Abstract
Aims
Glucagon like peptide-1 receptor (GLP-1R) agonists and leptin each exert anorexigenic effects. In combination, the intake inhibitory and weight loss effects are greater than either treatment alone, however the mechanisms unclear.
Materials and methods
Effects of liraglutide (a long-acting GLP-1 analogue) and leptin co-treatment, delivered in low or moderate doses subcutaneously (SC) or to the 3rd ventricle respectively, on cumulative intake, meal patterns, and hypothalamic expression of intracellular signaling proteins [phosphorylated signal transducer and activator of transcription-3 (pSTAT3) and protein tyrosine phosphatase-1B (PTP1B)] were examined in lean rats.
Results
A low-dose combination of liraglutide (25μg/kg) and leptin (0.75μg) additively reduced cumulative food intake and body weight, a result mediated predominantly through a significant reduction in meal frequency that was not present with either drug alone. Liraglutide treatment alone also reduced meal size; an effect not enhanced with leptin co-administration. Moderate doses of liraglutide (75μg/kg) and leptin (4μg) examined separately each reduced meal frequency, cumulative food intake, and body weight; only liraglutide reduced meal size. In combination these doses did not further enhance the anorexigenic effects of either treatment alone. Ex vivo immunoblot showed elevated pSTAT3 in hypothalamic tissue following liraglutide-leptin co-treatment, an effect greater than leptin treatment alone. In addition, SC liraglutide reduced expression of PTP1B (a negative regulator of leptin receptor signaling), revealing a potential mechanism for the enhanced pSTAT3 response following liraglutide-leptin co-administration.
Conclusions
Collectively, these results provide novel behavioral and molecular mechanisms underlying the additive reduction in food intake and body weight following liraglutide-leptin combination treatment.
Introduction
The prevalence of obesity in the United States has increased by 75% since the early 1980s (1, 2) with obesity-related US health care costs currently exceeding $140 billion per year (3). The sustained weight loss needed to substantially reduce risk for negative health co-morbidities in obese individuals is rarely achieved by diet and exercise alone. The incretin hormone glucagon-like peptide-1 (GLP-1) is a promising target biological system for the pharmacological treatment of obesity (4, 5). Liraglutide and exenatide (synthetic exendin-4) are long-acting GLP-1 receptor (GLP-1R) agonists that improve glycemic control and are FDA-approved for type-2 diabetes mellitus treatment. While not yet FDA-approved for obesity treatment, liraglutide and exenatide slow gastric emptying and reduce food intake and body weight in human populations and animal models (4, 6-8). These GLP-1 analogs produce some degree of weight loss as a mono-drug therapy; however, recent attention is on the potential for combination-based pharmacotherapies to increase the magnitude of weight loss effects (9, 10). Indeed, combined peripheral treatment of leptin, a hormone produced by adipose tissue with potent anorectic effects, and the GLP-1R agonist, exendin-4, produces greater intake reduction and weight loss in rats than either treatment alone (11, 12). Furthermore, central GLP-1R blockade via forebrain intracerebroventricular (ICV) delivery of exendin-(9-39) attenuates food intake suppression by ICV leptin (13, 14). Hindbrain neurons are a critical site for leptin receptor (LepRb) and GLP-1R interaction as exendin-4 and leptin co-delivered to the hindbrain (4th ICV) yields additive food intake and body weight reduction, and 4th ICV exendin-(9-39) attenuates the intake reduction by hindbrain leptin delivery (15). The hypothalamus is also critical for LepRb and GLP-1R interaction as leptin increases GLP-1 peptide (16) and GLP-1R mRNA expression (17) in hypothalamic neurons.
The behavioral mechanisms through which leptin and GLP-1R agonists combine to reduce feeding are unknown. Peripheral exendin-4 treatment in rhesus macaques reduces feeding through suppression of meal size, with no effect on meal frequency (18). In rodents, peripheral exendin-4 reduces both meal size and meal frequency (19), whereas exendin-4 administration to the ventral tegmental area (VTA) (20) or to the ventral hippocampus (21) reduces intake via a specific reduction in meal size. Similarly, peripheral liraglutide treatment reduces food intake via meal size reduction in minipigs (8).
Like GLP-1Rs, activation of LepRbs reduces food intake, at least in part, by reducing meal size. Chronic peripheral leptin treatment reduces meal size with minimal impact on meal frequency (22, 23). Similarly, leptin-deficient mice (24) and rats with virally mediated, chronic LepRb “knockdown” in NTS neurons (25) consume larger meals without substantially altered meal frequency compared to controls. In contrast to these findings, acute ICV leptin administration reduces meal frequency in rats with only minimal effect on meal size (26). It is unknown whether the additive anorectic effects of GLP-1R and LepRb co-administration occur by reducing meal size, meal frequency, or both. The present study examines the cumulative food intake, body weight, and meal pattern effects of low and moderate dose combinations of peripheral (SC) liraglutide and central (3rd ICV) leptin co-administration in rats.
Present experiments also pursue whether common or complementary intracellular signaling mechanisms underlie the additive food intake reduction achieved with combined GLP-1R and LepRb activation. LepRb binding induces rapid tyrosine-phosphorylation of signal transducer and activator of transcription-3 (pSTAT3), a transcription factor that is commonly used as a marker of LepRb-mediated neuronal activation (27). Recent data show that exendin-4 also elevates pSTAT3 in hypothalamic and hindbrain neurons following ICV administration (28). Therefore, the intracellular pSTAT3 signaling pathway is a potential molecular target underlying the enhanced food intake and body weight reduction by combined leptin and GLP-1R agonists. Using an ex vivo approach, experiments examined whether combined SC liraglutide and 3rd ICV leptin administration increases pSTAT3 beyond that observed following leptin treatment alone. The impact of SC liraglutide treatment on activation of protein tyrosine phosphatase-1B (PTP1B), a negative regulator of LepRb signaling, was investigated as a potential mechanism for the augmented leptin-mediated pSTAT3 effects following liraglutide-leptin co-treatment. Collectively our results reveal novel behavioral and neurobiological mechanisms through which leptin and GLP-1R agonist combination treatment enhance the feeding and body weight suppressive effects of either treatment alone.
Materials and Methods
Subjects
Adult male Sprague-Dawley rats (Charles River; 325-450g during experimental procedures) housed individually under a reverse 12h light/dark cycle (lights on 10.00h) for at least 2.5 weeks before procedures, had ad libitum access to chow (LabDiet; 5001) and water except where noted. All procedures conformed to and received approval from The University of Pennsylvania Institutional Animal Care and Use Committee.
Surgery
Under ketamine (90mg/kg), xylazine (2.7mg/kg), and acepromazine (0.64mg/kg) anesthesia and analgesia (Metacam 2mg/kg), guide cannulae (Plastics One; 26-guage) cemented to the skull using jewelers screws were implanted with its tip stereotaxically positioned 2.0mm above the 3rd ventricle at the following coordinates: 2.0mm caudal to bregma, 7.7mm ventral to skull surface, on midline. Anatomical positions of 3rd ICV injection sites were evaluated 1wk post-surgery by measurement of the cytoglucopenia-induced sympatho-adrenal mediated glycemic effect resulting from 210μg (2μl) of 5-thio-D-glucose (29).
Procedures
Food intake, meal patterns, and body weight analyses
Food was removed 30-min prior to the first injection and rats received 3rd ICV injections (1μL) of leptin (National Hormone & Peptide Program) or vehicle (NaHCO3). Fifteen minutes later, each rat received a SC injection (1mL/kg) of liraglutide (gift of Novo Nordisk, Bagsvaerd, Denmark) or vehicle (sterile saline), which occurred immediately before lights off. Pharmacological studies used a within-subjects design, with treatments separated by 3-4 days. Doses were selected to be in the low to moderate range for food intake reduction based on dose response curves for peripheral liraglutide (30) and ICV leptin (31). Cumulative intake was measured with an automated feeding system (DiaLog Instruments). Individually housed rats had access to a food cup on a load cell circuit that communicated with an interface and computer with customized software (LabVIEW, National Instruments). The weight of the food cup was measured every 10sec, enabling assessment of meal parameters. A meal was defined as an episode of feeding in which at least 0.25g was ingested, with meal termination criterion as the beginning of a pause in ingestion >10min, as previously described (25, 32). Data were objectively calculated using a custom Microsoft Excel macro.
Ex vivo immunoblot pSTAT3 and PTP1B analyses
For pSTAT3 immunoblot analyses, pharmacological treatments were carried out (four groups, n=6-7 per group), with the same injection timing parameters as described above, using a 6.0μg dose of leptin (ICV) and a 50μg/kg dose of liraglutide (SC). Consistent with our previous work that combined behavioral and ex vivo approaches (32, 33), higher doses were used for ex vivo signaling analyses compared to the behavioral analyses in order to optimize the ability to detect activated intracellular signaling pathways. 45-min following the SC injections (1hr following ICV injections), rats were sacrificed by decapitation. Brains were rapidly removed and the hypothalamus was extracted and flash frozen in isopentane and stored at -80°C. For PTP1B immunoblot analyses (n=9 per group), rats were sacrificed 45-min following SC injections.
Hypothalamic tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer. Hypothalamic lysates were subjected to SDS-PAGE and transferred to PVDF membranes for immunoblot analysis as previously described. Immunoreactivity was visualized using enhanced chemiluminescence (BioRad; Chemidoc XRS). Phosphorylated and total STAT3 antibodies (Cell Signaling; 1:1000 dilution) were used to evaluate pSTAT3 activity normalized to total STAT3. PTP1B (Santa Cruz; 1:500 dilution) protein expression was normalized to β Actin loading control. Blots were quantified using densitometry analysis in software (Image J; National Institute of Health).
Data and Statistical Analyses
All statistical analyses for behavioral measures employed repeated measures analysis of variance (ANOVA; separate analysis conducted for each time point) using ICV and SC drug as factors. When significant main effects of either drug were detected, Newman-Keuls posthoc tests were used to compare individual treatments. Immunoblot analyses were analyzed using One-Way ANOVA. Alpha level for significance was 0.05. Statistical analyses were conducted using Statsoft software (Statistica V10).
Results
Low dose combination: cumulative food intake, delta body weight, and meal patterns
SC liraglutide (25μg/kg) and ICV leptin (0.75μg) each reduced cumulative food intake relative to vehicle/vehicle treatment at 6hr, 12hr, and 24hr (Figure 1; Ps<0.05). Combined treatment yielded significantly greater intake reduction than either treatment alone at 6hr, 12hr, and 24hr (Ps<0.05 compared to each treatment). Body weight was significantly reduced 24hr after injections only by the combination treatment, which was significantly different from either drug treatment alone and from vehicle-vehicle treatment (Ps<0.05). SC liraglutide treatment and the combined treatment reduced meal size compared to vehicle-vehicle treatment at 6hr, 12hr, and 24hr (Figure 2, Ps<0.05). The combination treatment effect on meal size, however, was not different from SC liraglutide alone at any time point. Meal frequency was only reduced by the combination treatment at 12hr and 24hr, which was significantly different from each of the other three treatments at these time points (Figure 2, Ps<0.05).
Figure 1.
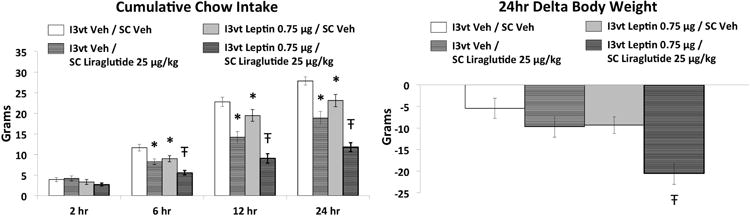
Cumulative chow intake and 24h delta body weight following 3rd ICV (I3vt) delivery of 0.75μg leptin and subcutaneous (SC) administration of 25μg/kg liraglutide. Liraglutide and leptin each significantly reduced chow intake. Co-administration of liraglutide and leptin further reduced chow intake and body weight such that these effects were significantly greater than that of either drug alone. * P<0.05 vs I3vt Veh/SC Veh, Ŧ P<0.05 vs all other treatments (I3vt Veh/SC Veh, I3vt Veh/SC Liraglutide, I3vt Leptin/SC Veh).
Figure 2.
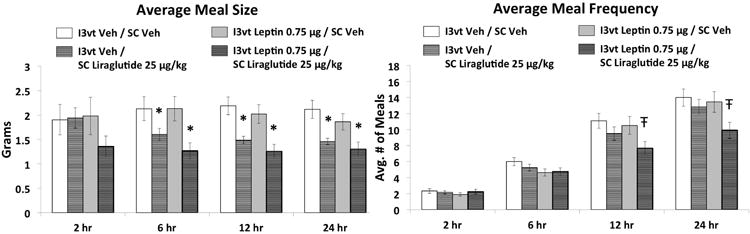
Average meal size and meal frequency following I3vt leptin (0.75μg) and SC liraglutide (25μg/kg). Liraglutide treatment alone reduced meal size. Meal frequency was significantly reduced by liraglutide and leptin co-administration, without any effects with either drug alone. * P<0.05 vs I3vt Veh/SC Veh, Ŧ P<0.05 vs all other treatments (I3vt Veh/SC Veh, I3vt Veh/SC Liraglutide, I3vt Leptin/SC Veh).
Moderate dose combination: cumulative food intake, delta body weight, and meal patterns
SC liraglutide (75μg/kg), ICV leptin (4μg), and the combination treatment each reduced cumulative food intake relative to vehicle/vehicle treatment at 6hr, 12hr, and 24hr, and delta body weight at 24hr (Figure 3; Ps<0.05). Combined treatment was not significantly different from either drug treatment alone at any time point for cumulative intake, nor for 24hr delta body weight. SC liraglutide treatment and combined treatment reduced meal size compared to vehicle-vehicle treatment at 12hr and 24hr (Figure 4, Ps<0.05), whereas the combination treatment was not different from SC liraglutide treatment alone at any time point. Meal frequency was reduced by each drug treatment alone and by the combination treatment at 6hr, 12hr and 24hr compared to vehicle-vehicle treatment (Figure 4, Ps<0.05), whereas the combined treatment did not differ from either drug treatment alone at any time point.
Figure 3.
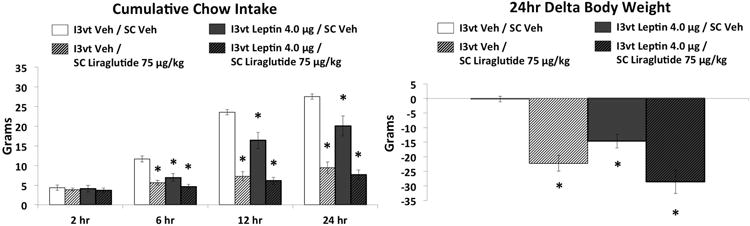
Cumulative chow intake and 24h delta body weight following I3vt leptin (4μg) and SC liraglutide (75μg/kg). Both liraglutide and leptin significantly reduced chow intake and body weight but the combinatorial effects were not greater than either drug alone. * P<0.05 vs I3vt Veh/SC Veh.
Figure 4.
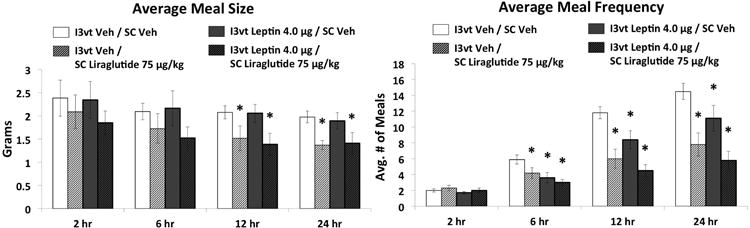
Average meal size and meal frequency following I3vt leptin (4μg) and SC liraglutide (75μg/kg). SC liraglutide reduced meal size and meal frequency while I3vt leptin reduced meal frequency only. Co-administration of liraglutide and leptin reduced meal size and meal frequency but the meal size effect was not greater than liraglutide alone and the meal frequency effect was not different from either liraglutide or leptin. * P<0.05 vs I3vt Veh/SC Veh.
Intracellular signaling pathways: pSTAT3 and PTP1B
ICV leptin treatment alone (6μg) increased pSTAT3 protein expression relative to vehicle-vehicle treatment, whereas SC liraglutide (50g/kg) alone had no effect (Figure 5, left, P<0.05). pSTAT3 activation was significantly higher following the combination treatment compared to either drug treatment alone (Ps<0.05). PTP1B expression following SC liraglutide (50μg/kg) was significantly lower than observed with vehicle treatment (Figure 5, right, P<0.05).
Figure 5.
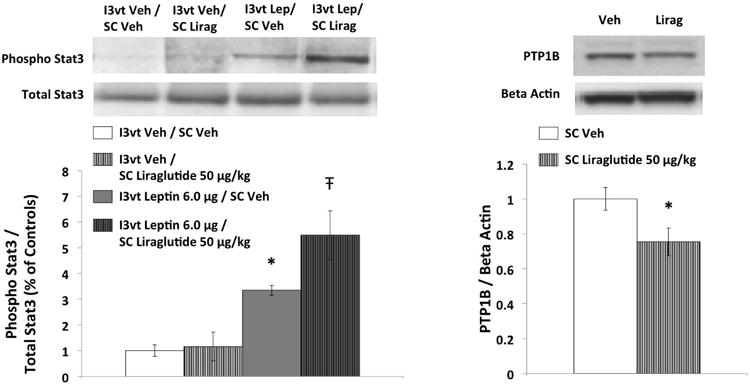
Phosphorylated STAT3 (pSTAT3) protein levels in hypothalamic tissue following I3vt leptin (6μg) and SC liraglutide (50μg/kg); and hypothalamic PTP1B protein following SC liraglutide (50μg/kg). Leptin alone significantly increased pSTAT3 levels. Hypothalamic pSTAT3 was further increased with co-administration of liraglutide and leptin. Liraglutide alone reduced PTP1B levels in the hypothalamus. Upper panel: representative immunoblots of pSTAT3, STAT3, PTP1B and beta actin. Lower panel: quantitative densitometric data of pSTAT3 normalized to total STAT3 (n=6-7 per group), and PTP1B normalized to beta actin (n=9 per group). * P<0.05 vs Vehicle, Ŧ P<0.05 vs all other treatments (I3vt Veh/SC Veh, I3vt Veh/SC Liraglutide, I3vt Leptin/SC Veh).
Discussion
Recent studies are beginning to explore the role of long acting GLP-1 analogues in combination pharmacotherapy, including its combination with leptin, as a strategy to enhance the feeding and body weight loss effects. Co-administration of leptin and GLP-1R agonists, delivered by different routes of administration, results in significant reductions in food intake and body weight that are greater than the effects of either drug alone (11, 12, 15). In the present study leptin was administered directly to the 3rd ventricle to achieve anatomically distributed (both forebrain and hindbrain) central effects on energy balance control, whereas liraglutide was delivered SC to model its delivery in a clinical setting. Consistent with previous findings, we report that combined acute treatment of low doses of leptin (0.75μg) and liraglutide (25μg/kg) reduced cumulative food intake for up to 24h at a significantly greater magnitude than the effects of either drug alone. Our study extends previous findings by showing that the additive food intake reduction results primarily from a significant reduction in meal frequency that was not observed in response to either treatment alone. This reduction in meal frequency following combined treatment may be mediated by reduced gastric emptying (and thereby increased inter-meal interval), as both peripheral liraglutide and central leptin reduce emptying rates (4, 34). Meal size, on the other hand, was significantly reduced by 25μg/kg liraglutide, but not by 0.75μg leptin. The combined effect of these two treatments on meal size was not significantly different than liraglutide treatment alone, although a trend was observed. Interestingly, while both leptin and liraglutide alone at these doses had no significant effect on body weight change 24h after administration, co-administration of leptin and liraglutide significantly reduced 24h body weight, supporting a role for liraglutide-leptin combination in amplifying body weight loss.
It was previously reported that the additive effect of intraperitoneal administration of leptin and GLP-1 on food intake and body weight only are obtained primarily with low dose combinations (12). Consistent with that finding, we found that the intake inhibitory and weight loss effects of a moderate dose liraglutide-leptin combination treatment (4μg leptin,75μg/kg liraglutide) were not greater compared to either treatment alone. It is possible that the intake and body weight effects of both leptin and liraglutide at these doses had reached a floor effect, thus limiting further reduction in food intake and body weight. However, in our previous work the food intake reduction produced by a high dose of 4th ICV leptin (20μg) was further augmented by 4th ICV exendin-4 (15), suggesting that the anorectic effects of high doses of central leptin can be further augmented by GLP-1R activation when both drugs are delivered to the hindbrain. Interestingly, present results also showed that the intake inhibitory effect of 4μg ICV leptin by itself is mediated by a reduction in meal frequency with no effect on meal size. These data contrast with those following chronic peripheral leptin treatment, which reduces meal size with minimal impact on meal frequency (22, 23).
Leptin's acute effect on meal pattern parameters following forebrain ICV delivery, to our knowledge, has only been reported in two other studies (26, 35). Consistent with present results, Zorilla and colleagues reported little effect of ICV leptin on meal size, whereas meal frequency reduction effects were observed with leptin doses ranging between 1-6.25μg, but not with 0.3μg leptin. This study used a breakpoint interval between intra-meal pauses and inter-meal intervals (inter-response interval; IRI) of 5min to define the end of a meal, whereas our present study employed a 10-min IRI criterion, both of which are widely accepted in the literature based on extensive behavioral observations and log survivorship analyses (26, 36-39). On the other hand, another study, using ∼30-min IRI, reported a reduction in meal size with no meal frequency reduction following ICV leptin. Thus, the contradictory finding of Flynn et al. may be based on the use of a much longer IRI (30-min) compared to our study (10-min) and the study by Zorilla and colleagues (5-min). Future work is needed to determine whether, unlike acute treatment, chronic ICV leptin has a more potent effect on meal size compared to meal frequency, as has previously been observed following chronic peripheral leptin administration.
SC liraglutide robustly reduces food intake and body weight in both lean and diet-induced obese (DIO) rats (30, 40-43), and reduces food intake in obese minipigs via meal size reduction. The effect of SC liraglutide on meal patterns in rats, to our knowledge, has not been previously reported. Here, we show that a moderate dose of acute SC liraglutide (75μg/kg) reduced both meal frequency and meal size. In previous studies, the effect of GLP-1R activation on meal patterns appears to be dependent on the route and site of administration (18-21, 44, 45), with more potent effects generally observed for meal size compared to meal frequency reduction. Given that acute liraglutide reduces food intake via activation of both peripheral and central GLP-1Rs following peripheral administration (41, 46), it is possible that the meal pattern effects observed in the present study are mediated by an action in the periphery, centrally, or both. It is also possible that nonspecific effects (e.g., nausea) contributed to the meal pattern effects of liraglutide (alone and in combination with leptin) given that under some conditions GLP-1 analogs produce conditioned flavor avoidance learning (30, 47).
We investigated the intracellular signaling pathways involved in the combined LepRb and GLP-1R activation in the hypothalamus, where receptors for both leptin and GLP-1 are abundantly expressed (48, 49). The intake inhibitory effect of LepRb signaling is mediated, in part, via the JAK-STAT pathway, where leptin binds to LepRb and results in the phosphorylation of Janus kinase-2 (pJAK2) and STAT3 (pSTAT3) (50). pSTAT3 is also increased following activation of inflammatory cytokine [e.g., interleukin (IL)-1, IL-6] receptors and central IL-1 and IL-6 signaling is thought to contribute to central GLP-1-induced anorexia (28). Our results showed that ICV leptin increased hypothalamic pSTAT3 signaling, and this effect was significantly amplified with SC liraglutide co-administration. However, we did not observe an increase pSTAT3 following SC liraglutide administration alone, an outcome that differs from a recent study reporting an increase in hypothalamic pSTAT3 following ICV Ex-4 (28). It is possible that the different route of administration (SC vs ICV), GLP-1 analog (liraglutide vs Ex-4), or relative dose strength used may have contributed to this difference.
Leptin resistance associated with obesity is often related to increased expression of negative regulators of leptin receptor signaling, such as PTP1B (51). PTP1B inhibits LepRb signaling by dephosphorylating JAK2 (52, 53). Further, PTP1B genetic deficiency and pharmacological inhibition of PTP1B is known to increase leptin sensitivity (54, 55), therefore highlighting the significance of PTP1B in energy balance control. We pursued a mediating role for PTP1B protein expression in the augmented pSTAT3 effect of leptin and liraglutide co-administration and found that peripheral liraglutide reduced hypothalamic PTP1B relative to vehicle treatment, which suggests a possible intracellular signaling interaction between leptin and liraglutide (Figure 6). The reduction in PTP1B protein was observed 45min after SC liraglutide treatment. Protein reduction within this short time frame is unlikely to be due to alterations in transcriptional events, which typically take hours to weeks (56). Other non-transcriptional mechanisms to account for PTP1B reduction by SC liraglutide include rapid protein degradation or re-localization of PTP1B protein to insoluble cellular compartments. However, since the lysis buffer used in the present study (RIPA) breaks down most cellular compartments, it is more likely that the PTP1B protein reduction following SC liraglutide administration is a result of rapid PTP1B degradation.
Figure 6.
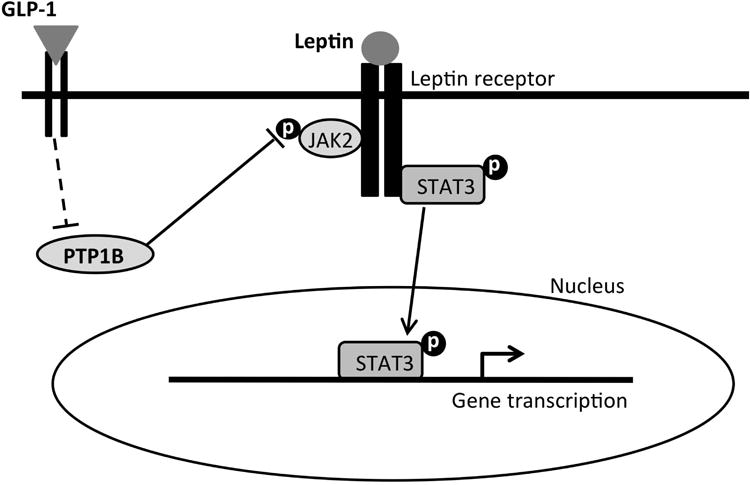
A simplified proposed summary of intracellular signaling mechanisms mediating the intake inhibitory and weight loss effects of leptin-liraglutide combination treatment. Leptin binds to the long form of leptin receptor (LepRb), which activates Janus kinase 2 protein (JAK2) and results in the phosphorylation of various tyrosine residues and the signal transducer and activator of transcription-3 (pSTAT3). pSTAT3 translocates to the nucleus to mediate the transcription of genes related to food intake and body weight control. GLP-1 on the other hand, binds to GLP-1 receptor and inhibits PTP1B via a direct or indirect pathway (broken lines). PTP1B normally dephosphorylates JAK2 and deactivates it. Inhibition of PTP1B therefore disinhibits pJAK2. The disinhibition of pJAK2 by GLP-1R activation, together with the activation of LepRb signaling, results in a combinatorial increase in pSTAT3, which is proposed to mediate the enhanced intake suppression and body weight loss effects of liraglutide and leptin co-administration.
It is unclear whether leptin and GLP-1 bind to receptors located on the same hypothalamic neurons or whether GLP-1 may be reducing PTP1B and augmenting pSTAT3 signaling via a secondary, indirect pathway. The difficulty in obtaining reliable GLP-1R and LepRb antibodies has made it technically difficult to provide anatomical data on the co-localization of both receptors in the hypothalamus. Nevertheless, earlier studies show that there is considerable overlap of leptin-induced and GLP-1-induced cFos-positive cells in the paraventricular nucleus of the hypothalamus (PVH) (57). Further, both leptin (58) and peripherally-injected liraglutide (59) bind to proopiomelanocortin- and cocaine- and amphetamine-regulated transcript -expressing neurons in the arcuate nucleus of the hypothalamus (ARH). Thus, the PVH and the ARH are potential hypothalamic targets for GLP-1R and LepRb interaction. It is important to note that the hypothalamus is not the only site of action for anorectic effects of leptin and GLP-1R agonists. Other target sites include the NTS, VTA, and the hippocampus, when leptin or GLP-1R agonists are administered directly at those sites (15, 21, 60-62). Future studies determining the behavioral and intracellular signaling effects following combinatorial leptin and GLP-1R agonists directed to other extrahypothalamic sites are warranted.
Present results identify PTP1B as one mechanism by which liraglutide and leptin co-administration increased pSTAT3. We also acknowledge the possibility of other alternative pathways mediating the enhanced intake inhibitory and weight loss effects of liraglutide-leptin combination treatment. For example, mRNA expression of fibroblast growth factor 21 (FGF21), a growth factor important for the regulation of glucose and lipid homeostasis, increases in the liver following peripheral liraglutide administration (63, 64). FGF21 increases leptin sensitivity in DIO mice (65) and the metabolic effects of FGF21 appear to be, in part, centrally mediated (66). Therefore, it is possible that liraglutide-induced increases in FGF21 also contribute to the augmented hypothalamic LepRb signaling (increased pSTAT3) following acute liraglutide-leptin co-administration. Alternatively, this combinatorial elevation in pSTAT3 may also be mediated by an increase in LepRb expression as a result of liraglutide treatment. This putative mechanism is less likely, however, given that sub-chronic peripheral liraglutide treatment does not alter LepRb expression in the ARH of DIO rats (59). Nevertheless, these hypotheses remain to be extensively explored.
Results from the present study show that ICV leptin and SC liraglutide when combined at lower, but not moderate doses increased the food intake and body weight reducing effects of either drug alone. This additive food intake effect following combined administration was based on a significant reduction in meal frequency that was not observed in response to either treatment alone. In conjunction with these behavioral effects we showed for the first time that SC liraglutide increased leptin-driven pSTAT3 activation in hypothalamic neurons, an effect that may be based, in part, on liraglutide-mediated reduction in hypothalamic PTP1B expression. Future studies are required to examine whether these intake and body weight reducing effects of leptin and liraglutide combination treatment are present following other routes of administration, long-term chronic administration, and persist in DIO rodent models.
Acknowledgments
We thank the following individuals for notable contributions: Dr. Kendra Bence, Derek Zimmer, Polly Van den Berg, Jennifer Gilbert, Jeffrey Chen, and Amber Alhadeff.
Funding and Disclosure: This work was funded by Novo Nordisk (HJG), and by NIH grants DK21397 (HJG), DK097147 (SEK), and DK102478 (SEK).
Footnotes
Conflict of interest details: S.E.K., Z.Y.O., and H.J.G. did the conception and design of the research; S.E.K., Z.Y.O., S.M.F., E.S. performed the experiments; S.E.K., Z.Y.O., S.M.F., and E.S.S. analyzed the data; S.E.K., Z.Y.O., S.M.F., E.S.S., and H.J.G. interpreted the results of the experiments; S.E.K. prepared the figures; S.E.K., and Z.Y.O drafted the manuscript; S.E.K., Z.Y.O., S.M.F., E.S.S., and H.J.G. edited and revised the manuscript; S.E.K., Z.Y.O., S.M.F., E.S.S., and H.J.G. approved the final version of the manuscript.
Authorship details: None
References
- 1.Center for Disease Control and Prevention; 2014. Overweight and Obesity: Data and Statistics [database on the Internet] Available from: http://www.cdc.gov/obesity/data/index.html. [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. Epub 2010/01/15. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28(5):w822–31. doi: 10.1377/hlthaff.28.5.w822. Epub 2009/07/29. [DOI] [PubMed] [Google Scholar]
- 4.Jelsing J, Vrang N, Hansen G, Raun K, Tang-Christensen M, Knudsen LB. Liraglutide: short-lived effect on gastric emptying -- long lasting effects on body weight. Diabetes, obesity & metabolism. 2012;14(6):531–8. doi: 10.1111/j.1463-1326.2012.01557.x. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, et al. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes research and clinical practice. 2012;97(2):258–66. doi: 10.1016/j.diabres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 2011;19(7):1342–9. doi: 10.1038/oby.2011.50. Epub 2011/03/19. [DOI] [PubMed] [Google Scholar]
- 7.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–16. doi: 10.1016/S0140-6736(09)61375-1. Epub 2009/10/27. [DOI] [PubMed] [Google Scholar]
- 8.Raun K, von Voss P, Knudsen LB. Liraglutide, a once-daily human glucagon-like peptide-1 analog, minimizes food intake in severely obese minipigs. Obesity (Silver Spring) 2007;15(7):1710–6. doi: 10.1038/oby.2007.204. Epub 2007/07/20. [DOI] [PubMed] [Google Scholar]
- 9.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nature reviews Endocrinology. 2010;6(10):578–88. doi: 10.1038/nrendo.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009;17(1):30–9. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 11.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55(12):3387–93. doi: 10.2337/db06-0558. Epub 2006/11/30. [DOI] [PubMed] [Google Scholar]
- 12.Bojanowska E, Nowak A. Interactions between leptin and exendin-4, a glucagon-like peptide-1 agonist, in the regulation of food intake in the rat. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2007;58(2):349–60. [PubMed] [Google Scholar]
- 13.Goldstone AP, Mercer JG, Gunn I, Moar KM, Edwards CM, Rossi M, et al. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett. 1997;415(2):134–8. doi: 10.1016/s0014-5793(97)01103-4. Epub 1997/11/14. [DOI] [PubMed] [Google Scholar]
- 14.Nowak A, Bojanowska E. Effects of peripheral or central GLP-1 receptor blockade on leptin-induced suppression of appetite. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2008;59(3):501–10. [PubMed] [Google Scholar]
- 15.Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. International journal of obesity. 2012;36(12):1522–8. doi: 10.1038/ijo.2011.265. Epub 2012/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstone AP, Morgan I, Mercer JG, Morgan DG, Moar KM, Ghatei MA, et al. Effect of leptin on hypothalamic GLP-1 peptide and brain-stem pre-proglucagon mRNA. Biochemical and biophysical research communications. 2000;269(2):331–5. doi: 10.1006/bbrc.2000.2288. [DOI] [PubMed] [Google Scholar]
- 17.Sanz C, Vazquez P, Navas MA, Alvarez E, Blazquez E. Leptin but not neuropeptide Y up-regulated glucagon-like peptide 1 receptor expression in GT1-7 cells and rat hypothalamic slices. Metabolism: clinical and experimental. 2008;57(1):40–8. doi: 10.1016/j.metabol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293(3):R983–7. doi: 10.1152/ajpregu.00323.2007. [DOI] [PubMed] [Google Scholar]
- 19.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–7. doi: 10.1210/en.2008-1045. Epub 2008/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. American journal of physiology Endocrinology and metabolism. 2013;305(11):E1367–74. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 Receptors Influence Food Intake, Meal Size, and Effort-Based Responding for Food through Volume Transmission. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol. 1998;275(1 Pt 2):R186–93. doi: 10.1152/ajpregu.1998.275.1.R186. Epub 1998/08/05. [DOI] [PubMed] [Google Scholar]
- 23.Kahler A, Geary N, Eckel LA, Campfield LA, Smith FJ, Langhans W. Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. Am J Physiol. 1998;275(1 Pt 2):R180–5. doi: 10.1152/ajpregu.1998.275.1.R180. Epub 1998/08/05. [DOI] [PubMed] [Google Scholar]
- 24.Ho A, Chin A. Circadian feeding and drinking patterns of genetically obese mice fed solid chow diet. Physiol Behav. 1988;43(5):651–6. doi: 10.1016/0031-9384(88)90221-1. Epub 1988/01/01. [DOI] [PubMed] [Google Scholar]
- 25.Kanoski SE, Zhao S, Guarnieri DJ, Dileone RJ, Yan J, De Jonghe BC, et al. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. American journal of physiology Endocrinology and metabolism. 2012;303(4):E496–503. doi: 10.1152/ajpendo.00205.2012. Epub 2012/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorrilla EP, Inoue K, Valdez GR, Tabarin A, Koob GF. Leptin and post-prandial satiety: acute central leptin more potently reduces meal frequency than meal size in the rat. Psychopharmacology (Berl) 2005;177(3):324–35. doi: 10.1007/s00213-004-1952-1. Epub 2004/12/21. [DOI] [PubMed] [Google Scholar]
- 27.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent progress in hormone research. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 28.Shirazi R, Palsdottir V, Collander J, Anesten F, Vogel H, Langlet F, et al. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(40):16199–204. doi: 10.1073/pnas.1306799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213(4506):451–2. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 30.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62(5-6):1916–27. doi: 10.1016/j.neuropharm.2011.12.022. Epub 2012/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cusin I, Rohner-Jeanrenaud F, Stricker-Krongrad A, Jeanrenaud B. The weight-reducing effect of an intracerebroventricular bolus injection of leptin in genetically obese fa/fa rats. Reduced sensitivity compared with lean animals. Diabetes. 1996;45(10):1446–50. doi: 10.2337/diab.45.10.1446. [DOI] [PubMed] [Google Scholar]
- 32.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin Signaling in the Ventral Hippocampus Stimulates Learned and Motivational Aspects of Feeding via PI3K-Akt Signaling. Biological psychiatry. 2013;73(9):915–23. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011;13(3):320–30. doi: 10.1016/j.cmet.2011.02.001. Epub 2011/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cakir B, Kasimay O, Devseren E, Yegen BC. Leptin inhibits gastric emptying in rats: role of CCK receptors and vagal afferent fibers. Physiological research / Academia Scientiarum Bohemoslovaca. 2007;56(3):315–22. doi: 10.33549/physiolres.930865. [DOI] [PubMed] [Google Scholar]
- 35.Flynn MC, Scott TR, Pritchard TC, Plata-Salaman CR. Mode of action of OB protein (leptin) on feeding. Am J Physiol. 1998;275(1 Pt 2):R174–9. doi: 10.1152/ajpregu.1998.275.1.R174. [DOI] [PubMed] [Google Scholar]
- 36.Castonguay TW, Kaiser LL, Stern JS. Meal pattern analysis: artifacts, assumptions and implications. Brain research bulletin. 1986;17(3):439–43. doi: 10.1016/0361-9230(86)90252-2. [DOI] [PubMed] [Google Scholar]
- 37.Demaria-Pesce VH, Nicolaidis S. Mathematical determination of feeding patterns and its consequence on correlational studies. Physiol Behav. 1998;65(1):157–70. doi: 10.1016/s0031-9384(98)00159-0. [DOI] [PubMed] [Google Scholar]
- 38.Kissileff HR. Free feeding in normal and “recovered lateral” rats monitored by a pellet-detecting eatometer. Physiol Behav. 1970;5(2):163–73. doi: 10.1016/0031-9384(70)90060-0. [DOI] [PubMed] [Google Scholar]
- 39.Clifton PG. Meal patterning in rodents: psychopharmacological and neuroanatomical studies. Neuroscience and biobehavioral reviews. 2000;24(2):213–22. doi: 10.1016/s0149-7634(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 40.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 2011;19(7):1342–9. doi: 10.1038/oby.2011.50. Epub 2011/03/19. [DOI] [PubMed] [Google Scholar]
- 41.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152(8):3103–12. doi: 10.1210/en.2011-0174. Epub 2011/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummings BP, Stanhope KL, Graham JL, Baskin DG, Griffen SC, Nilsson C, et al. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes. 2010;59(10):2653–61. doi: 10.2337/db09-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56(1):8–15. doi: 10.2337/db06-0565. Epub 2006/12/29. [DOI] [PubMed] [Google Scholar]
- 44.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(41):14453–7. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–81. doi: 10.1210/en.2008-1221. Epub 2008/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. The Journal of clinical investigation. 2014 doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22(23):10470–6. doi: 10.1523/JNEUROSCI.22-23-10470.2002. Epub 2002/11/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. The Journal of comparative neurology. 1998;395(4):535–47. [PubMed] [Google Scholar]
- 49.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of comparative neurology. 1999;403(2):261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. Epub 1999/01/14. [DOI] [PubMed] [Google Scholar]
- 50.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annual review of physiology. 2008;70:537–56. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y, Yu Y, Szabo A, Han M, Huang XF. Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet. PloS one. 2014;9(3):e92618. doi: 10.1371/journal.pone.0092618. Epub 2014/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. The Journal of biological chemistry. 2001;276(51):47771–4. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 53.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2(4):489–95. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 54.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nature medicine. 2006;12(8):917–24. doi: 10.1038/nm1435. Epub 2006/07/18. [DOI] [PubMed] [Google Scholar]
- 55.Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, et al. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology. 2007;148(1):433–40. doi: 10.1210/en.2006-0672. Epub 2006/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Remage-Healey L. Frank Beach Award Winner: Steroids as neuromodulators of brain circuits and behavior. Hormones and behavior. 2014;66(3):552–60. doi: 10.1016/j.yhbeh.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Dijk G, Thiele TE, Donahey JC, Campfield LA, Smith FJ, Burn P, et al. Central infusions of leptin and GLP-1-(7-36) amide differentially stimulate c-FLI in the rat brain. Am J Physiol. 1996;271(4 Pt 2):R1096–100. doi: 10.1152/ajpregu.1996.271.4.R1096. [DOI] [PubMed] [Google Scholar]
- 58.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–4. doi: 10.1038/35078085. Epub 2001/05/25. [DOI] [PubMed] [Google Scholar]
- 59.Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. The Journal of clinical investigation. 2014 doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake. Endocrinology. 2012;153(2):647–58. doi: 10.1210/en.2011-1443. Epub 2011/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–10. doi: 10.1016/j.neuron.2006.08.023. Epub 2006/09/20. [DOI] [PubMed] [Google Scholar]
- 62.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(9):1859–70. doi: 10.1038/npp.2011.70. Epub 2011/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nonogaki K, Hazama M, Satoh N. Liraglutide suppresses obesity and hyperglycemia associated with increases in hepatic fibroblast growth factor 21 production in KKAy mice. BioMed research international. 2014;2014:751930. doi: 10.1155/2014/751930. Epub 2014/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang M, Zhang L, Wang C, Liu H, Boden G, Yang G, et al. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PloS one. 2012;7(11):e48392. doi: 10.1371/journal.pone.0048392. Epub 2012/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muller TD, Sullivan LM, Habegger K, Yi CX, Kabra D, Grant E, et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. Journal of peptide science : an official publication of the European Peptide Society. 2012;18(6):383–93. doi: 10.1002/psc.2408. Epub 2012/05/09. [DOI] [PubMed] [Google Scholar]
- 66.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59(7):1817–24. doi: 10.2337/db09-1878. Epub 2010/04/02. [DOI] [PMC free article] [PubMed] [Google Scholar]


