Abstract
Objectives
To study the association of adiposity with longitudinal kidney function change in 544 HIV-infected persons in Study of Fat Redistribution and Metabolic Change in HIV infection (FRAM) cohort over 5 years of follow-up.
Methods
Regional distribution of muscle and adipose tissue was quantified by whole-body MRI, and total adiponectin and leptin levels were measured in serum. Kidney function was assessed by estimated glomerular filtration rate from serum cystatin C (eGFRCys), obtained at baseline and follow-up. Rapid kidney function decline was defined as annual loss of eGFRCys ≥ 3 ml/min/1.73m2, and incident chronic kidney disease (CKD) was defined at eGFRCys < 60 ml/min/1.73m2. Multivariate regression analysis was adjusted for age, race, gender, glucose, antihypertensive use, serum albumin, baseline and change in HIV viral load.
Results
At baseline, mean age was 43 years, mean eGFRCys 86 ml/min/1.73m2, and 21% had albuminuria. Mean (standard deviation) eGFRCys decline was −0.11 ± 4.87 ml/min/1.73m2 per year; 23% of participants had rapid kidney function decline, and 10% developed incident CKD. Lowest tertile of visceral adipose tissue and highest tertile of adiponectin were both marginally associated with annual kidney function decline of −0.5 ml/min/1.73m2 each, but these associations were not statistically significant after adjustment. We found no statistically significant associations of MRI-measured regional adiposity or serum adipokines with rapid kidney function decline or incident CKD (all p-values > 0.1 in adjusted models).
Conclusions
Contrary to findings in the general population, adiposity did not have a substantial association with longitudinal change in kidney function among HIV-infected persons.
Keywords: adiposity, FRAM, HIV, kidney decline
Introduction
Manifestations of chronic kidney disease (CKD) in HIV-infected adults have evolved with wider application of combination antiretroviral therapy. CKD due to hypertensive vascular disease, diabetes, co-infection with hepatitis C, and certain antiretroviral drugs is now more common than HIV-associated nephropathy.1, 2 Risk factors for CKD in HIV remain under active investigation. In addition to traditional kidney disease risk factors (e.g. hypertension and albuminuria), HIV-related risk factors (e.g. lower CD4 lymphocyte count, higher HIV RNA level, and co-infection with hepatitis C) contribute to progression and onset of CKD.3, 4, 6, 7
In the general population, obesity has been associated with a higher risk for both incident CKD8–10 and end-stage renal disease (ESRD).11, 12 High serum leptin concentration has been shown to be independently associated with higher prevalence of CKD, even after accounting for body mass index (BMI)13; and high adiponectin level is associated with faster decline of kidney function. 14, 15 In HIV infection, high visceral and upper trunk subcutaneous fat, and low leg subcutaneous fat are associated with increased mortality16, insulin resistance17, proatherogenic and dyslipidemic profile18, and higher cardiovascular disease risk. 19 While leptin similarly correlates with adiposity in HIV-infected and uninfected populations, 20, 21 decreased adiponectin associated with antiretroviral drugs induced loss of subcutaneous fat in the leg is unique to HIV-infected persons.21 It is not clear how regional adiposity and serum adipokines in HIV-infected persons relate to kidney disease progression.
Therefore, we performed this analysis to assess the association of MRI-measured regional distribution of fat and muscle and two serum adiposity markers (leptin and adiponectin) with longitudinal kidney function change in HIV-infected persons over 5 years of follow-up.
Methods
Subjects
This prospective study included 554 HIV-infected participants followed over 5 years in the FRAM study. FRAM was initially designed to study the association of HIV infection and its treatments with progression of fat and metabolic changes. The methods have been previously described.22 Briefly, HIV-infected participants were recruited between June 2000 and September 2002 from 16 geographically diverse sites in the United States. The demographics of the FRAM cohort are representative of the HIV-infected adults in the United States.22 Follow-up data were obtained from October 2004 through August 2007, with an average of 5 years since the baseline visit. Of 1183 HIV-infected participants at baseline, 581 were seen at follow-up. This study included 554 participants with measurements of cystatin C at baseline and follow-up. Exclusion criteria for FRAM and this analysis were age less than 18 years, current or planned pregnancy within 3 months of enrollment, contraindication to the MRI scan, or failure to follow-up. The protocol was approved by institutional review boards at all sites.
Predictors
Whole-body magnetic resonance imaging (MRI) was performed to quantify body composition in terms of regional subcutaneous adipose tissue, visceral adipose tissue, and total skeletal muscle volume in all participants using a standard protocol, as described previously.22 Volume of each tissue depot was calculated by means of a mathematical algorithm.23 Tissue volume was quantified at the following sites: visceral adipose tissue (VAT), total skeletal muscle (SM), total adipose tissue (total fat), arm and leg subcutaneous adipose tissue (arm and leg SAT), upper trunk SAT including chest and back, and lower trunk SAT including abdomen and back. Total adiponectin and leptin levels were measured by RIA at Linco Research Inc. (St. Louis, MO). The sensitivity of assays, and interassay and intraassay coefficients of variation were published previously.21
Other measurements
Age, gender, ethnicity, and medical history were determined by self-report; and tobacco and illicit drug use were assessed by a standardized questionnaire. Diabetes was defined as hypoglycemic medication use or fasting glucose ≥ 126. Height, weight, and blood pressure were measured by standardized protocols. Laboratory data (CD4 lymphocyte count, HIV RNA, HCV RNA, cystatin C, serum direct low-density lipoprotein (D-LDL), high-density lipoprotein (HDL), triglycerides, serum albumin, urine microalbumin and creatinine) were measured at baseline, as previously described.16, 22 Research associates performed medical chart abstraction of medications and medical history at HIV sites.
Primary outcomes
Kidney function was assessed by estimated glomerular filtration rate from serum cystatin C (eGFRCys) level on two collections (baseline and a follow-up visit) according to the CKD-EPI equation. The characteristics of the assay and equation have been previously published.6, 24 Cystatin C has shown stronger associations with mortality in the HIV population compared to creatinine.25
Changes in kidney function were evaluated as continuous and dichotomized variables. Annual change in eGFRCys from baseline to the follow-up visit was calculated from the difference in two measurements and elapsed time between them, and expressed in ml/min/1.73m2 per year. Rapid kidney function decline was defined a priori as loss of eGFRCys ≥ 3 ml/min/1.73m2 per year from baseline to the follow-up visit. This cutoff has been shown to be associated with change in HIV viremia and mortality.6, 26 Incident CKD was defined as development of eGFRCys < 60ml/min/1.73m2 and decline in eGFRCys of > 1ml/min/1.73m2 per year at follow-up visit in persons without CKD at baseline.
Statistical analysis
We first determined annual change in eGFR for each tertile of baseline body composition measures and adipokines using least-squares means (LS-means) from an age-adjusted linear regression model. We next used multivariable linear regression models to evaluate associations with annual change in eGFR, in separate models for each candidate measure of body composition or adipokine. Multivariable logistic regression models were used to estimate odds ratios for rapid kidney function decline and incident CKD. Persons with CKD at baseline (N = 47) were excluded from incident CKD analysis.
Covariates were selected as previously described,6 using stepwise regression with p ≤ 0.05 for entry and retention. The fully adjusted model included demographic (self-reported age, race, and gender), HIV-related risk factors (HIV RNA level at baseline and change from baseline during follow-up, CD4 count change from baseline to follow-up, and duration of saquinavir exposure), and kidney disease related risk factors (baseline glucose measurement, diagnosis of diabetes mellitus, use of medications (hypoglycemic, antihypertensive, hypolipidemic), D-LDL, HDL, and serum albumin). Because of their skewed distribution, body composition and adipokines measures were log-transformed. Multiple imputation utilizing the Markov chain Monte Carlo method for arbitrary missing data was used to impute missing covariates prior to covariate selection.27 There was no interaction of diabetes and hypertension with the associations of predictors and outcomes. For example, in analyses of VAT and rapid kidney function decline, interaction was quantified at p = 0.84 for diabetes and 0.96 for hypertension.
To account for those with missing follow-up data, we adjusted estimates using an inverse probability weighting approach by modeling the participant’s probability of having a non-missing outcome at the follow-up visit.28 The inverse of this probability was then used as a weight applied to persons with known outcome in the multivariable regression analyses of changes in kidney function.
Results
Baseline characteristics of FRAM participants are presented in Table 1. Median age at baseline was 43 years old, and median eGFRCys was 86 ml/min/1.73m2; 30% were women, 43% African American, and 48% Caucasian; 6% of participants had coronary artery disease, 8% diabetes, 28% hypertension, 8% CKD by eGFRCys < 60 ml/min/1.73m2, and 21% had albuminuria. The median and baseline CD4 count was 126 and 385 respectively, 69% had a prior or current history of AIDS, 87% reported taking combination antiretroviral therapy currently or in the past, and 45% had currently undetectable plasma HIV RNA.
Table 1.
Baseline characteristics of HIV-infected FRAM participants
| Parameter | N = 554 |
|---|---|
| Baseline Age (y) | 43 (37–48) |
| Female | 167 (30%) |
| Race | |
| African American | 236 (43%) |
| Caucasian | 266 (48%) |
| Other | 52 (9%) |
| History of CAD | 34 (6%) |
| Cigarette smoking | |
| Current | 206 (37%) |
| Past | 123 (22%) |
| Never | 225 (41%) |
| Diabetes mellitus | 45 (8%) |
| Systolic BP (mmHg) | 116 (107–124) |
| Diastolic BP (mmHg) | 78 (71–85) |
| Antihypertensive use | 114 (21%) |
| Hypertension | 153 (28%) |
| LDL (mg/dL) | 109 (79–137) |
| HDL (mg/dL) | 41 (34–52) |
| TG (mg/dL) | 153 (97–279) |
| BMI (kg/m2) | 25 (22–28) |
| Waist Circumference (cm) | 88 (81–97) |
| Ever HAART use | 480 (87%) |
| Current CD4 count | 385 (232–560) |
| Nadir CD4 count | 126 (30–251) |
| History of AIDS | 383 (69%) |
| Plasma HIV RNA: | |
| ≤ 80 | 247 (45%) |
| 81–1999 | 120 (22%) |
| 2000–9999 | 59 (11%) |
| >10000 | 128 (23%) |
| Hepatitis C | 122 (22%) |
| Current heroin use | 107 (20%) |
| Albuminuria* | 95 (21%) |
| ACR (mg/g) | 7.4 (4.8–17.3) |
| eGFRCys< 60 | 47 (8%) |
| eGFRCys(ml/min/1.73m2) | 86 (72–103) |
Data are presented as Median (IQR) or numbers (percent). ACR – albumin to creatinine ratio.
defined as a positive urine dipstick result (≥1+) or urine albumin-creatinine ratio >30 mg/g.
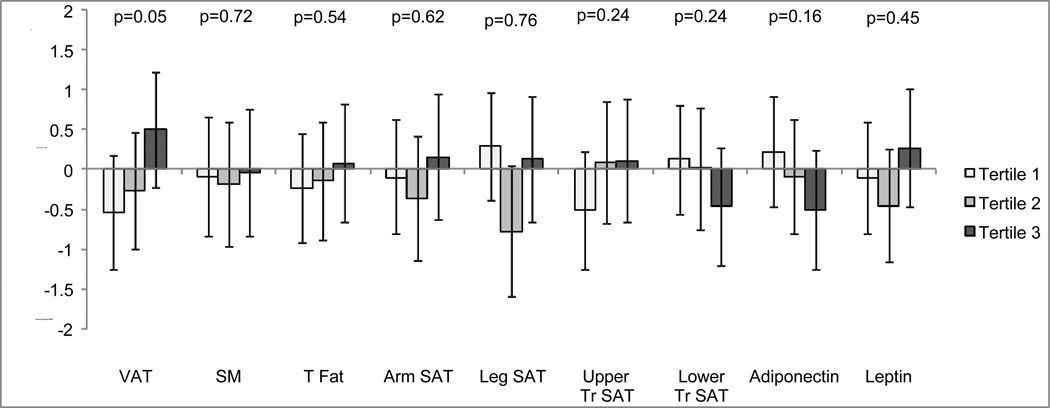
We compared the age-adjusted annual change in eGFRCys by tertiles of each body depot and adipokine, separately. In the age-adjusted model, persons in the highest tertile of baseline VAT had a small improvement in kidney function of +0.50 ml/min/1.73m2/yr (−0.23, 1.22), while those in the lowest tertile experienced decline by −0.54 ml/min/1.73m2/yr (−1.25, 0.18); with p = 0.05 for difference across tertiles. By contrast, the highest baseline tertile of adiponectin was associated with decline in kidney function at follow-up, however the association did not reach statistical significance (p = 0.2). The other baseline body composition regions and leptin showed little association with annual change in eGFRCys (Figure 1). After multivariable adjustment for HIV-related and kidney disease risk factors, associations of body depots and adipokines with annual change in kidney function remained weak and statistically non-significant (Table 2). For example, the association of baseline VAT with annual change in eGFR was + 0.074 (−0.26, 0.41) ml/min/1.73m2 for each doubling of VAT (p = 0.7).
Figure 1.
Age-adjusted associations of baseline body composition measures and adipokines (in tertiles) with annual change in eGFRCys in HIV-infected FRAM participants (N=554).
Data are represented as bars – medians, whiskers – 95% CI for the median. VAT – visceral adipose tissue, SM – total skeletal muscle, T Fat – total fat, Arm SAT – arm subcutaneous adipose tissue, Leg SAT – leg subcutaneous adipose tissue, Upper and Lower Tr SAT – upper and lower trunk subcutaneous adipose tissue.
Table 2.
Association of baseline body composition measures and adipokines with annual eGFRCys change* in HIV-infected FRAM participants (N = 554).
|
(results are per doubling) |
Unadjusted Estimate (95%CI) |
Adjusted Estimate (95%CI)** (single measures) |
|---|---|---|
| MRI-measured body composition: | ||
| VAT | 0.22 (−0.10, 0.54) p=0.18 |
0.07 (−0.26, 0.41) p=0.67 |
| Skeletal Muscle | 0.18 (−1.62, 1.98) p=0.84 |
0.17 (−1.60, 1.93) p=0.85 |
| Total Fat | 0.10 (−0.36, 0.56) p=0.67 |
0.18 (−0.33, 0.69) p=0.50 |
| Arm SAT | 0.12 (−0.47, 0.71) p=0.69 |
0.24 (−0.43, 0.91) p=0.48 |
| Leg SAT | 0.05 (−0.36, 0.46) p=0.81 |
0.27 (−0.24, 0.79) p=0.30 |
| Upper Trunk SAT | 0.19 (−0.25, 0.64) p=0.39 |
0.22 (−0.26, 0.69) p=0.37 |
| Lower Trunk SAT | −0.09 (−0.44, 0.27) p=0.64 |
−0.05 (−0.44, 0.35) p=0.81 |
| Adipokines: | ||
| Adiponectin | −0.28 (−0.63, 0.080) p=0.13 |
−0.16 (−0.53, 0.21) p=0.39 |
| Leptin | 0.03 (−0.27, 0.33) p=0.84 |
0.11 (−0.27, 0.49) p=0.57 |
Annual change in eGFR in ml/min/1.73m2
Covariates in fully adjusted model include baseline age, race, gender, glucose, antihypertensive use, hypolipidemic use, HDL, D-LDL, viral load, change in viral load, change in CD4 count, duration of saquinavir exposure.
In this cohort, 125 (23%) participants met our definition of rapid kidney function decline during follow-up. In unadjusted analysis, each doubling of adiponectin was associated with an 18% higher odds of rapid decline in kidney function, but the association weakened (OR 1.11, p = 0.3) when fully adjusted. (Table 3) Finally, 51 (10%) participants developed incident CKD at follow up. Regional distribution of fat and muscle, and level of adipokine showed little association with incidence of CKD. (Table 3)
Table 3.
Association of baseline body composition measures and adipokines with rapid decline* and incident CKD**by cystatin C in HIV-infected FRAM participants
| Rapid decline (N = 125) | Incident CKD (N = 51) | |
|---|---|---|
|
(results are per doubling) |
Adjusted odds ratio (95% CI) *** single measures) |
Adjusted odds ratio (95% CI) *** (single measures) |
| MRI-measured body composition: | ||
| VAT | 0.91 (0.76, 1.08) p=0.29 |
0.94 (0.72, 1.22) p=0.65 |
| Skeletal Muscle | 0.62 (0.23, 1.65) p=0.33 |
0.65 (0.16, 2.6) p=0.54 |
| Total Fat | 0.88 (0.66, 1.16) p=0.36 |
0.95 (0.63, 1.44) p=0.82 |
| Arm SAT | 0.83 (0.57, 1.20) p=0.32 |
0.81 (0.47, 1.41) p=0.45 |
| Leg SAT | 0.87 (0.65, 1.17) p=0.37 |
1.03 (0.67, 1.57) p=0.89 |
| Upper Trunk SAT | 0.85 (0.65, 1.10) p=0.21 |
0.82 (0.55, 1.20) p=0.30 |
| Lower Trunk SAT | 0.96 (0.76, 1.20) p=0.70 |
1.12 (0.80, 1.56) p=0.51 |
| Adipokines: | ||
| Adiponectin | 1.11 (0.90, 1.38) p=0.33 |
1.11 (0.80, 1.53) p=0.54 |
| Leptin | 0.93 (0.75, 1.14) p=0.49 |
1.03 (0.75, 1.41) p=0.85 |
Rapid decline defined as annual eGFR loss ≥3 mL/min/1.73m2
CKD defined as eGFR< 60ml/min/1.73m2 and decline in eGFR of > 1ml/min/year. Persons with CKD at baseline are excluded from analysis. Multiple imputation is used to impute missing covariates.
covariates in fully adjusted model include age, race, gender, DM, albumin, viral load, change in viral load.
Discussion
In this nationally-representative cohort of HIV-infected adults, we found that MRI measures of fat and muscle depots and serum adipokines had insignificant associations with longitudinal kidney function decline and incident CKD. This finding is important because of the current public health emphasis on prevention of chronic diseases, such as CKD. Extrapolation of epidemiologic findings from the general population with regard to obesity may not be appropriate in the setting of HIV. Prior literature has suggested that, in the general population, obesity may be a risk factor for kidney function decline, but data are conflicting. While some studies show an association of high BMI with incident ESRD11, 12 and CKD10, others report these findings to be confounded by cardiovascular disease risk factors. 9, 29 Other studies have suggested that central adiposity rather than BMI is a risk factor for decline in kidney function.8, 30 Our study expands on this literature by assessing obesity markers in HIV-infected persons, a unique and high risk population for development and progression of CKD. It has been previously shown that measures of adiposity are associated with increased overall mortality16 and cardiovascular disease risk factors19 in the HIV population. In addition, central obesity has been associated with higher prevalence of hypertension in HIV-infected persons regardless of presence or absence of peripheral HIV-associated lipoatrophy.31 However, this study, found no significant association of MRI-measured regional distribution of fat and muscle volume and levels of serum adipokines with kidney function outcomes. It is likely that other factors, such as HIV viremia and an associated inflammatory state have stronger bearing on kidney prognosis. Prior studies in FRAM demonstrated that effective viremic control was associated with improvement in kidney function.6 Additionally, the metabolic and hemodynamic damage from adiposity may become clinically evident only with severe obesity, which was not captured in this cohort. Whether changes in adiposity would lead to a change in kidney disease risk in the HIV population cannot be answered by this study.
Among the strengths of this study are the geographic and racial diversity of the cohort, objective measurements of body composition and serum adipokines, and longitudinal follow-up. Prevalence of CKD and albuminuria were low at baseline, which provided the opportunity to study risk factors prior to development of kidney disease. However, we were limited by a relatively short follow-up period. Additionally, this cohort captured generally non-obese individuals with a median BMI 25 kg/m2so the distribution was lower than that of the general population. Also, the majority of FRAM participants had a prior or current diagnosis of AIDS, and many were treated with early forms of antiretroviral therapy. Therefore, the lower BMI values in this cohort may signify complications of HIV disease, rather than optimal health due to the absence of obesity. Finally, we are limited by use of indirect measures of GFR because direct measurements are not practical in large epidemiologic studies. However, prior studies have demonstrated superiority of cystatin C as a surrogate measure of eGFR in the HIV population. 3, 5, 32
In summary, our findings suggest that neither regional distribution of adipose tissue nor serum adipokines are significantly associated with change in kidney function in the HIV-infected population. Future studies with more HIV-infected but non-AIDS participants and longer follow-up period.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the FRAM study for their valuable contributions. Dr. Peralta is funded by grants 1K23DK082793-01 and 1R03DK095877-01 from NIDDK. These funding sources had no involvement in the design or execution of this study.
Footnotes
There are no potential conflicts of interest.
References
- 1.Fernando SK, Finkelstein FO, Moore BA, Weissman S. Prevalence of chronic kidney disease in an urban hiv infected population. The American journal of the medical sciences. 2008;335:89–94. doi: 10.1097/MAJ.0b013e31812e6b34. [DOI] [PubMed] [Google Scholar]
- 2.Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, Shlipak MG. Association of tenofovir exposure with kidney disease risk in hiv infection. Aids. 2012;26:867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, Shlipak MG. Cystatin c level as a marker of kidney function in human immunodeficiency virus infection: The fram study. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, Wohl D, Shlipak MG. Microalbuminuria in hiv infection. Aids. 2007;21:1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrella MM, Parekh RS, Astor BC, Bolan R, Evans RW, Palella FJ, Jr, Jacobson LP. Chronic kidney disease and estimates of kidney function in hiv infection: A cross-sectional study in the multicenter aids cohort study. Journal of acquired immune deficiency syndromes. 2011;57:380–386. doi: 10.1097/QAI.0b013e318222f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longenecker CT, Scherzer R, Bacchetti P, Lewis CE, Grunfeld C, Shlipak MG. Hiv viremia and changes in kidney function. Aids. 2009;23:1089–1096. doi: 10.1097/QAD.0b013e32832a3f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for esrd in hiv-infected individuals: Traditional and hiv-related factors. Am J Kidney Dis. 2012;59:628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, Levey AS, Weiner DE. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52:29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Boer IH, Katz R, Fried LF, Ix JH, Luchsinger J, Sarnak MJ, Shlipak MG, Siscovick DS, Kestenbaum B. Obesity and change in estimated gfr among older adults. Am J Kidney Dis. 2009;54:1043–1051. doi: 10.1053/j.ajkd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bash LD, Astor BC, Coresh J. Risk of incident esrd: A comprehensive look at cardiovascular risk factors and 17 years of follow-up in the atherosclerosis risk in communities (aric) study. Am J Kidney Dis. 2010;55:31–41. doi: 10.1053/j.ajkd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Shankar A, Syamala S, Xiao J, Muntner P. Relationship between plasma leptin level and chronic kidney disease. International journal of nephrology. 2012;2012:269532. doi: 10.1155/2012/269532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsnefes M, Kartal J, Khoury P, Daniels S. Adiponectin in children with chronic kidney disease: Role of adiposity and kidney dysfunction. Clin J Am Soc Nephrol. 2007;2:46–50. doi: 10.2215/CJN.02790806. [DOI] [PubMed] [Google Scholar]
- 15.Kollerits B, Fliser D, Heid IM, Ritz E, Kronenberg F. Gender-specific association of adiponectin as a predictor of progression of chronic kidney disease: The mild to moderate kidney disease study. Kidney international. 2007;71:1279–1286. doi: 10.1038/sj.ki.5002191. [DOI] [PubMed] [Google Scholar]
- 16.Scherzer R, Heymsfield SB, Lee D, Powderly WG, Tien PC, Bacchetti P, Shlipak MG, Grunfeld C Study of Fat R. Metabolic Change in HIVI. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in hiv infection. Aids. 2011;25:1405–1414. doi: 10.1097/QAD.0b013e32834884e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunfeld C, Rimland D, Gibert CL, Powderly WG, Sidney S, Shlipak MG, Bacchetti P, Scherzer R, Haffner S, Heymsfield SB. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and hiv-infected subjects in the fram study. Journal of acquired immune deficiency syndromes. 2007;46:283–290. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wohl D, Scherzer R, Heymsfield S, Simberkoff M, Sidney S, Bacchetti P, Grunfeld C, Investigators FS. The associations of regional adipose tissue with lipid and lipoprotein levels in hiv-infected men. Journal of acquired immune deficiency syndromes. 2008;48:44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lake JE, Wohl D, Scherzer R, Grunfeld C, Tien PC, Sidney S, Currier JS. Regional fat deposition and cardiovascular risk in hiv infection: The fram study. AIDS care. 2011;23:929–938. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 21.Kosmiski LA, Bacchetti P, Kotler DP, Heymsfield SB, Lewis CE, Shlipak MG, Scherzer R, Grunfeld C. Relationship of fat distribution with adipokines in human immunodeficiency virus infection. The Journal of clinical endocrinology and metabolism. 2008;93:216–224. doi: 10.1210/jc.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in hiv infection (fram): Methods, design, and sample characteristics. American journal of epidemiology. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen W, Wang Z, Tang H, Heshka S, Punyanitya M, Zhu S, Lei J, Heymsfield SB. Volume estimates by imaging methods: Model comparisons with visible woman as the reference. Obesity research. 2003;11:217–225. doi: 10.1038/oby.2003.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating gfr using serum cystatin c alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with ckd. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driver TH, Scherzer R, Peralta CA, Tien PC, Estrella MM, Parikh CR, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Abraham A, Shlipak MG. Comparisons of creatinine and cystatin c for detection of kidney disease and prediction of all-cause mortality in hiv-infected women. Aids. 2013;27:2291–2299. doi: 10.1097/QAD.0b013e328362e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer JL. Multiple imputation: A primer. Statistical methods in medical research. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 28.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an aids clinical trial with inverse probability of censoring weighted (ipcw) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 29.Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, Levy D, Fox CS. Overweight, obesity, and the development of stage 3 ckd: The framingham heart study. Am J Kidney Dis. 2008;52:39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young JA, Hwang SJ, Sarnak MJ, Hoffmann U, Massaro JM, Levy D, Benjamin EJ, Larson MG, Vasan RS, O'Donnell CJ, Fox CS. Association of visceral and subcutaneous adiposity with kidney function. Clin J Am Soc Nephrol. 2008;3:1786–1791. doi: 10.2215/CJN.02490508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freitas P, Carvalho D, Santos AC, Madureira AJ, Xerinda S, Martinez E, Pereira J, Sarmento A, Medina JL. Central/peripheral fat mass ratio is associated with increased risk of hypertension in hiv-infected patients. Journal of clinical hypertension (Greenwich, Conn.) 2012;14:593–600. doi: 10.1111/j.1751-7176.2012.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, Szczech LA, Grunfeld C, Shlipak MG. Cystatin c, albuminuria, and 5-year all-cause mortality in hiv-infected persons. Am J Kidney Dis. 2010;56:872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]