Abstract
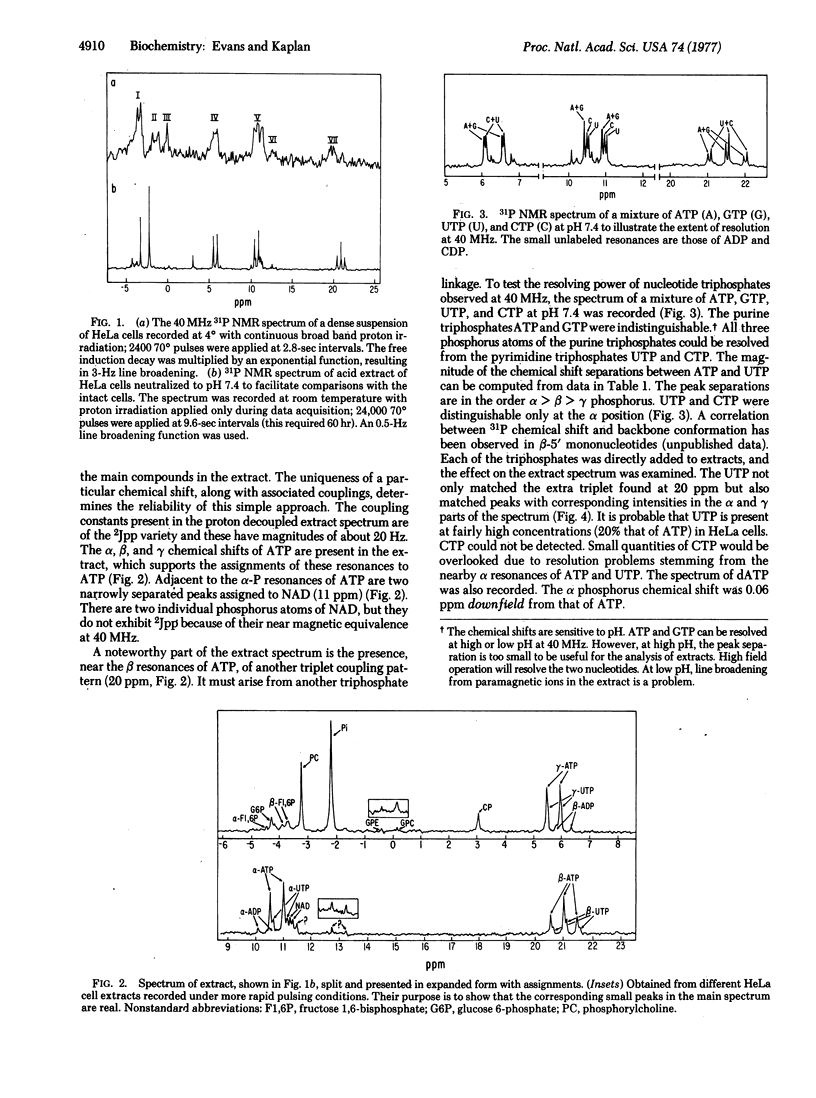
A survey of phosphorus compounds present in HeLa cells and their acid extracts has been carried out by 31P nuclear magnetic resonance spectroscopy at 40 MHz. The proton decoupled 31P spectrum of the neutralized extract had resolution adequate to enable the identification of the main phosphate compounds. The spectral intensities were converted to concentrations. The lower detection limit with extensive signal averaging was 0.02 μmol for the extract. The composition, listed in order of decreasing concentration, was: inorganic phosphate, ATP, phosphorylcholine, creatine phosphate, UTP, NAD+, glucose 6-phosphate, β-D-fructose 1,6-bisphosphate, α-D-fructose 1,6-bisphosphate, ADP, α-glycerophosphorylcholine, and α-glycerophosphorylethanolamine. UTP made up ⅕ of the total nucleotide triphosphate content. The composition was compared to the 31P spectrum of an extract from a human astrocytoma grown in athymic mice. The signal from P-containing macromolecules such as nucleic acids was not detected in the intact HeLa cell spectrum because of broad lines. Effects of the glycolysis inhibitor iodoacetic acid could be clearly shown in spectra of both the intact cell and the extract as buildup of fructose 1,6-bisphosphate at the expense of ATP, UTP, and creatine phosphate.
Keywords: human astrocytoma, acid-soluble phosphates, metabolic inhibitors
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burt C. T., Glonek T., Bárány M. Analysis of living tissue by phosphorus-31 magnetic resonance. Science. 1977 Jan 14;195(4274):145–149. doi: 10.1126/science.188132. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Colman A., Gadian D. G. 31P nuclear-magnetic-resonance studies on the developing embryos of Xenopus laevis. Eur J Biochem. 1976 Jan 15;61(2):387–396. doi: 10.1111/j.1432-1033.1976.tb10032.x. [DOI] [PubMed] [Google Scholar]
- Gadian D. G., Hoult D. I., Radda G. K., Seeley P. J., Chance B., Barlow C. Phosphorus nuclear magnetic resonance studies on normoxic and ischemic cardiac tissue. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4446–4448. doi: 10.1073/pnas.73.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- Henderson T. O., Costello A. J., Omachi A. Phosphate metabolism in intact human erythrocytes: determination by phosphorus-31 nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2487–2490. doi: 10.1073/pnas.71.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis D. P., Nunnally R. L., Jacobus W. E., Taylor G. J., 4th Detection of regional ischemia in perfused beating hearts by phosphorus nuclear magnetic resonance. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1086–1091. doi: 10.1016/0006-291x(77)91493-0. [DOI] [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Taylor G. J., 4th, Hollis D. P., Nunnally R. L. Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature. 1977 Feb 24;265(5596):756–758. doi: 10.1038/265756a0. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. 31P nuclear magnetic resonance studies of Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):87–91. doi: 10.1073/pnas.74.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]


