Abstract
The intact neuromotor system prepares for object grasp by first opening the hand to an aperture that is scaled according to object size and then closing the hand around the object. After cervical spinal cord injury (SCI), hand function is significantly impaired, but the degree to which object-specific hand aperture scaling is affected remains unknown. Here we hypothesized that persons with incomplete cervical SCI have a reduced maximum hand opening capacity but exhibit novel neuromuscular coordination strategies that permit object-specific hand aperture scaling during reaching. To test this hypothesis, we measured hand kinematics and surface electromyography (EMG) from seven muscles of the hand and wrist during attempts at maximum hand opening as well as reaching for four balls of different diameters. Our results showed that persons with SCI exhibited significantly reduced maximum hand aperture compared to able-bodied (AB) controls. However, persons with SCI preserved the ability to scale peak hand aperture with ball size during reaching. Persons with SCI also used distinct muscle coordination patterns that included increased co-activity of flexors and extensors at the wrist and hand compared to AB controls. These results suggest that motor planning for aperture modulation is preserved even though execution is limited by constraints on hand opening capacity and altered muscle co-activity. Thus, persons with incomplete cervical SCI may benefit from rehabilitation aimed at increasing hand opening capacity and reducing flexor-extensor co-activity at the wrist and hand.
Keywords: Spinal Cord Injury, Reaching, Motor Control, Hand shaping, Muscle coordination
Introduction
The ability to open the hand for grasping objects is considerably compromised after cervical spinal cord injury (SCI). Object-specific hand opening is a highly complex visuomotor task that involves transformation of visual information about the size and shape of objects into motor commands that coordinate the muscle activity necessary for hand shaping. The intact neuromotor system prepares for object grasp by first opening the hand to an aperture that is scaled according to object size and then closing the hand around the object (Jeannerod 1986; Marteniuk et al. 1990). Cervical SCI damages the descending pathways that provide the requisite volitional activation of arm and hand muscles required for hand opening, thereby impairing hand function. While disruption of descending pathways, such as the corticospinal tract, may result in a broad range of altered hand and finger function (Muir and Lemon 1983; Lang and Schieber 2004), spared neural networks and neuromuscular abundancy allow for reorganization and preservation of some hand function (Topka et al. 1991; Laffont et al. 2000; Koshland et al. 2005; Laffont et al. 2007; Oudega and Perez 2012; Di Rienzo et al. 2014). Nevertheless, the extent to which neuromuscular strategies may enable modulation of hand opening after cervical SCI remains unknown.
Lesions to supraspinal structures provide great insight into the pathways underlying the control of hand opening. In humans, damage to the anterior intraparietal sulcus as well as the superior parietal lobe (i.e. optic ataxia) showed deficits in hand pre-shaping for object grasp such that the scaling of aperture with object size was lost (Jeannerod 1986; Binkofski et al. 1998). Similarly, inactivation of the intraparietal sulcus or the F5 area of the primary motor cortex in monkeys severely impaired hand aperture shaping (Gallese et al. 1994; Fogassi et al. 2001), suggesting that the F5 area and anterior intraparietal sulcus are heavily involved in the visuomotor transformation required for appropriate hand opening during reaching. However, supraspinal injuries outside of these regions, such as Parkinson's disease, cerebellar injury, or cortical stroke, do not impair hand aperture scaling despite significant impairments in other aspects of hand function (e.g., reduced coordination and speed) (Alberts et al. 2000; Michaelsen et al. 2009). These results suggest that supraspinal circuits play a major role in hand opening during reaching, but how neural reorganization and/or disrupted descending motor commands that must traverse the damaged cord alter hand opening after SCI is not entirely clear.
Neural reorganization involving multiple supraspinal structures is evident after SCI (Di Rienzo et al. 2014). The nervous system has the capacity to engage parallel corticospinal pathways for mediating hand dexterity after SCI (Darian-Smith et al. 1999) due in part to neural reorganization (Topka et al. 1991; Bruehlmeier et al. 1998; Oudega and Perez 2012). In particular, the corticospinal tract shows significant plasticity (Fouad et al. 2001; Bareyre et al. 2004; Wrigley et al. 2009; Oudega and Perez 2012). Thus, the neuromuscular system controlling hand movement is highly redundant, which permits new, distinct neuromuscular strategies to be used to achieve the same motor goal (e.g., hand aperture or shape). Suggesting that the human motor system has the capacity to redistribute commands and allow for emergence of novel muscle coordination strategies to preserve function after spinal injury is reasonable. Indeed, Koshland and colleagues found that after complete SCI different arm muscle coordination strategies emerge to produce arm reaches with kinematic features similar to able-bodied persons (Koshland et al. 2005). A similar possibility may be evident for the hand (Laffont et al. 2007). Laffont and colleagues showed that, despite increased difficulty in reach-to-grasp tasks as evidenced by longer times to complete the task and increased failure rate, many aspects of hand configuration, such as the number of fingers used and their orientation, were preserved (Laffont et al. 2007). However, the extent to which novel muscle coordination strategies also exists for preserving modulation of hand opening after incomplete SCI is not fully understood. Further, there is a paucity of research quantifying the limitations on hand opening after injury (Harvey et al. 2001), as studies on post-SCI hand function focus on hand closing (i.e. grasp) rather than hand opening or release (Popovic et al. 2006; Kapadia et al. 2013; Mateo et al. 2013; Di Rienzo et al. 2014).
The purpose of this study was to quantify the extent to which object-specific hand aperture modulation is preserved in persons with incomplete cervical SCI. Due to the preservation of supraspinal structures and the potential for neural reorganization after injury, we hypothesized that persons with cervical SCI preserve object-specific scaling of hand aperture during reaching while exhibiting unique neuromuscular coordination strategies. This hypothesis was tested by measuring hand kinematics and surface electromyography (EMG) of wrist and hand muscles during maximum hand opening and reaching for objects of different sizes. The results of this study have implications for understanding the potential of the nervous system to exploit new strategies in an effort to retain hand function after cervical SCI. The capacity for persons with SCI to modify their coordination strategies for hand opening promotes the use of rehabilitation therapies to guide development of task-appropriate muscle coordination strategies through task practice (Beekhuizen and Field-Fote 2005; Edgerton et al. 2006).
Methods
Subjects
We performed experiments on eight adult subjects (37±4.4 years, mean ± standard error; Table 1) with incomplete cervical SCI and eight able-bodied (AB) subjects of comparable age (26.6±2.4 years; p > 0.05). All protocols were approved by the Emory University Institutional Review Board and conducted in accordance with the Helsinki Declaration. Potential participants with SCI underwent an evaluation by a licensed physical therapist to determine eligibility.
Table 1. Participant Characteristics.
| Subject | Gender | Age (yrs) | AIS Level | Years Since Injury | Dominant Hand | Testing Hand | UEMS (L/R) | Box & Blocks Test ‡ | Jebsen-Taylor Test § | Medications |
|---|---|---|---|---|---|---|---|---|---|---|
| SCI1 | M | 53 | C5-C7 | 16 | R | R | 17/17 | 56 | 48 | None |
| SCI2 | M | 32 | C5 | 3 | R | R | 10/20 | 15 | 171 | Lyrica Neurotin |
| SCI3 | M | 32 | C5-C7 | 2 | R | R | 23/22 | 53 | 66 | Baclofen |
| SCI4 | M | 20 | C5-C7 | 3 | L | R | 14/13 | 43 | 82 | Baclofen |
| SCI5 | M | 31 | C5-C7 | 12 | R | R | 24/24 | 59 | 48 | None |
| SCI6 | M | 54 | C5-C7 | 39 | R | R | 13/15 | 46 | 79 | None |
| SCI7 | F | 28 | C4-C6 | 5 | R | R | 7/24 | 42 | 70 | Baclofen Dantrolene |
| SCI8 | M | 46 | C5-C7 | 7 | R | R | 22/22 | 54 | 69 | Baclofen Gabatrine Colace Oxybutia |
| AB1 | F | 21 | R | R | ||||||
| AB2 | M | 25 | R | R | ||||||
| AB3 | M | 21 | R | R | ||||||
| AB4 | F | 21 | R | R | ||||||
| AB5 | M | 32 | R | R | ||||||
| AB6 | M | 22 | R | R | ||||||
| AB7 | M | 34 | R | R | ||||||
| AB8 | M | 37 | L | R |
Abbreviations: AIS = American Spinal Cord Injury Association Impairment Scale; UEMS = upper extremity motor scores.
Box-and-blocks Test score = total number of blocks moved in 1 min
Jebsen-Taylor Test score = time in seconds for the dominant hand
Persons with SCI were included in the study if they had a single spinal injury more than 1 year ago (i.e., chronic), between levels C5 and C7 per medical chart review, had voluntary shoulder motion opposing gravity, as well as, visible voluntary movement of the elbow and wrist joints. Participants also had ability to extend first finger and thumb against gravity, and could follow verbal and visual commands. Potential participants were excluded if they had a progressive injury to spinal cord, brain injury or memory loss (> 24/30 on the mini-mental exam), change in spasticity medication < 1 month, upper limb joint contractures, and/or were undergoing concurrent physical therapy. AB participants had no history of upper limb neurological or muscular injuries or medical problems that interfere with the measurements of the study.
We characterized injury severity as well as upper extremity strength and dexterity using a set of standard clinical tests (Table 1). The American Spinal Injury Association Impairment Scale (AIS) was used to categorize neurological injury level and completeness. Strength was assessed using the Upper Extremity Motor Score (UEMS) from the AIS (Marino et al. 1999). The Box and Blocks Test (Patterson Medical Holdings, Inc. USA) (Platz et al. 2005) and Jebsen-Taylor Hand Function Test (Sammons Preston, Bolingbrook, IL USA) were used as clinical measures of hand dexterity and speed (Jebsen et al. 1969).
Protocols
Participants sat comfortably with their trunk securely strapped to an adjustable chair using padded chest and lap straps (Figure 1a). They maintained an upright posture with the base of their xiphoid process 18 cm away from the table and approximately 5 cm above the table. Two experiments were performed: 1) maximum hand opening and 2) reaching. For both experiments, participants were asked to respond as quickly as possible to a visual “go-signal”, which was a two-way light that changed from red to green. During maximum hand opening (experiment 1), we instructed individuals to place their hand on top of a wooden ball (diameter=7.6 cm, Figure 1c) while resting their forearm on a padded portion of the table. We recorded a minimum of 10 trials with a random rest interval of 3-5 seconds between each trial. Upon the visual go-signal, participants were encouraged to maximally open their hand for 3 seconds while their palm maintained contact with the ball. During reaching (experiment 2), participants repeatedly reached for four wooden balls of varying sizes with their right hand (diameter: A=1.9 cm, B=5.1 cm, C=7.6 cm, D=10.2 cm). We chose the right hand to ensure that all enrolled SCI participants were able to perform the experiments 1 and 2. Specific to experiment 2, the hand was initially placed on a table with shoulder at 70 degrees of flexion and 45 degrees of abduction, the elbow joint at 90 degrees, and the forearm pronated. The thumb was positioned approximately 19 cm from the xiphoid process with the first finger and thumb approximately 45 degrees apart. This position was marked on the table to ensure a consistent starting position across trials. Participants were then instructed to reach for the target ball, which was placed 36.8 cm away, as quickly as possible at the onset of the visual cue. When the ball was contacted, a synchronized trigger signal (“touch signal”, 3V) was sent if the ball was displaced and recorded to indicate successful completion of the task. The target ball was then repositioned in the target location and the hand was returned to the initial configuration. Trials were separated by random intervals of 3-5 seconds, and a minimum of 10 trials was collected for each ball size. Ball presentation order was block randomized, and participants were required to rest for a minimum of 30 seconds between each ball size to avoid fatigue. Experiment 1 was always performed first, followed by experiment 2.
Figure 1. Experimental Setup.
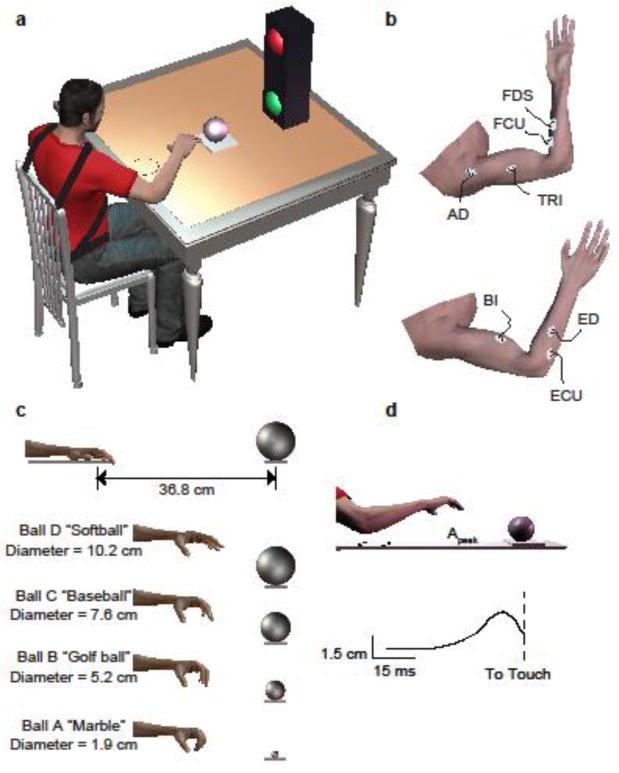
(a) Subjects were seated and placed their hand in an initial position. Upon the green “go” signal, the subject reached for the ball. As depicted in b, Surface electromyography (EMG) was recorded from 7 muscles. In c, Four target ball sizes and representative scaled hand opening. (d) Example of reaching with mean peak aperture trace calculated from the finger and thumb marker data across multiple trials.
Data collection and processing
Quantifying peak aperture
To quantify peak aperture in experiment 1 and 2, we recorded displacements between the first finger and thumb using two optical motion analysis systems (Optotrak 3020 and Certus; Northern Digital, Waterloo, Ontario), with resolutions of 0.1mm. Infrared LEDs were attached securely to the first finger nail and thumbnail. Kinematic data were collected at 100 Hz and interpolated to 2.5 kHz to match the sampling rate of the electromyography (EMG) system. Post-hoc analyses were performed on trials having markers with >95% visibility. We removed trials if 1) aperture was not calculable during active reaching, 2) no response to the go-signal was detected, 3) a detectable touch signal was not received indicating the ball was not displaced, and/or 4) peak aperture value was an outlier. Outliers were defined as values greater than 2.7 standard deviations above or below the mean for each subject and ball size. Based on these criteria, approximately 1% of trials were deemed unusable. Maximum hand aperture (experiment 1) was defined as the greatest distance between the finger and thumb during maximum voluntary hand opening (Jeannerod 1981; Haggard and Wing 1998). Similarly, peak hand aperture during reaching (experiment 2) was defined as the greatest distance between the finger and the thumb occurring between the onset of movement and within 90% of the reach time.
Quantifying muscle activity
We recorded surface EMG from seven muscles spanning the shoulder, elbow, wrist, and fingers. The recorded muscles included the anterior deltoid (AD), biceps brachii (BI), lateral head of triceps brachii (TRI), flexor carpi ulnaris (FCU), extensor carpi ulnaris (ECU), extensor digitorum (ED), and flexor digitorum superficialis (FDS), which represent the majority of extrinsic arm and hand muscles active during reach and hand opening (Weiss and Flanders 2004). Standard skin preparation techniques were applied prior to the application of self-adhesive Ag/AgCl bipolar surface electrodes with a 2 cm inter-electrode distance (model #272, Noraxon USA, Scottsdale, AZ). We used SENIAM guidelines to standardize longitudinal placement of surface EMG electrodes (Hermens et al. 2000). Palm contact with the table and balls, as well as EMG motion artifact and noise from the Optotrak LED wires, prohibited us from recording muscle activity of the intrinsic thumb and finger muscles (i.e., thenar eminence, pollicis brevis, first dorsal interrosseus).
EMG signals were amplified using a Bortec® AMT-16 system (Bortec Biomedical, Calgary, AB), which has an input impedance of 10GΩ a Common Mode Rejection Ratio of 115 dB at 60Hz, and a bandwidth of 10-1000 Hz. Analog signals were anti-alias filtered using custom-built, differential input, 4th order Bessel filters with a cutoff frequency of 500Hz and then sampled at 2.5 kHz by a 16 channel, 16 bit analog-to-digital data converter (NI PCI-6289; National Instruments, Austin, Texas), and collected using custom software developed using Matlab xPC (Mathworks, Inc, Natick, MA). Maximum voluntary contractions (MVCs) were recorded for shoulder adduction, elbow flexion and extension, and wrist/finger flexion and extension. Standard muscle testing procedures were used to isolate the activity of each target muscle during these MVCs (Delagi and Perotto 1979). Post-hoc, EMG data were demeaned (relative to the mean background EMG for each trial), rectified, and low-pass zero-phase lag filtered with a fourth order butterworth filter. EMG data were then normalized to maximal voluntary contractions for each muscle and participant to provide a measure of EMG relative to maximum volitional activation. During maximum hand opening and reaching tasks, muscle activity corresponding to peak aperture was defined as a 100ms window centered at 100ms prior to peak aperture time (Cavanagh and Komi 1979). A common clock and trigger were used to synchronize the collection of EMG and kinematic data.
To understand how muscle co-activity differs between AB and SCI and how this might contribute to hand opening impairments, we also assessed levels of EMG co-activity between agonist and antagonist muscles for the wrist and hand. Co-activity ratios for the hand were calculated as a ratio of the difference between mean hand extensor (ED) EMG activity and mean hand flexor (FD) EMG activity activities over the sum of the mean ED and FD EMG activities. Similarly, wrist co-activation ratios were calculated as the ratio of the mean wrist extensor (ECU) EMG activity and mean flexor (FCU) EMG activity over the sum.
To compare muscle coordination strategies, muscle coordination patterns across all muscles were identified using a factor analysis technique in which principle component analysis (PCA) is first applied to determine dimensionality followed by independent component analysis (ICA) to extract independent components (ICA/PCA, described previously in Trumbower, 2010). EMG data from the time of go-signal to the time of ball contact for three trials for each ball size and each participant were considered in this analysis. Prior to running the ICA/PCA algorithm, data were compressed by down-sampling the rectified EMG into 10% bins for each trial. We then applied PCA to each participant's data to identify the number of components required to account for > 90% data variance on average for AB subjects and for SCI subjects (see Figure 6). This number of components was then extracted from the pooled data from all AB participants and from the pooled data from all SCI participants using ICA. Resulting components from ICA represented muscle coordination patterns that describe the relative contributions of each muscle to that coordination pattern and each muscle can contribute to more than one patterns. Muscle coordination patterns can be flexibly combined to create the muscle EMGs observed for each participant in that group.
Figure 6. Similar number of coordination patterns between AB and SCI groups.
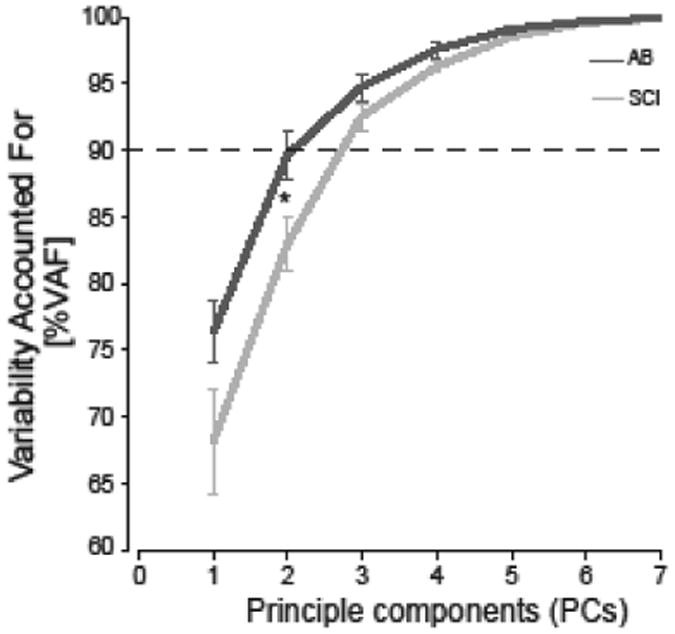
Coordination patterns were estimated using principle component analysis/independent component analysis (ICA/PCA) on the EMG data for the SCI and AB groups. Horizontal dashed line depicts 90% variance accounted for of the observed muscle activity. An asterisk indicates statistical significant differences between groups (p<0.05).
To assess whether group muscle coordination patterns adequately represented each individual within the group, we performed a within-group cross reconstruction analysis. For each group (AB and SCI), each participant's data was reconstructed using muscle coordination patterns extracted from the data from all remaining participants in that group. If the variability accounted for (VAF) by the group patterns was not significantly different from 90%, then the group patterns were considered representative and group extraction valid. To assess the differences in muscle coordination between the AB and SCI groups, we also tested how well the independent components identified for one subject group could characterize the muscle EMG from the alternate group using between-group cross reconstructions in which one group's patterns are used to reconstruct the EMG data from each participant in the alternate group. Similar patterns of muscle coordination were identified for each group but not between groups (see Results Section). We therefore used a common set of independent components to represent each group.
We also considered how the relative activation of each coordination pattern varied across ball size. To compare activations, the activation profiles were normalized by the root-mean-squared activation across all balls for each participant and each pattern. Normalized activations were compared between the ball sizes.
Statistical Analyses
Statistical analyses were performed in SPSS 20 statistical software (SPSS, USA) and considered significant if p<0.05. Results are reported as mean ± 1 standard error (SE). Before performing any statistical test, we inspected our experimental data of interest for normalcy using the Shapiro-Wilk test (Shapiro and Wilk 1965) and homogeneity of variance using the Levene test (Levene 1960) to ensure use of the appropriate statistic models.
In the first experiment, we compared voluntary maximum hand opening, normalized EMG for each muscle, and co-activation ratios at the wrist and hand between AB and SCI using independent t-tests. Relationships of peak aperture to EMG co-activity indices and to clinical metrics of hand function (i.e., Jebsen and Box-and-Blocks) were assessed using linear regression models.
In the second experiment, we used a repeated-measure ANOVA with Bonferroni post-hoc corrections to test our primary hypothesis that scaling of hand aperture during reaching is preserved after SCI. Specifically, we used ball size and group as the factors and peak aperture as the repeated measure to test for differences in peak aperture between and within groups. For each group, does peak aperture differ between balls? For each ball, does peak aperture differ between groups? In addition, we compared the scaling of peak aperture with ball size using linear regression models on the mean peak aperture for each ball size for each group, and regression slopes were compared between groups using an ANCOVA. Finally, the co-activation ratios at the wrist and hand were compared between AB and SCI groups and within groups across ball sizes using a linear mixed model with co-activity as the repeated measure, group as a between-subject factor, and ball size as a within-subject factor.
For muscle coordination patterns, we used several non-parametric tests. To assess how well group muscle coordination patterns represented individual data, the variance accounted for (VAF) in each participant's EMG data by patterns extracted from the remaining participants in the group was compared to the 90% cutoff level using non-parametric Wilcoxon Signed Rank tests. To assess the differences in muscle coordination between the AB and SCI groups, we compared the VAF of the cross-reconstructions using Mann-Whitney U-tests. Finally, we used Kruskal-Wallis ANOVA to assess how the activation of muscle coordination patterns scaled with ball size. If activations differed across ball sizes, post-hoc pairwise comparisons were made with Bonferroni corrections.
Results
Maximum voluntary hand opening is altered in persons with incomplete cervical SCI
Maximum hand opening was reduced in SCI participants as compared to controls (Figure 2a, p=0.002). All SCI participants had reduced hand opening despite a broad range of hand impairments. We found no correlation between maximum voluntary hand opening and hand function as measured using the Box-and-Blocks Test (p=0.934) and total Jebsen-Taylor Hand Function (p=0.701).
Figure 2. Maximum hand aperture and corresponding EMG during maximum voluntary hand opening is reduced after SCI.
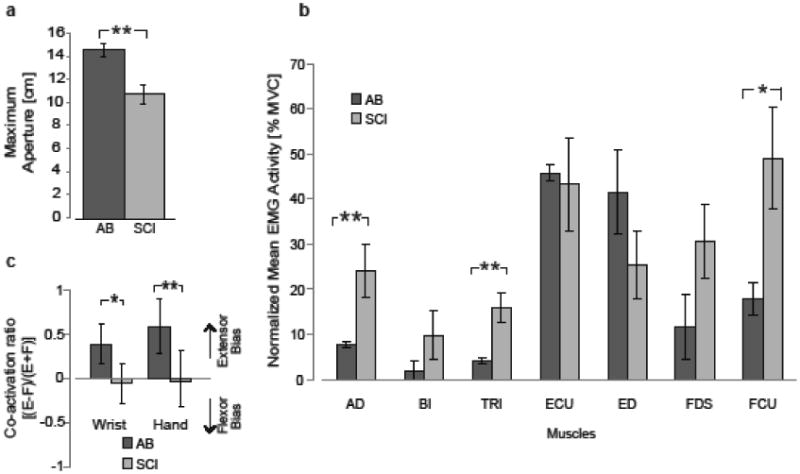
All bars represent mean ± 1 standard error. Statistical significance is indicated by an asterisk (p< 0.05) and double asterisk (p < 0.01) for between-group comparisons. (a) Mean maximum aperture was significantly greater for AB compared to SCI subjects. (b) Mean EMG activity across all subjects in the AB and SCI groups for a 100ms period that occurs 100ms prior to maximum aperture. EMG activation is normalized to maximum voluntary contraction (MVC) for each muscle and subject. SCI subjects show greater activation of proximal arm and hand flexor muscles. In (c), co-activation ratio represents the relative activation of flexors and extensors at the wrist or hand. Positive values represent greater proportional extensor (E) activity, while negative values represent greater flexor (F) activity. SCI subjects use more equal co-activation of flexors and extensors, while AB subjects use task-appropriate extensor-biased activity.
Decreased maximum hand opening after SCI was accompanied by altered EMG activity. The SCI group showed greater normalized EMG activity of proximal muscles and hand flexors compared to the AB group. SCI participants had greater EMG activity in AD (p=0.018), TRI (p=0.009), FCU (p=0.020), and FDS (p=0.051) as compared to AB group (Figure 2b). EMG co-activity of agonist (i.e., EDS, ECU) and antagonist (i.e., FDS, FCU) muscles for maximum voluntary hand opening differed between groups (Figure 2c). In AB group, co-activity ratios were positive, indicating greater normalized EMG activity of extensor muscles of the wrist and the hand, while co-activity ratios in SCI group were close to zero, indicating normalized EMG activity of flexors and extensors were similar. Co-activation ratios at wrist and hand were reduced for the SCI group compared to the AB group (wrist: p=0.030, hand: p=0.006) indicating greater agonist-antagonist EMG coactivity in the SCI group. Increased EMG coactivity of agonists and antagonists may limit maximum hand opening ability since wrist and hand co-activation ratios were correlated with maximum hand opening (wrist p=0.041, hand p=0.026).
Hand aperture modulation is preserved in persons with incomplete cervical SCI
Peak hand aperture during reaching increased with ball size for AB and SCI groups (Figure 3a; AB p≤0.001, SCI p=0.004). Within AB and SCI groups, peak aperture was different between ball sizes (all p≤0.006). Between AB and SCI groups, peak aperture for balls B, C and D were larger in the AB group than the SCI group (all p≤0.042), while peak aperture for the smallest ball A was not different (p=0.163). Peak aperture for ball D approached the overall maximum hand aperture for SCI group suggesting that SCI participants may reach an asymptote at the larger ball sizes. Peak aperture for the largest ball D sometimes even exceeded maximum hand aperture observed during experiment 1, possibly owing to the order of the experiments in which subjects performed maximum hand opening first thereby possibly improving with practice or with repeated stretch of tight flexor muscles. AB participants operate well below their maximum hand aperture with greater aperture scaling with ball size as compared to SCI participants (p=0.004). However, when peak aperture was normalized to overall maximum aperture (Figure 3b), regression slopes for normalized peak aperture versus ball size were similar between groups (p=0.895). Within AB and SCI groups, the normalized peak aperture increased with increasing ball size (all p≤0.003). However, AB participants utilized less of their aperture range compared to the SCI group (p=0.035); peak apertures for the three smaller ball sizes were different between groups (p≤0.003).
Figure 3. Aperture modulation with ball size is preserved within the limits of available aperture range.
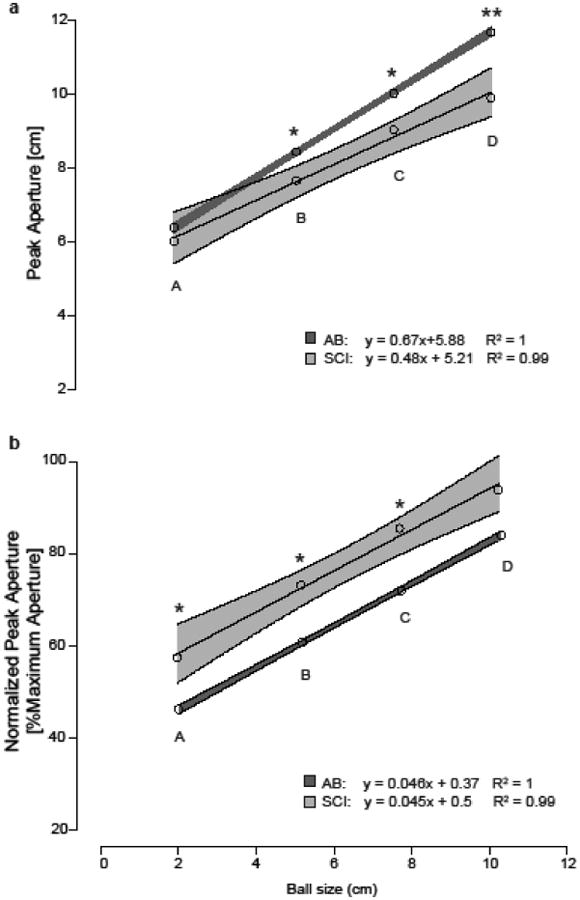
Linear regression models were used to describe the relationship between mean peak aperture during reach and ball size for AB and SCI groups. Light grey corresponds to the SCI group and dark grey to the AB group; means are represented as circles and shaded area represents 95% confidence intervals (CIs). Significant differences in peak aperture for a given ball size are indicated by an asterisk (p<0.05) or double asterisk (p<0.01). (a) AB control group show a steeper increase in absolute peak hand aperture across ball size compared to the SCI group. There iss no significant difference in mean peak aperture for the smallest ball size (A), but peak aperture differed significantly for balls B, C, and D. (b) When normalized to overall maximum aperture, the SCI group show similar slope and aperture modulation to AB group, with the SCI group using a greater percentage of their aperture range.
Muscle coordination during hand opening is altered in persons with incomplete cervical SCI
Representative EMG activity during the reaching task from representative SCI and AB participants is shown in Figure 4. SCI participant (S08) exhibited greater EMG activity of the AD, TRI, ECU and FCU muscles during reaching compared to the AB participant (S07), consistent with the differences seen during maximum hand opening. As seen during maximum hand opening (Figure 5), co-activation ratios at wrist and hand were greater for AB compared to SCI for all ball sizes (all p≤0.010 for the wrist; all p≤0.043 for the hand). The AB group wrist and hand co-activation ratios did not differ significantly across ball sizes. However, the SCI group tended to decrease their co-activation ratios (i.e. less extensor and more flexor activity) across ball sizes, particularly for the hand. Hand co-activation was reduced when reaching for ball C compared to ball A (p=0.023) and ball B (p=0.003).
Figure 4. Persons with SCI show greater proximal arm and hand extensor muscle activity during reaching.
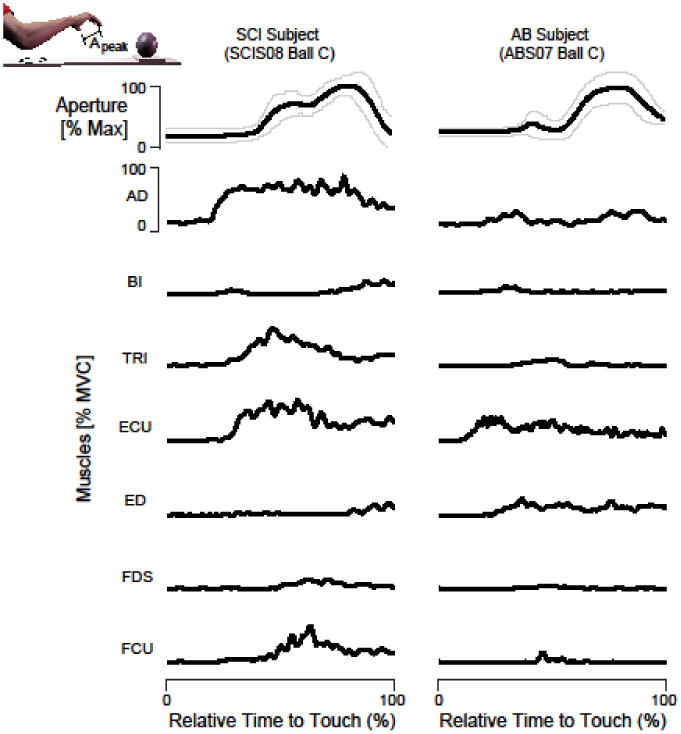
Representative EMG responses normalized to MVC during reaching for ball C (diameter 7.6 cm) from a representative SCI subject (left, SCI S08) and an AB subject (right, AB S07). The first row shows the aperture between the index finger and the thumb normalized to maximum aperture and subsequent rows show the corresponding muscle EMG normalized to MVC. The black lines correspond to the average and the thin gray lines represent the standard error. The SCI subject exhibits greater relative activation of the AD, TRI, ECU and FCU muscles compared to the AB subject.
Figure 5. Persons with SCI use greater co-activation of flexors and extensors during reaching.
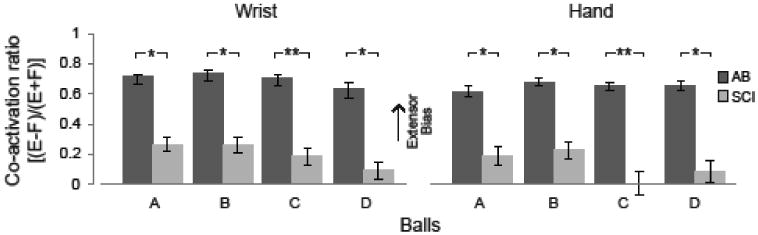
The co-activation of the hand and wrist muscles during reaching for balls A-D. The bars represent mean ± 1 standard error. Statistical significance between groups is indicated by an asterisk (p< 0.05) and double asterisk (p<0.01). A positive value represents greater proportional extensor (E) activity, while a negative value represents greater flexor (F) activity.
There was no difference in the number of muscle coordination patterns (SCI 2.9±0.2, AB 2.5±0.3; p=0.227, Mann Whitney U Test) as both groups required three patterns to sufficiently account for greater than 90% of EMG data variance (Figure 6). We pooled data within groups since the VAF in the individual data by the group patterns from the remaining participants were not different from 90% for either group (Figure 7a); AB group patterns explained 88.8±3.2% of the variance in individual AB EMG data (Wilcoxon Signed Rank vs 90%, p=0.89), while SCI group patterns explained 88.8±1.3% of the variance in individual SCI EMG data (p=0.40).
Figure 7. Inter-group ICA/PCA reconstructions reveal differences in SCI and AB group coordination patterns.
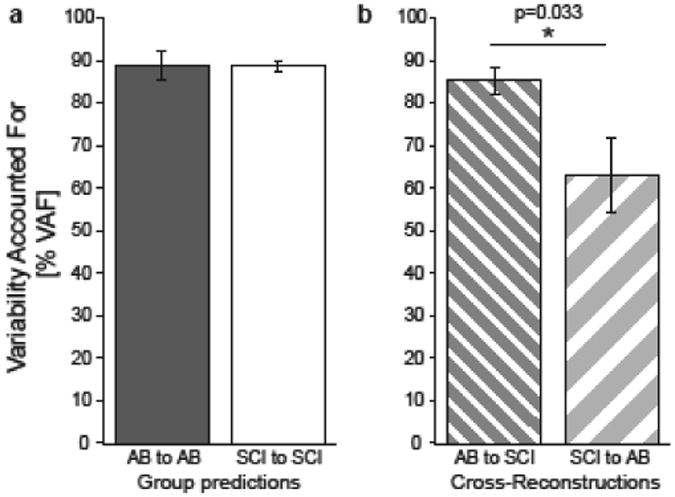
Each bar represents the mean ± standard error variability accounted for (VAF) when one groups' muscle coordination patterns are used to reconstruct the data from the individuals in that group (left) or the other group (right). Statistical significance is indicated by an asterisk (p< 0.05) and double asterisk (p<0.01) for between-group comparisons. For both groups, the VAF by group patterns is not different from 90% (p>0.05). SCI muscle coordination patterns have lower VAF when reconstructing AB group EMG data (p=0.003).
Wrist and finger extensor muscles dominated muscle coordination patterns in the AB group (Figure 8). The wrist extension pattern consisted primarily of ECU and accounted for 67.9±3.7% of the variance in the EMG data across the AB subjects. The finger extension pattern included significant contributions from ED and ECU and accounted for 201.0± 3.9% of the data variance. The shoulder flexion pattern was dominated by activation of the AD muscle but also included ED activation. This pattern accounted for 11. 1±1.5% of the data variance.
Figure 8. Comparison of muscle coordination patterns between AB and SCI groups.
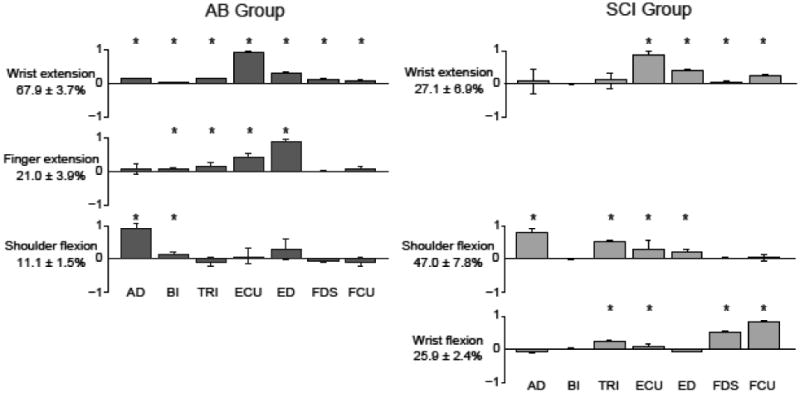
Muscle coordination patterns are shown for the AB control (left) and SCI (right) groups. Muscles in each coordination pattern are indicated at the bottom for each bar. Bar heights correspond to the relative activation of each muscle normalized to unity for a given pattern. Percentages at the left of each pattern indicate the relative variance accounted for by that pattern across the whole group dataset. Both groups use a shoulder flexion and a wrist extension pattern. However, the SCI group exhibits a unique wrist flexion pattern, while the AB group showed a more task-appropriate finger extension pattern.
When patterns were extracted from grouped SCI data, a distinct wrist flexion coordination pattern emerged for the SCI group that was dominated by the wrist and finger flexor muscles (Figure 8). This distinct pattern was dominated by the FCU and to a lesser extent FDS, and accounted for 25.9±2.4% of the data variance. Unlike in AB group, the shoulder flexion pattern accounted for the most variance (47.0±7.8%) for the SCI group. Finally, the wrist extension pattern, which included activity of wrist and hand extensors ECU and ED, only accounted for 27.1±6.9% of the SCI data variance.
We quantified differences in muscle coordination patterns between the two groups using cross-reconstruction analyses, in which one groups' patterns were used to reconstruct the EMG data from the other group. The coordination patterns for the SCI group were less effective at reconstructing the AB subjects' data compared to vice versa (p=0.038, Figure 7b). The SCI group patterns only accounted for 63.1±8.9% of the variance of the AB subjects' data, while the AB group patterns accounted for 85.4±3.2% of the variance of the SCI subjects' data, suggesting that SCI subjects may have a limited repertoire to coordinate muscles during reaching.
Finally, we assessed how the activation of muscle coordination patterns scaled with increasing ball size. For the AB group, both the wrist extension (p=0.002) and finger extension (p=0.030) muscle coordination patterns showed a significant increase in activation across ball sizes, as would be expected for a hand opening task. For both groups, the shoulder flexion pattern was not modulated with ball size. For the SCI group, the wrist flexion muscle coordination pattern (p=0.002) increased activation as the ball size increased (Figure 9), suggesting that greater antagonist flexor activity increases as SCI subjects attempt to further open their hand. The wrist extension muscle coordination pattern also showed a non-significant trend to increase activation as ball size increased. These results agree with the decrease co-activation ratios shown in Figure 5, suggesting that flexor activity increases with ball size more than extensor activity yielding a near-zero co-activation ratio at larger ball sizes.
Figure 9. Participants with SCI increased wrist flexor muscle activity with ball size.
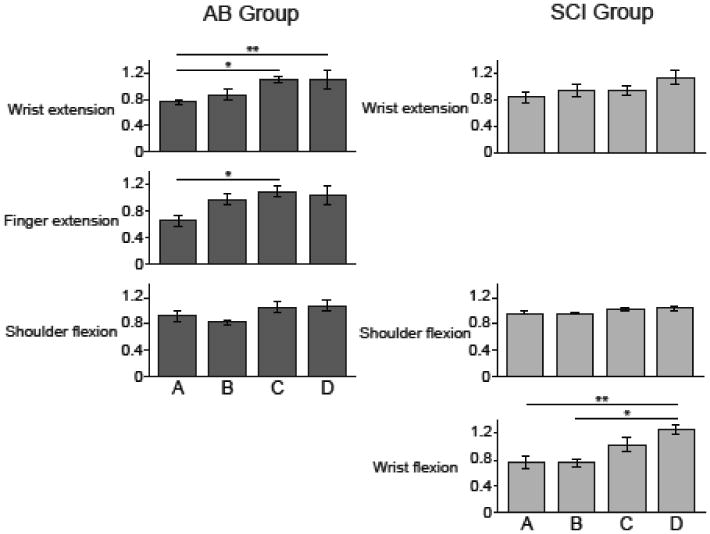
Normalized root-mean-square (rms) pattern activations during reaching for the wrist flexion pattern in the SCI group across the four ball sizes. Significant difference in activation between ball sizes is denoted by a single asterisk (p<0.05) or double asterisk (p<0.01). There is a significant increase in activation of this pattern with activation for the D ball being significantly greater than activation for the A and B balls.
Discussion
The purpose of this study was to examine the effect of incomplete cervical SCI on hand opening and modulation of hand aperture during reaching. In particular, we were interested in determining the extent to which hand opening is preserved and how changes in muscle coordination patterns may contribute to altered hand opening during reaching. Our results demonstrate that individuals with cervical SCI preserved the ability to scale hand aperture with object size (e.g., ball diameter), yet the magnitude of hand opening and the degree of aperture scaling as well as muscle coordination patterns they used were distinct from AB subjects. Together, these findings suggest that changes in muscle coordination may contribute to novel neuromotor strategies for modulating peak hand aperture after cervical SCI.
Participants with incomplete cervical SCI demonstrated reduced maximum hand opening as compared to AB subjects. Maximum hand aperture was approximately 36% less in the SCI group as compared to the AB group, a finding consistent with clinical observations where reduced voluntary hand opening is often a significant challenge to overcome after cervical SCI. Yet, the reduction in opening ability was not correlated to hand function, suggesting clinical scores reflect a more global measure of hand function not only hand opening ability.
Despite limitations in maximum hand aperture from all our participants with SCI, they modulated peak hand aperture during reaching. Indeed, we found no difference in modulation of normalized peak hand aperture between groups. Thus, the preservation of peak hand aperture modulation suggests the emergence of novel control strategies within a damaged neuromotor system. A striking finding from this study was that individuals with SCI preserved kinematic features of hand opening (i.e. peak aperture) while using altered muscle coordination patterns. We found that comparable aperture scaling between SCI and AB groups was not simply a result of similar EMG muscle coordination patterns between groups. Although both groups exhibited consistent shoulder flexion patterns for opposing gravity, the SCI participants required greater activity of their proximal anti-gravity muscles (e.g., anterior deltoid, triceps) compared to AB participants. The between-group difference is likely due to SCI-induced weakness and has been previously suggested by increased shoulder girdle motion and scapular displacement (Laffont et al. 2000). Although both groups exhibited a wrist extension pattern, the SCI group did not exhibit an additional hand extension pattern. Instead, the SCI group exhibited a merging of coordination patterns involving shoulder flexion and wrist extension patterns that included EMG activity of ED. Thus, SCI participants may have a reduced ability to individually control the hand and wrist extensors but rather co-activate them with other muscles (see Figure 8). The altered coordination co-activity patterns may constrain the ability to execute the precise muscle coordination required for hand opening and modulated aperture sculpting after injury. SCI participants also exhibited a unique wrist flexion pattern that would presumably oppose finger extension, possibly limiting their hand opening capacity especially at larger ball sizes; a behavior not observed in AB subjects. While AB participants appropriately rely on extensors for hand opening, we found those with SCI defaulted to increased flexor EMG activity at both the hand and wrist that increased with the requirement for greater hand opening (i.e., larger ball size). This possibility is seen in the reduced co-activation ratios (Figure 5) and increased activation of the flexor pattern (Figure 9) across ball sizes. It should be noted that wrist flexion could provide some passive finger extension contributing to hand opening, but the co-activation of wrist extensors with wrist flexors likely stiffened the wrist, reducing the likelihood of this possibility. However, future studies including wrist kinematics would be required to rule this out as a possible strategy.
Results from this study provide evidence for neuromotor redundancy after SCI, which denotes the nervous system's inherent ability to achieve a kinematic outcome using distinct neuromuscular strategies (Koshland et al. 2005; Latash et al. 2010). Such redundancy provides opportunity for functional recovery after SCI by allowing for flexibility so that the nervous system can reorganize and adapt when neural pathways become disrupted. For example, the nervous system may have rerouted a learned motor plan for hand opening, sending commands via spared pathways and developing new coordination patterns that traverse residual spinal circuitry. Evidence in animal models and humans suggests that such reorganization is possible both at the supraspinal level as well as below the lesion in the spinal motor networks (Raineteau and Schwab 2001; Bareyre et al. 2004; Girgis et al. 2007; Wrigley et al. 2009; Oudega and Perez 2012). Previous studies have shown similar preservation of reaching kinematics in neurologically injured groups with corresponding changes in muscle activations (Koshland et al. 2005; Rand et al. 2006), suggesting the prospect that such plasticity and goal-directed reorganization is possible across a range of neurological injuries. Further, understanding which features of hand shaping are preserved by the nervous system at the cost of others may suggest important targets for rehabilitation.
Preserved hand opening has important implications for hand rehabilitation after SCI. The ability to modulate hand aperture for object grasp is preserved within a limited capacity to open the hand. Quite often persons with cervical SCI have limited functional range of hand opening, compromising their ability to perform daily living activities. As a result, motor planning for aperture modulation is intact, but execution is limited. Thus, persons with incomplete SCI may benefit from rehabilitation aimed expanding the range of hand opening and addressing pathological co-activity of flexor muscles. Therapeutic approaches such as mass practice (Beekhuizen and Field-Fote 2005), botulinum toxin injections to reduce wrist/hand flexor coactivity (Marciniak et al. 2008), and electrical stimulation for grasp retraining (Popovic et al. 2006; Ragnarsson 2008) may be merited. However, the observed increase in EMG co-activity also may serve to stiffen the wrist joint for ensuring greater stability (Enoka 1997; Osu et al. 2002; Selen et al. 2006) and, thus, may in part represent a compensatory strategy.
While our findings provide new insight into the control of hand opening during reaching after SCI, several key questions remain. First, to what extent do intrinsic hand muscles (e.g., thenar eminence) contribute to the unique neuromuscular strategies that emerge after SCI? We speculate that thumb opposition is important in peak aperture modulation and quantifying their contribution is an important next step. Additional manual muscle testing to carefully characterize differences in muscle function between subjects might also provide insight into the varying degrees of hand opening abilities and differences in strategies used between subjects to achieve aperture modulation. Second, what compensatory strategies of the arm might be used in preparation for reach-to-grasp? Quantifying whole limb kinematics of the upper extremity may help uncover the which features of reach are altered after SCI, such as during transport versus during hand shaping (Jeannerod 1986) and ways to reinforce appropriate strategies to maximize preservation of hand function (Koshland et al. 2005). Because hand opening during reaching is controlled via distributed networks that involve supraspinal and spinal structures, different injuries may preserve different kinematic features and/or employ different neuromuscular strategies. The present study does not clarify the extent to which preservation of motor pathways is necessary. We found no relationship between maximum hand aperture and clinical measures of hand function impairment. A larger sample size from a heterogeneous population may allow for stratification to investigate these possibilities. Third, does the capacity to open the hand affect the functionality of grasp? Although our study provides evidence that persons with SCI preserve hand opening modulation during reach, how they execute object grasp remains unclear. The linkage between hand opening during reach and grasp function is not stereotypical after SCI due to heterogeneity of injury. For instance, some persons with cervical SCI may have limited ability to volitionally open their hand and extend their fingers yet achieve object grasp through compensatory strategies. In one case, they may position the hand on a stiff object and passively extend fingers around the object using more proximal effort from muscles less affected by the spinal injury. Successful grasp of the object may then be achieved via tenodesis without need for voluntary hand opening during reach. Despite use of compensatory strategies, a majority of persons with cervical SCI do not regain normal functioning grasp (Laffont et al. 2007). Thus, future quantitative studies that examine how, what, and why compensatory strategies used during hand opening and grasp are necessary to help identify sensorimotor deficits for which to target during SCI rehabilitation.
The neural substrates that contribute to hand opening after SCI remain unclear. Prior studies showed supraspinal structures, such as the intraparietal sulcus and anterior parietal lobe contribute to modulation of hand opening and are responsible for visuomotor transformation and planning of hand pre-shaping (Binkofski et al. 1998; Castiello 2005). One may speculate that after partial spinal injury, these same structures may provide the necessary commands to preserve hand opening. However, from our results the differences in coordination patterns between groups suggests alternative strategies that likely require new commands to preserve modulation of hand aperture during reaching. Although intact supraspinal pathways have the capacity release motor commands (e.g., via corticospinal tract) for hand opening, injury to spinal circuitry likely disrupts execution. Alternative possibilities may involve plasticity-inducing mechanisms of neural reorganization that exploits the motor system's inherent abundancy. For example, plasticity-driven changes along the neuraxis that includes propriospinal system (Pierrot-Deseilligny 1996; Alstermark et al. 2007; Pettersson et al. 2007) and spared corticospinal tracts (Oudega and Perez 2012) may unmask networks to successfully perform a skilled motor task. Although spontaneous plasticity of the central nervous system is limited in the adult human after SCI, motor recovery can occur for several years post-injury, with the degree of recovery dependent upon the reorganization of pathways spared by the lesion (Green et al. 1999). Nevertheless, further studies are warranted to identify the neural mechanisms that contribute to preserved hand opening after SCI.
Overall, this study revealed the unique neuromuscular constraints and strategies for controlling hand opening after incomplete SCI. Despite the altered coordination patterns, subjects with SCI preserved maximum hand aperture modulation, a vital feature for successful object grasp. These findings may help guide new directions for rehabilitation and intervention development for persons with cervical SCI.
Acknowledgments
This work was supported in part by NIH grant K12 HD055931 and Craig H. Neilsen Foundation. The authors are very grateful to the participants. The authors would also like to thank Ian Cooke, Matthew Freeman, and Dennis Valerstain for their assistance with data collection.
References
- Alberts JL, Saling M, Adler CH, Stelmach GE. Disruptions in the reach-to-grasp actions of Parkinson's patients. Exp Brain Res. 2000;134:353–362. doi: 10.1007/s002210000468. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Pettersson LG, Sasaki S. The C3-C4 propriospinal system in the cat and monkey: a spinal pre-motoneuronal centre for voluntary motor control. Acta Physiol (Oxf) 2007;189:123–140. doi: 10.1111/j.1748-1716.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Beekhuizen KS, Field-Fote EC. Massed practice versus massed practice with stimulation: effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil Neural Repair. 2005;19:33–45. doi: 10.1177/1545968305274517. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci. 1998;10:3918–3922. doi: 10.1046/j.1460-9568.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- Castiello U. The neuroscience of grasping. Nat Rev Neurosci. 2005;6:726–736. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I, Burman K, Darian-Smith C. Parallel pathways mediating manual dexterity in the macaque. Exp Brain Res. 1999;128:101–108. doi: 10.1007/s002210050824. [DOI] [PubMed] [Google Scholar]
- Delagi EF, Perotto A. Anatomical Guide for the Electromyographer. Charles C. Thomas; Springfield: 1979. [Google Scholar]
- Di Rienzo F, Guillot A, Mateo S, Daligault S, Delpuech C, Rode G, Collet C. Neuroplasticity of prehensile neural networks after quadriplegia. Neuroscience. 2014;274:82–92. doi: 10.1016/j.neuroscience.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP, Roy RR. Rehabilitative therapies after spinal cord injury. J Neurotrauma. 2006;23:560–570. doi: 10.1089/neu.2006.23.560. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Neural strategies in the control of muscle force. Muscle Nerve Suppl. 1997;5:S66–69. [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: A reversible inactivation study. Brain. 2001;124:571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Fouad K, Dietz V, Schwab ME. Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res Brain Res Rev. 2001;36:204–212. doi: 10.1016/s0165-0173(01)00096-0. [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport. 1994;5:1525–1529. doi: 10.1097/00001756-199407000-00029. [DOI] [PubMed] [Google Scholar]
- Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical motor reorganization after paraplegia: an EEG study. Neurology. 1999;53:736–743. doi: 10.1212/wnl.53.4.736. [DOI] [PubMed] [Google Scholar]
- Haggard P, Wing A. Coordination of hand aperture with the spatial path of hand transport. Exp Brain Res. 1998;118:286–292. doi: 10.1007/s002210050283. [DOI] [PubMed] [Google Scholar]
- Harvey LA, Batty J, Jones R, Crosbie J. Hand function of C6 and C7 tetraplegics 1 - 16 years following injury. Spinal Cord. 2001;39:37–43. doi: 10.1038/sj.sc.3101101. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Intersegmental coordination during reaching at natural visual objects. In: Baddeley RLA, editor. Attention and performance IX. Erlbaum Associates; Hillsdale, NJ: 1981. pp. 153–169. [Google Scholar]
- Jeannerod M. The Formation of Finger Grip during Prehension - a Cortically Mediated Visuomotor Pattern. Behavioural Brain Research. 1986;19:99–116. doi: 10.1016/0166-4328(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- Kapadia N, Zivanovic V, Popovic MR. Restoring voluntary grasping function in individuals with incomplete chronic spinal cord injury: pilot study. Top Spinal Cord Inj Rehabil. 2013;19:279–287. doi: 10.1310/sci1904-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland GF, Galloway JC, Farley B. Novel muscle patterns for reaching after cervical spinal cord injury: a case for motor redundancy. Experimental Brain Research. 2005;164:133–147. doi: 10.1007/s00221-005-2218-9. [DOI] [PubMed] [Google Scholar]
- Laffont I, Briand E, Dizien O, Combeaud M, Bussel B, Revol M, Roby-Brami A. Kinematics of prehension and pointing movements in C6 quadriplegic patients. Spinal Cord. 2000;38:354–362. doi: 10.1038/sj.sc.3100999. [DOI] [PubMed] [Google Scholar]
- Laffont I, Hoffmann G, Dizien O, Revol M, Roby-Brami A. How do C6/C7 tetraplegic patients grasp balls of different sizes and weights? Impact of surgical musculo-tendinous transfers. Spinal Cord. 2007;45:502–512. doi: 10.1038/sj.sc.3102047. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- Latash ML, Friedman J, Kim SW, Feldman AG, Zatsiorsky VM. Prehension synergies and control with referent hand configurations. Exp Brain Res. 2010;202:213–229. doi: 10.1007/s00221-009-2128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene H. Robust Tests for Equality of Variances, in Contributions to Probability and Statistics. Stanford University Press; 1960. [Google Scholar]
- Marciniak C, Rader L, Gagnon C. The use of botulinum toxin for spasticity after spinal cord injury. Am J Phys Med Rehabil. 2008;87:312–317. doi: 10.1097/PHM.0b013e318168ceaf. quiz 318-320, 329. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Ditunno JF, Jr, Donovan WH, Maynard F., Jr Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil. 1999;80:1391–1396. doi: 10.1016/s0003-9993(99)90249-6. [DOI] [PubMed] [Google Scholar]
- Marteniuk RG, Leavitt JL, Mackenzie CL, Athenes S. Functional-Relationships between Grasp and Transport Components in a Prehension Task. Human Movement Science. 1990;9:149–176. doi: 10.1016/0167-9457(90)90025-9. [DOI] [Google Scholar]
- Mateo S, Revol P, Fourtassi M, Rossetti Y, Collet C, Rode G. Kinematic characteristics of tenodesis grasp in C6 quadriplegia. Spinal Cord. 2013;51:144–149. doi: 10.1038/sc.2012.101. [DOI] [PubMed] [Google Scholar]
- Michaelsen SM, Magdalon EC, Levin MF. Grip aperture scaling to object size in chronic stroke. Motor Control. 2009;13:197–217. doi: 10.1123/mcj.13.2.197. [DOI] [PubMed] [Google Scholar]
- Muir RB, Lemon RN. Corticospinal neurons with a special role in precision grip. Brain Res. 1983;261:312–316. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- Osu R, Franklin DW, Kato H, Gomi H, Domen K, Yoshioka T, Kawato M. Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J Neurophysiol. 2002;88:991–1004. doi: 10.1152/jn.2002.88.2.991. [DOI] [PubMed] [Google Scholar]
- Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol. 2012;590:3647–3663. doi: 10.1113/jphysiol.2012.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson LG, Alstermark B, Blagovechtchenski E, Isa T, Sasaski S. Skilled digit movements in feline and primate--recovery after selective spinal cord lesions. Acta Physiol (Oxf) 2007;189:141–154. doi: 10.1111/j.1748-1716.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Prog Neurobiol. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Platz T, Pinkowski C, van Wijck F, Kim IH, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin Rehabil. 2005;19:404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord. 2006;44:143–151. doi: 10.1038/sj.sc.3101822. [DOI] [PubMed] [Google Scholar]
- Ragnarsson KT. Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord. 2008;46:255–274. doi: 10.1038/sj.sc.3102091. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Rand MK, Smiley-Oyen AL, Shimansky YP, Bloedel JR, Stelmach GE. Control of aperture closure during reach-to-grasp movements in Parkinson's disease. Exp Brain Res. 2006;168:131–142. doi: 10.1007/s00221-005-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selen LP, Beek PJ, van Dieen JH. Impedance is modulated to meet accuracy demands during goal-directed arm movements. Exp Brain Res. 2006;172:129–138. doi: 10.1007/s00221-005-0320-7. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An Analysis of Variance Test for Normality (Complete Samples) Biometrika. 1965;52:591. doi: 10.2307/2333709. [DOI] [Google Scholar]
- Topka H, Cohen LG, Cole RA, Hallett M. Reorganization of corticospinal pathways following spinal cord injury. Neurology. 1991;41:1276–1283. doi: 10.1212/wnl.41.8.1276. [DOI] [PubMed] [Google Scholar]
- Weiss EJ, Flanders M. Muscular and postural synergies of the human hand. J Neurophysiol. 2004;92:523–535. doi: 10.1152/jn.01265.2003. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, et al. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]


