Abstract
The Cre/loxP system has been widely used to generate tissue-specific gene knockout mice. Inducible (Tet-off) Osx-GFP::Cre (Osx-Cre) mouse line that targets osteoblasts is widely used in the bone research field. In this study, we investigated the effect of Osx-Cre on craniofacial bone development. We found that newborn Osx-Cre mice showed severe hypomineralization in parietal, frontal, and nasal bones as well as the coronal sutural area when compared to control mice. As the mice matured the intramembranous bone hypomineralization phenotype became less severe. The major hypomineralization defect in parietal, frontal, and nasal bones had mostly disappeared by postnatal day 21, but the defect in sutural areas persisted. Importantly, Doxycycline treatment eliminated cranial bone defects at birth which indicates that Cre expression may be responsible for the phenotype. In addition, we showed that the primary calvarial osteoblasts isolated from neonatal Osx-Cre mice had comparable differentiation ability compared to their littermate controls. This study reinforces the idea that Cre positive litter mates are indispensable controls in studies using conditional gene deletion.
Keywords: Osx-Cre, Intramembranous bone, Craniofacial, Suture, Hypomineralization
INTRODUCTION
The Cre/loxP system has been widely used to generate tissue-specific gene knockout mice. Two strains of mice, one with a Cre insertion and the other with a loxP insertion, are bred to generate offspring with the tissue-specific gene knockout. The application of this technology has greatly advanced our understanding of the functions of numerous genes in a wide range of disciplines including bone research. Bone is a heterogeneous tissue dynamically regulated by osteoblasts and osteoclasts as well as other cell types. The availability of Cre mouse lines targeting various bone cells has increased our ability to dissect cell type specific roles by targeting genes in both a cell and development stage specific manner.
In the past decade several Cre mouse lines have been generated which target osteoblasts [1–5]. Osx-GFP::Cre (Osx-Cre) mice are commonly used to target osteoblasts; they have a tTA and a tetracycline responsive element-controlled GFP/Cre fusion protein under the control of the Osterix (Sp7) promoter [2]. It has been shown that Osx-Cre is expressed during embryonic development from E14.5 and postnatally in both osteoblasts and sporadically in hypertrophic chondrocytes [2]. Breeding Osx-Cre mice with a floxed mouse line, where the target gene loci is flanked by loxP sites, generates a Cre/loxP knockout mouse with targeted gene deletion occurring in osteoblasts. Treatment with doxycycline can prevent the expression of the GFP-Cre fusion protein and recombination of the target gene. Therefore, this system allows cell type- and time-specific gene targeting [2, 6].
It is ideal to include both floxed and Cre transgenic littermate controls in Cre/loxP breeding experiments to ensure accurate interpretation of any observed phenotype. Mating schemes capable of generating genotypes including flox, Cre control, and conditional knockout (CKO) mice are inefficient at producing Cre control and CKO in one litter. For this practical reason many researchers did not include a Cre control group and used only a flox control group instead. This is indeed true for most of the gene targeting research with the Osx-Cre mouse line. When we used the Osx-Cre transgenic mouse line to generate conditional knockout mice for our gene of interest we found severely defective intramembranous bone development when compared to the flox control mice. Interestingly, a similar defective intramembranous bone development phenotype has been reported in several other CKO mouse models using Osx-Cre [7–9]. It has been documented that there is decreased body weight in young Osx-Cre transgenic mice resulting in delayed cortical bone expansion and accrual [10]. It is known that the Cre transgene itself may have non-specific effects [11]. This prompted us to question whether defective bone development in our CKO mouse models was due to the Osx-Cre itself. Therefore, we investigated the effect of Osx-Cre on early postnatal bone development. We showed that the Osx-Cre transgene itself had no effect on the trabecular bone parameters and minimal effect on the cortical parameters for one month old C57BL/6 background mice [12]. However, we noted that there is defective intramembranous bone development in Osx-Cre mice. This finding is of particular importance considering the increasing use of the Osx-Cre mouse line to generate osteoblast-specific knockouts. This study underscores the importance of including Cre positive littermate controls for comparison with CKOs.
MATERIALS AND METHODS
Animals and Genotyping
The Osx-Cre transgenic mouse line was described previously [2]. Osx-Cre mice were bred with Rosa 26 reporter mice [13] to generateRosa26;Osx-Cre mice that bring reporter gene LacZ. Osx-Cre mice have been backcrossed to C57BL/6 background for at least 9 generations. Rosa 26 reporter mice have been backcrossed to C57BL/6 background for at least 5 generations. All mice used in this study were maintained and handled according to local, state, and federal regulation, and were approved by the Institutional Animal Care and Use Committee at the University of Michigan. Mice were fed with 5001 or 5008 rodent diet (LabDiet). To suppress Cre activity, designated breeders were fed a diet containing 625 mg/kg doxycycline (Harlan, USA) to deliver a daily dose of 2–3 mg/mouse of doxycycline according to manufacturer’s instruction. Mice were genotyped by PCR using tail DNA and primers (Cre and Rosa26) as previously described [14]. Primer sequences are available upon request.
Skeleton Preparation and Staining
Skeleton preparation and staining of mice at different ages was described in previous studies [15]. Brie y, after fixation with 95% ethanol for 48h, skeletons were stained with 0.015% Alcian blue in 80% ethanol and 20% acetic acid solution for overnight, and then digested by 2% KOH for 24h, followed by 0.015% Alizarin red in (1% KOH solution) staining for 24 h. Samples were finally maintained in 1:1 mixture of glycerol and 95% ethanol for analysis.
Primary Calvaria Culture and Histochemical Assay
Isolation and culture of calvarial cells from neonatal mice were described previously [12]. Cells were plated and cultured in 6-cm dish in alpha MEM containing 10% Heat-inactivated FBS till analysis. Alkaline phosphatase staining (ALP) was performed for osteoblast differentiation using a commercially kit (Sigma Diagnostics, USA), all procedures were according to the manufacturer’s instructions. Modi ed Alizarin red staining (AR) was performed for mineralization analysis. In brief, cells were xed for 1 hour with cold 70% ethanol and stained with 40 mM AR-S, pH 4.2, at room temperature for 10 minutes with rotation.
Histology and Fluorescence Microscopy
Histology was performed as described previously [12]. Primary calvarial osteoblasts isolated from Osx-Cre transgenic mice were observed with uorescence microscope directly.
RNA extraction and qRT-PCR
RNA extraction and qRT-PCR were performed as previously described [12]. Total RNA of mouse frontal and nasal bones was extracted using the Trizol reagent (Invitrogen, USA), and 1 ug of total RNA (equal amount) per sample was reverse transcribed using first strand RT kit (Invitrogen, USA). Quantitative real time PCR (qRT-PCR) was performed using SYBR Green Supermix (Qiagen, USA) with primers that sequence information are available upon request. The level of target gene expression was normalized to the level of the reference gene 18S rRNA as described previously [12].
X-gal staining
X-gal (5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside) staining was performed for β-galactosidase activity assay. In brief, calvariae were dissected and rinsed with PBS, fixed in 4% PFA for 20 minutes at room temperature, rinsed with PBS again, and then stained 5 hours in X-gal solution containing 1 mg/mL X-gal (Invitrogen, USA), 5 mM potassium ferricyanide, 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% NP-40 at 37°C.
Statistical analysis
Statistical differences were evaluated by Mann-Whitney test (Student’s t-test). A value of P<0.05 was considered to be statistical significance.
RESULTS
Intramembranous bone formation is defective in Osx-Cre mice
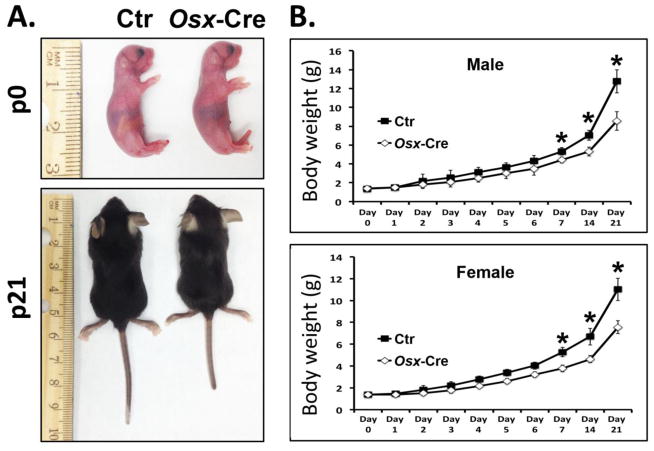
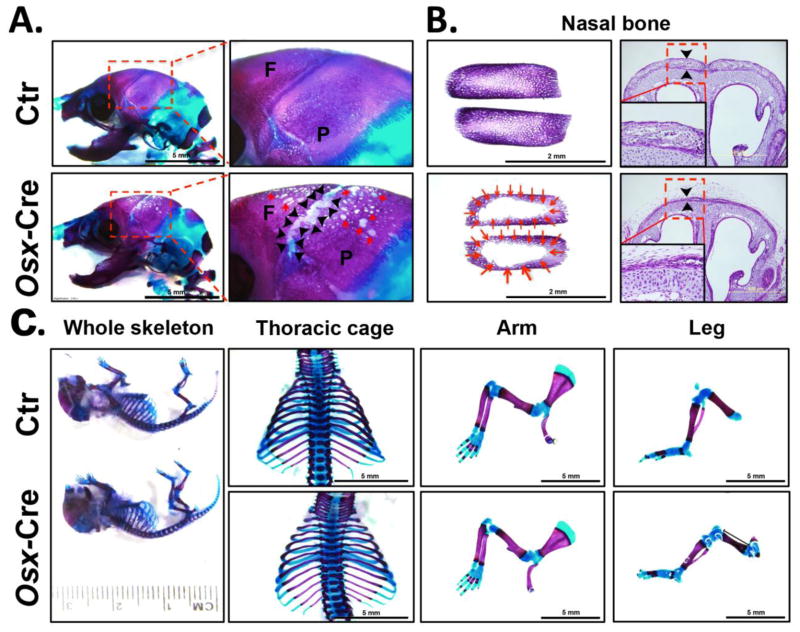
We previously characterized the effect of Osx-Cre transgene on the endochondrally formed femur and vertebra in one month old mice and found no adverse effect on the trabecular compartment of either bone[12]. In this study, we focused on the effect of the Osx-Cre transgene on intramembranous bone formation in the first three weeks postnatally. At birth there is no body weight difference between control mice and Osx-Cre mice (Fig 1). However, the body weight of Osx-Cre mice is significantly decreased after one week. As a first step to investigate the early bone development in these mice, we performed whole skeleton staining in newborn pups. Surprisingly, severe hypomineralization of nasal, frontal and parietal bone was observed in Osx-Cre positive skulls compared to controls (Fig 2A, 2B). This indicates defective intramembranous bone formation. The coronal sutural area had severe hypomineralization in Osx-Cre mice (Fig 2A). We performed skeleton staining on more than 20 Osx-Cre newborn pups and all pups showed a similar phenotype which indicates high penetrance. Histological examination in the nasal bone area showed significantly diminished mineralized tissue in the nasal bone of Osx-Cre mice (Fig 2B). Except for the craniofacial bone defects Osx-Cre mice had no obvious developmental defects in other major skeletal elements we examined including the humerus, radius and ulna, femur, tibia, fibula, ribs and vertebrae (Fig 2C) suggesting that endochondral bone formation was not affected at birth.
Figure 1. Osx-Cre mice have decreased body weight after postnatal day 7.
(A) Representative images of control (Ctr) and Osx-Cre mice at birth (p0) and postnatal day 21 (p21). (B) Body weight at indicated time points. *p<0.05, n= 7 per group. Data are mean ± SD.
Figure 2. Intramembranous bone formation is defective in Osx-Cre mice at birth.
(A) There was severe hypomineralization in the frontal and parietal bone (arrows) and coronal sutural area (arrow heads) in Osx-Cre mice. (B) There was significant less mineralized area in the nasal bone of Osx-Cre mice (left panel). Arrow indicates the alizarin red negative staining area; H&E staining (right panel) showed much thinner nasal bone (arrowhead) in Osx-Cre mice. Inserts were the enlargement of indicated area. (C) Osx-Cre mice had normal whole skeleton, thoracic cage, arm, and leg development at birth.
Hypomineralization of intramembranous bone in Osx-Cre mice gradually recovers during development
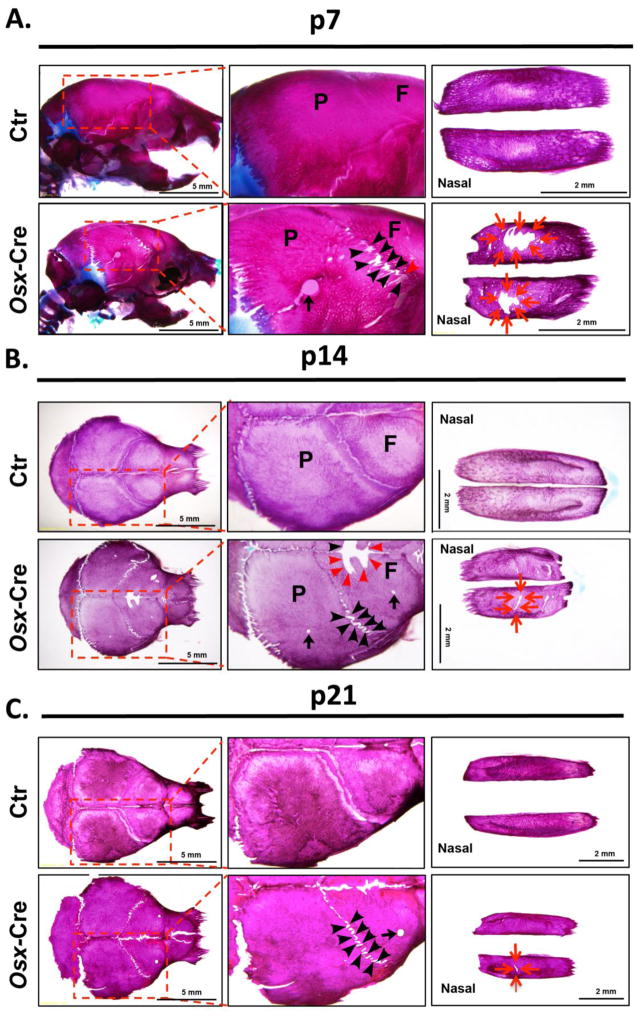
To further determine the extent of hypomineralization in Osx-Cre mice, we assessed skull development by performing skeleton staining at different postnatal time points. At day 7 hypomineralization in frontal, parietal and nasal bones of Osx-Cre mice persisted; however, it was much less severe compared to the defects present at birth (Fig 3A). The mineralization defect in coronal sutural area was the most prominent. At this stage, the staining pattern in other parts of the body was normal except that Osx-Cre mice had slightly shorter tibia and humerus (data not shown). At day 14 hypomineralization in frontal, parietal, and nasal bones was further diminished (Fig 3B). Minor, but still noticeable, defects existed in frontal and parietal bones. Hypomineralization persisted in coronal sutural area and started to become evident in the metopic sutural area. At day 21, the parietal bone in Osx-Cre mice appears normal. Very few minor porous spots were noticed in the frontal and nasal bones. The hypomineralization in coronal and metopic sutural areas continued to be evident (Fig 3C).
Figure 3. Hypomineralization in Osx-Cre mice gradually recovers during development.
Whole skull skeleton preparation of Osx-Cre and control mice at day7 (A), day 14 (B) and day 21 (C). P: Parietal bone; F: Frontal bone. Black arrows point to the sporadic porous holes in calvaria. Red arrows point to the unmineralized nasal bone. Black arrow heads point to the hypomineralized coronal sutural area. Red arrow heads point to the hypomineralized metopic sutural area.
Cre and Osterix expression in Osx-Cre mice at different development stages
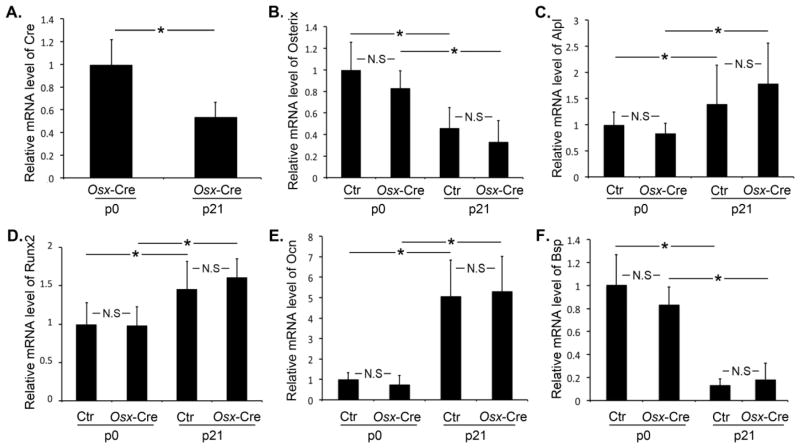
To determine the contribution Cre expression has in the observed hypomineralization phenotype, we measured Cre expression in the calvaria of Osx-Cre mice and found that there was higher Cre expression at birth relative to day 21 (Fig 4A). The association between Cre expression level and hypomineralization phenotype severity suggests higher Cre expression at birth may contribute to the phenotype. In addition, we measured the Osterix mRNA expression and found that Osterix mRNA levels did not differ between control and Osx-Cre mice (Fig 4B). We found decreased Osterix mRNA expression at day 21 compared to that at birth which is consistent with Cre expression. We measured osteoblast differentiation marker expression to determine to what extent defective osteoblast differentiation may contribute to the hypomineralization. As expected, the expression of Alkaline phosphatase, liver/bone/kidney (Alpl), Runt-related transcription factor 2 (Runx2), and Bone gamma carboxyglutamate protein (also known as osteocalcin, Ocn) was increased at later development stage (day 21) compared to at birth in both control and Osx-Cre groups (Fig 4C–4E). In contrast, Bone sialoprotein (Bsp) expression was decreased at later development stage (Fig 4F), indicating that calvaria at early development stage has more robust Bsp expression. However, we found no differential expression in any of those genes between control and Osx-Cre mice at either time point.
Figure 4. Cre and osteoblast differentiation marker gene expression at different development stages.
Quantitative PCR analysis of the mRNA expression of Cre (A), and osteoblast differentiation marker gene (B–F): Osterix (B), Alpl (C), Runx2 (D), Ocn (E), and Bsp (F) of parietal bone of 7-day-old control and Osx-Cre mice. *p<0.05, N.S: Not statistically significant, n=5–7 per group. Data are mean ± SD.
Doxycycline treatment rescues hypomineralization phenotype in Osx-Cre mice
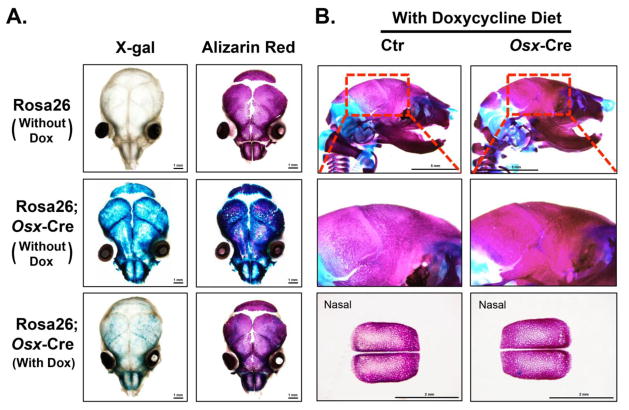
To determine whether the hypomineralization phenotype in Osx-Cre mice was due to the expression of Cre protein, we took advantage of the inducible nature of Cre gene in Osx-Cre mice. The Cre expression in Osx-Cre mice is regulatable in a tet-off manner [2]. Therefore, treatment with doxycycline can prevent the expression of the GFP-Cre fusion protein and recombination of the target gene. We fed the Osx-Cre mice in mating units with doxycycline food in a continuous manner and analyzed the skeleton of the offspring of these mice. To determine whether indeed doxycycline treatment could prevent the expression of Cre recombinase, we crossed Osx-Cre mice with Rosa26 reporter mice and examined the effect of doxycycline treatment on LacZ expression. As expected, there was strong X-Gal staining in the frontal, parietal, nasal bone of Rosa26;Osx-Cre mice without doxycycline treatment. However, there was only minimal X-Gal positive staining in these areas in Rosa26;Osx-Cre mice with doxycycline treatment, indicating that doxycycline treatment effectively inhibited Cre expression (Fig 5A). Next, we performed skeleton staining for the control and Osx-Cre mice at birth with doxycycline treatment. We found that the hypomineralization phenotype in Osx-Cre mice was completely rescued after doxycycline treatment (Fig 5B), suggesting that the phenotype may be caused by the Cre expression.
Figure 5. Doxycycline treatment can rescue hypo-mineralization phenotype of Osx-Cre mice.
The mating units were fed with or without doxycycline food as indicated. (A) X-gal staining and subsequent Alizarin Red staining of Rosa26 and Rosa26;Osx-Cre mice at birth. (B) Skull skeleton preparation of control and Osx-Cre mice fed with doxycycline food at birth.
Osx-Cre did not affect osteoblast in vitro differentiation
We previously showed that deletion of gene of interest with Osx-Cre greatly compromised in vitro osteoblast differentiation [12]. This prompted us to ask whether this was due to the effect of Osx-Cre itself. We digested primary osteoblasts from the parietal bone of neonatal control and Osx-Cre mice. In Osx-Cre mice, the Cre is fused with GFP under the control of Osterix promoter [2]. As expected, the primary calvarial osteoblasts isolated from Osx-Cre mice had positive nucleus GFP signal while it was absent in control cells (Fig 6A). We cultured these calvarial osteoblasts in osteoblast differentiation medium and perform alkaline phosphatase staining at day 7 and Alizarin red staining at day 21. We observed similar staining pattern in Cre expressing osteoblast culture compared to that in control culture, suggesting that Cre expression in primary calvaria osteoblasts had no negative effect on both early and late osteoblast differentiation in vitro.
Figure 6. Osx-Cre does not affect osteoblast in vitro differentiation.
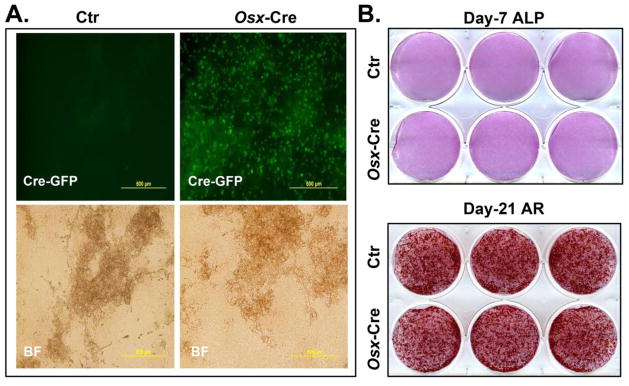
Primary calvarial osteoblasts were isolated from neonatal control (Ctr) and Osx-Cre mice. (A) Green fluorescence (Cre-GFP) and bright field (BF) images of cultured osteoblasts at day 21. (B) Alkaline phosphatase (ALP) staining at day 7. (C) Alizarin red S (AR) staining at day 21.
DISCUSSION
Davey et al. reported a cortical bone phenotype in skeletally immature Osx-Cre mice [10]. Razidlo et al. reported a minor craniofacial defect in the skulls of 5.5 week old Osx-Cre mice [7]. In this study, we reported a severe intramembranous bone formation defect in Osx-Cre mice during the early postnatal stage. Interestingly, the mineralization defect in these mice gradually recovered during development and largely disappeared by three weeks of age. Furthermore, doxycycline treatment can completely rescue these defects.
Cre recombinase is known to have possible toxic effects that can compromise normal cell cycle and survival. High levels of Cre recombinase expression in the nucleus of neuronal progenitors can compromise normal brain development [16]. High level myocardial expression of Cre recombinase can lead to dilated cardiomyopathy [17]. A similar mechanism may explain the hypomineralization phenotype in Osx-Cre mice. To support this notion, we found levels of Cre expression in the calvaria of Osx-Cre mice were more than two fold higher at birth than at three weeks of age. Increased calvarial Cre expression in newborn Osx-Cre mice is consistent with a higher Osterix expression level. Osterix mRNA levels did not differ between control and Osx-Cre mice at either age which suggests that introducing the GFP::Cre construct into exon 1 of the Osterix gene did not alter its endogenous expression. Importantly, we found that doxycycline treatment could completely rescue the defective mineralization phenotype with concomitant inhibition of Cre recombinase expression which further supports the idea that the hypomineralization phenotype in Osx-Cre mice was due to the Cre expression. Davey et al. also suggested that the Cre expression may directly contribute to the cortical bone phenotype in the skeletally immature Osx-Cre mice [10]. In Osx-Cre mice there is a Tet-controlled transactivator, tTA, cassette placed upstream of GFP::Cre. tTA is a fusion protein of an Escherichia coli Tet repressor (TetR) and the transcriptional transactivation domain of herpes simplex virus protein 16 (VP16AD) [18]. It has been reported that overexpression of tTA can result in disruption of transcriptional machinery and thus decreases the expression of other genes [19]. tTA expression in Osterix-expressing osteoblasts may lead to similar cellular damage; however, the rescue of the phenotype by doxycycline treatment argues against this possibility. tTA expression is under the control of the endogenous Osterix promoter and is not affected by doxycycline treatment which only prevents the binding of tTA to tetracycline-responsive element (TRE). This suggests that the expression of rTA is not responsible for functional defects in osteoblasts of Osx-Cre mice.
Although it is widely accepted that Osterix is expressed specifically by osteoblasts, some recent studies identified the Osterix expression in several non-osteoblast-lineage cells including olfactory glomerular cells, a subset of the gastric and intestinal epithelium, adipocytes, and perivascular cells in the bone marrow [20–22]. This raised the possibility that the craniofacial bone development phenotype in Osx-Cre mice may be indirectly due to the non-osseous expression of GFP::Cre. Although it remains a possibility, normal body weight and general skeletal development observed in newborn Osx-Cre mice indicated defective localized intramembranous bone formation in craniofacial bones is not likely due to the systemic or indirect effect of Cre expression in other tissues.
Cre and GFP are fusion proteins under the control of the Osterix promoter, so higher levels of Cre expression detected shortly after birth could suggest increased GFP expression which may contribute to the hypomineralization phenotype. Although expression of GFP is considered to be innocuous for cells, cytotoxic effects have been demonstrated in both in vitro and in vivo studies [23, 24]. It remains to be determined the extent to which Cre or GFP contributed to the in vivo hypomineralization phenotype. Nevertheless, our current finding as well as others [10] underscored the importance of using appropriate controls for data analysis when the Cre/loxP system is used to generate tissue-specific gene knockout mice. Ideally, all CKO mice should have Cre positive littermate controls; however, the efficiency of producing both Cre control and CKO of the same gender in one litter from breeding Flox/+;Cre mice and Flox/+ mice is extremely low. This practical limitation precluded the ideal approach for many study designs. If we thoroughly characterize the tissue specific effect due to Cre expression in a given tissue of interest, then we will have greater confidence when reaching conclusions from breeding experiments where Flox/Flox mice are used as controls. For example, we and others have shown that Osx-Cre itself has no effect on trabecular bone parameters [10, 12, 25]. Thus, any trabecular bone parameter changes observed in a given CKO mice with Osx-Cre is likely due to the deletion of target gene but not the expression of Osx-Cre itself. On the other hand, attributing any cortical bone parameter changes or intramembranous bone development at early stages to the gene of interest should be done cautiously. Osx-Cre positive littermate controls would be required to draw definitive conclusion in the latter case.
Primary calvaria osteoblast culture is a well-established cell culture system to study in vitro osteoblast differentiation. The observation that Osx-Cre caused significantly compromised osteoblast mineralization function in vivo raised the question about whether it would affect in vitro osteoblast differentiation and mineralization. It is important to address this because the conclusion will affect the interpretation for all in vitro primary calvarial osteoblast differentiation experiments which utilize Osx-Cre mice to delete any gene of interest. Our data demonstrated that both the early and terminal differentiation of primary calvarial osteoblasts isolated from neonatal Osx-Cre mice was comparable to control cells. It is unclear why Osx-Cre expression had adverse effect on mineralization in early postnatal calvaria in vivo but not in isolated primary calvarial osteoblasts in vitro. It remains to be determined whether the difference between in vitro culture and in vivo bone is due to the different Cre expression level. Nevertheless, our data validated the application of this in vitro osteoblast differentiation culture system when Osx-Cre is used to delete the gene of interest.
Acknowledgments
We thank Dr. Andrew McMahon for providing the Osx-Cre mice; Dr. Taylor Snider for critically reading and editing the manuscript. This study was funded by the National Institute Of Arthritis And Musculoskeletal And Skin Diseases of the National Institutes of Health under Award Number R01AR062030 (to F.L.) and R01DE020843 (to Y.M.).
Footnotes
DISCLOSURE
Dr. Fei Liu reports grant from NIH during the conduct of the study.
Dr. Yuji Mishina reports grants from NIH/NIDCR, personal fees from NIH/NICHD, other from NIH/NIEHS, personal fees from Shriners Hospital, grants from Department of Defense during the conduct of the study.
Dr. Li Wang has nothing to disclose.
References
- 1.Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M, Kream BE. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol. 2004;48:645–653. doi: 10.1387/ijdb.041816fl. [DOI] [PubMed] [Google Scholar]
- 2.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 3.Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224:245–251. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 5.Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schutz G, Tuckermann JP. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11:517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27:2344–2358. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, Secreto FJ, Knutson SK, Hiebert SW, Westendorf JJ. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One. 2010;5:e11492. doi: 10.1371/journal.pone.0011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121:3492–3504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu W, Liang G, Huang Z, Doty SB, Boskey AL. Conditional inactivation of the CXCR4 receptor in osteoprecursors reduces postnatal bone formation due to impaired osteoblast development. J Biol Chem. 2011;286:26794–26805. doi: 10.1074/jbc.M111.250985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey RA, Clarke MV, Sastra S, Skinner JP, Chiang C, Anderson PH, Zajac JD. Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic Res. 2012;21:885–893. doi: 10.1007/s11248-011-9581-z. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, Goldstein SA, Krebsbach PH, Guan JL. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res. 2013;28:2414–2430. doi: 10.1002/jbmr.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Lee JY, Wei H, Tanabe O, Engel JD, Morrison SJ, Guan JL. FIP200 is required for the cell-autonomous maintenance of fetal hematopoietic stem cells. Blood. 2010;116:4806–4814. doi: 10.1182/blood-2010-06-288589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- 16.Forni PE, Scuoppo C, Imayoshi I, Taulli R, Dastru W, Sala V, Betz UA, Muzzi P, Martinuzzi D, Vercelli AE, Kageyama R, Ponzetto C. High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J Neurosci. 2006;26:9593–9602. doi: 10.1523/JNEUROSCI.2815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J Card Fail. 2006;12:392–398. doi: 10.1016/j.cardfail.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Nishijima H, Yasunari T, Nakayama T, Adachi N, Shibahara K. Improved applications of the tetracycline-regulated gene depletion system. Biosci Trends. 2009;3:161–167. [PubMed] [Google Scholar]
- 19.Kuhnel F, Fritsch C, Krause S, Mundt B, Wirth T, Paul Y, Malek NP, Zender L, Manns MP, Kubicka S. Doxycycline regulation in a single retroviral vector by an autoregulatory loop facilitates controlled gene expression in liver cells. Nucleic Acids Res. 2004;32:e30. doi: 10.1093/nar/gnh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JS, Baek WY, Kim YH, Kim JE. In vivo expression of Osterix in mature granule cells of adult mouse olfactory bulb. Biochem Biophys Res Commun. 2011;407:842–847. doi: 10.1016/j.bbrc.2011.03.129. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, Maye P. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8:e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One. 2014;9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ. Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun. 1999;260:712–717. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- 24.Huang WY, Aramburu J, Douglas PS, Izumo S. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med. 2000;6:482–483. doi: 10.1038/74914. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]