D: lighter skinned individuals can produce larger amounts of 25(OH)D with a fixed amount of ultraviolet B radiation.13 This supports our observation of a higher proportion of non-Caucasians in the 25(OH)D-deficient group. The observation that patients with 25(OH)D deficiency had significantly higher body mass indexes compared to those with sufficient levels of 25(OH)D supports research that suggests sequestering of 25(OH)D in adipose tissue and decreasing the amount of circulating 25(OH)D.14
Although vitamin D deficiency has been strongly associated with CV risk factors, inflammation, and adverse CV outcomes, randomized trials have not yet been done to demonstrate that normalizing vitamin D levels will improve CV health and prognosis. However, vitamin D deficiency adversely affects many aspects of general health, especially with regard to musculoskeletal and immunologic function.2 Thus, it is reasonable to screen patients with AMI with a vitamin D level and correct deficient levels according to nationally established consensus guidelines to optimize overall health.
Some of the other observations made in this study, such as the tendency for 25(OH)D-deficient subjects to lack health insurance, lack social support, or have lower activity levels, have no direct link to 25(OH)D deficiency. Perhaps those subjects with no health insurance or lacking social support also lack the means to supplement their diet with nutritional supplements. Subjects with lower activity levels living more sedentary lifestyles may stay indoors more and have less sunlight exposure for adequate 25(OH)D production. No significant differences between 25(OH)D-deficient and nondeficient groups were observed in seasonal variation and geographic location.
Some limitations of this study include the small number of enrollees during 1 part of the year. Very few patients were enrolled in the spring, and no patients were enrolled from January to May. Although we lacked enrollees from the months when 25(OH)D deficiency should be at its peak, we were still able to show a large proportion of patients with 25(OH)D deficiency. The addition of the winter months would almost certainly show higher prevalence rates of 25(OH)D deficiency. And although we had a relatively small number of enrollees, we still had a diverse group enrolled from 20 of the 24 centers participating in the TRIUMPH registry. Another important limitation was the lack of an adequate control group with normal vitamin D levels. Because of the overwhelming proportion of patients who were vitamin D insufficient or deficient, we did not have an adequate group for comparison.
In conclusion, vitamin D deficiency is present in almost all patients with AMI in a ulticenter United States cohort. Prospective studies are needed to investigate the benefits of screening and treatment of this very common vitamin deficiency.
Acknowledgment
We wish to thank Lori J. Wilson for her help in the preparation of this report.
References
- 1.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)—evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 8.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Binkley N, Lensmeyer G. 25-Hydroxyvitamin D assays and their clinical utility. In: Bendich A, ed. Vitamin D: Physiology, Molecular Biology, and Clinical Applications. Springer. 2010:383–399. [Google Scholar]
- 10.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 11.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 12.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29:722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol. 1991;127:536–538. [PubMed] [Google Scholar]
- 14.Lin E, Armstrong-Moore D, Liang Z, Sweeney JF, Torres WE, Ziegler TR, Tangpricha V, Gletsu-Miller N. Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity (Silver Spring) 2011;19:588–594. doi: 10.1038/oby.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]