Abstract
Background
Platelets are critical cells for maintaining vascular hemostasis but their activities in other processes are becoming apparent. Specifically, the ability of platelets to recognize and respond to infectious agents is an important area of investigation. To understand the physiological roles of platelets in vivo, most researchers have used antibody-mediated platelet depletion, which has certain limitations.
Objective
To develop an optimal system to study the contribution of platelets to protection from S. aureus blood infection.
Methods
Here we describe a novel experimental model of conditional platelet depletion based on the Cre-recombinase cell ablation system. Using this technology, the simian diphtheria toxin receptor was expressed in platelet factor 4 (PF4) positive cells (megakaryocytes and platelets).
Results
Systemic administration of diphtheria toxin (DT) every 48 hours results in reduced platelet numbers that become undetectable after six days. While platelets are depleted, no other blood cells are affected. Using this newly-developed model, the functional contributions of platelets in protection against Staphylococcus aureus (S. aureus) bacteremia was examined. Platelet-depleted mice succumbed to infection more rapidly than wild-type (WT) mice and contained significantly higher bacterial burden in kidneys, increased serum markers of kidney damage and elevated levels of cytokines indicative of septic shock.
Conclusions
Here we illustrate a new mouse model for conditional platelet depletion and implicate platelets as important participants of the immune response to bacterial blood infections.
Keywords: blood platelets, bacteria, Staphylococcus aureus, sepsis, septic shock
Introduction
Platelets are the anucleate products of megakaryocytes, and have established roles in detection of endothelial damage leading to clot formation and maintenance of hemostasis (reviewed in [1]). In addition to activation by thrombotic stimuli such as fibrinogen (αIIbβ3), collagen (GPVI), ADP (P2Y12) and thrombin (PAR 1 & 4), platelets express a panel of receptors capable of recognizing pathogenic and immunologic molecules almost identical to those expressed by professional phagocytes [2]. For example, Toll-like receptors expressed by platelets trigger specific responses after ligation [3, 4]. Activated platelets release over 300 known secretory products, including the anti-microbial products β-defensin 1, thrombocidins and kinocidins [5–7].
Platelets are now appreciated for their contributions to both innate and adaptive immunity [2, 8]. For instance, platelets participate in inflammatory conditions such as rheumatoid arthritis and virus-induced hepatitis in mice [9, 10]. Platelets also bind various pathogens including human immunodeficiency virus (HIV) and S. aureus [11]. Although not considered professional phagocytes, platelets are capable of internalizing targets resulting in killing of various bacterial species including E. coli and S. aureus [12–14]. In addition to their direct bactericidal activities, platelets can also affect both innate and adaptive immune responses through interactions with various types of leukocytes, most notably neutrophils [15–17]. Importantly, platelets induce the production of neutrophil extracellular traps, which is important for containing infection [18, 19]. Moreover, a recent report shows that platelets assist in clearing bacterial blood infections in mice via interaction with liver Kupffer cells [20] and platelets protect from LPS-induced septic shock by modulating macrophage-induced inflammation [21].
One of the most studied platelet-pathogen interactions is between platelets and S. aureus. S. aureus infection ranges from mild skin infections to life threatening conditions such as infective endocarditis and sepsis [22–24]. Platelets can actively phagocytose S. aureus, release granule contents in response to S. aureus α-hemolysin, and form platelet-neutrophil aggregates (PNA) in response to S. aureus exposure [11, 25]. While this interaction has been widely observed, these results were studied only in vitro.
Due to the importance of platelets in vascular development, mutant mice incapable of forming platelets die from hemorrhage in utero or shortly after birth [26]. Therefore, understanding platelet functions in vivo has been elusive, resulting in most experiments performed in vitro. Recently, in vivo models of antibody-mediated depletion of platelets have been described. Although effective at clearing platelets through Fcγ receptors, certain limitations exist. For example, large amounts of foreign antibody (~80µg per animal) are typically needed to deplete platelets and multiple doses would need to be administered to maintain depletion. This could lead to adverse effects such as type III hypersensitivity reactions and other pathological responses to foreign proteins thus limiting the duration of depletion [27, 28].
To better assess the biological activities of platelets under various pathological settings, we developed a transgenic mouse strain in which platelets can be conditionally depleted utilizing a Cre-recombinase-mediated cell ablation system. To this end, we created mice that express simian inducible diphtheria toxin receptor (iDTR) in PF4 positive cell populations (megakaryocytes and platelets)[27, 29]. Upon DT exposure, these cells are susceptible to DT-induced apoptosis leading to depletion of the specific cell type [27]. To test the usefulness of this new mouse strain, we examined the contribution of platelets in a well-established model of S. aureus USA300 blood infection.
Our new findings indicate that platelets are selectively depleted in this new mouse strain, while numbers of all other hematopoietic cells remain unchanged. Furthermore, we show that platelets are essential for efficient control of S. aureus bacteremia and in preventing organ damage associated with these infections. These observations likely have implications in other blood-associated infections and our novel mouse model is a powerful tool that may be broadly useful in platelet research.
Materials and Methods
Animal Care and Maintenance
Two mouse strains were utilized to generate PF4-DTR mice. C57BL/6 mice transgenic for the simian iDTR containing a LoxP-flanked stop sequence inserted into the Gt(ROSA)26Sor locus (Jackson Laboratories, Bar Harbor, ME strain #007900) were crossed with C57BL/6 mice expressing Cre recombinase under the control of the PF4 promoter (Jackson Laboratories strain #008535). PCR-based genotyping was performed on ear punch samples using primers corresponding to the Jackson Labs database (Figure 1A). All experiments were performed on male and female mice of 6–12 weeks of age. Wild-type (WT) C57BL/6 mice along with mice positive for both the iDTR and PF4 transgenes (PF4-DTR) were then administered via i.p. injection with either 100µl phosphate buffered saline (PBS) (HyClone, Logan, UT) or 400ng/animal DT (Sigma Aldrich, St. Louis, MO) every 48h to induce platelet depletion. Platelet depletion was monitored by obtaining blood from the facial vein into EDTA-coated tubes (BD Biosciences, San Jose, CA) and complete blood count (CBC) analysis using an Abaxis VetScan HM2 (Abaxis, Union City, CA). To confirm CBC results, flow cytometric analysis of CD41+ cell populations was performed. Blood was incubated with anti-CD41-APC (BD Biosciences) diluted 1:400 for 30min on ice protected from light. Samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences) using Cell Quest Pro (BD Biosciences) and interpreted with FlowJo (Tree Star, Ashland, OR). Animals were housed in microisolator cages, kept on a 12:12-h dark-light cycle and provided water and standard chow ad libitum. The Institutional Animal Care and Use Committee (IACUC) at the University of Toledo approved all procedures.
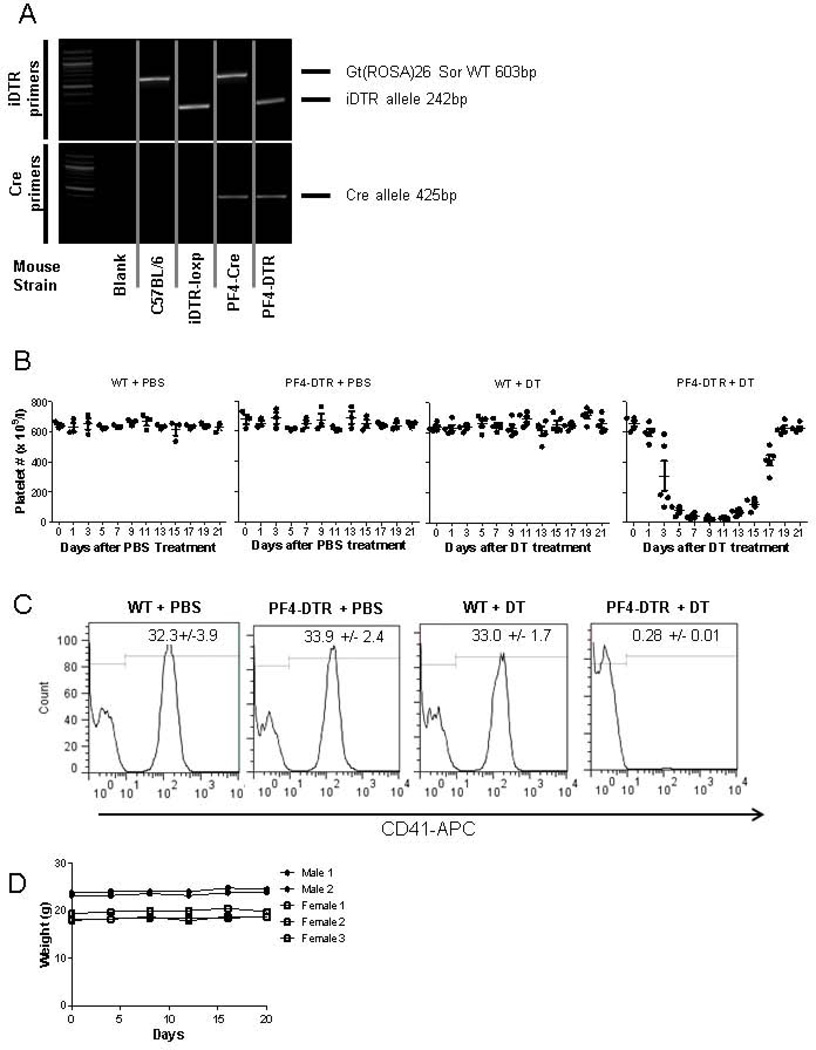
Figure 1. Genotyping and platelet numbers in PF4-DTR mice.
A) PCR products of iDTR and Cre alleles in WT and PF4-DTR mice. B) Platelet depletion and recovery in WT and PF4-DTR mice over 21 days after PBS or DT injection (N=3/group for PBS and N=5/group for DT). C) Representative flow cytometric analysis of blood samples obtained after three injections of PBS or DT and stained with anti-CD41 (n=5). D) Mouse weight monitored over a period of 25 days after DT administration (N=5, each line represents a mouse)
Purification of Platelets and Bone Marrow
Blood was drawn from facial vein as described above and purified as previously reported [30]. Platelets were either resuspended in Trizol reagent (Life Technologies, Grand Island, NY) and stored at −80°C until RNA extraction, or resuspended in buffer for functional assays as described [31]. For bone marrow extraction, mice were euthanized and femurs and tibias were removed and bone marrow was flushed using 10ml of PBS. Bone marrow was disrupted by passing through a 25-guage needle and centrifuged at 1000×g for 10min. Supernatant was aspirated and erythrocytes were lysed using ACK lysing buffer (Life Technologies). Bone marrow cells were enumerated (hemocytometer) and either Trizol was added and stored at −80°C or samples were imaged using a Zeiss Axiovert 200 microscope (Carl Zeiss, Thornwood, NY). Metamorph software (Molecular Devices, Downingtown, PA) was used to acquire images which were then processed with ImageJ software [32] using the cell counter plugin..
RNA Isolation and cDNA Synthesis
Cell pellets were stored in 400µl Trizol reagent (Life Technologies) at −80°C then lysed using the freeze thaw method. RNA extraction was performed according to manufacturer instructions (Life Technologies). RNA was resuspended in DEPC water and quantified using Nanodrop 2000 (Thermo Scientific, Wilmington, DE). 1µg of RNA was used for cDNA synthesis using the SuperScriptIII reverse transcriptase system as instructed by the manufacturer (Life Technologies). cDNA was then diluted to a concentration of 100ng/µl for PCR analysis.
PCR Analysis
PCR was performed on 100ng of cDNA per sample using primers specific for the simian iDTR and mouse CD41 using Taq polymerase from Empirical Bioscience (Grand Rapids, MI).
iDTR-F: CACTGGTGACTGGCGAGAGC
iDTR-R: CGATTTTCCACTGGGAGGCT
CD41-F: TCCGTCTATGCAGGTCCCAAT
CD41-R: TGCTGTTGTCGTAGGTGAAGC
Platelet Activation Assay
Mice were treated with 2 doses of 400ng DT or PBS and platelets were purified as described above. Purified platelets (100µl aliquots) were placed in a 37°C water bath in the presence (0.1U/ml) or absence (0U/ml) of thrombin (Chronolog, Havertown, PA) for 15 minutes and prepared for flow cytometry as described [33].
S. aureus Infection
A derivative of a previously characterized clinical isolate of S. aureus USA300 [34] was provided by Dr. R.M. Blumenthal (University of Toledo, Toledo, OH). Bacteria were grown overnight at 37°C in tryptic soy broth (Becton Dickinson, Sparks, MD), centrifuged at 10,000×g for 5 minutes, washed in PBS and enumerated via hemacytometer. Colony forming units (CFU) of the inoculum were measured by serial dilution on tryptic soy agar (TSA) plates.
Mice were infected with 108 CFUs S. aureus in 100µl of PBS via injection into the retro-orbital sinus. Following infection, mice were monitored for signs of severe illness characterized by scruffy fur, hunched posture, and inability to rear According to our IACUC protocol, mice exhibiting all the above signs of severe illness were euthanized via isoflurane inhalation and cervical dislocation. Euthanization due to severe illness was included as events in survival curves. Mice were monitored for 14 days after infection and percent survival was calculated using GraphPad Prism software (GraphPad Software, San Diego California USA).
Blood Chemistry and Plasma Cytokines
Blood chemistry was performed on whole blood samples collected from the facial vein 48 hours post-infection with S. aureus. Briefly, 100µl of whole blood was collected and analyzed on the Abaxis VetScan VS2 using a Comprehensive Diagnostic Rotor (Abaxis, Union City, CA) to assess the protein levels and blood chemistry. Plasma cytokine levels were determined 48h post-infection. Blood was collected via transcutaneous cardiac puncture into a syringe containing acid citrate-dextrose (ACD) buffer. Blood samples were centrifuged at 500×g for 10min at 4°C. Plasma was then collected and used for cytokine analysis using the Bio-Plex Pro Mouse Cytokine TH1/TH2 Assay (BioRad, Hercules, CA) following manufacturer’s instructions. Samples were analyzed on a Luminex 200 (Luminex, Austin, TX) instrument using the Bio-Plex Manager standard edition software v4.1.1 (BioRad, Hercules, CA). IL-6 was quantitated by ELISA (R & D Systems, Minneapolis, MN).
Bacterial Burden
Mice were euthanized 48h post-infection via i.p. injection of ketamine/xylazine (100mg/kg/10mg/kg). Blood was collected via transcutaneous cardiac puncture into a syringe containing ACD buffer. Liver, kidney, spleen, and heart were removed aseptically, weighed and homogenized in 1–2ml of sterile PBS. Blood and tissue homogenates were plated, along with 10-fold serial dilutions on TSA plates, incubated at 37°C overnight and bacterial colonies were counted using an aCOLyte automated colony counter (Synbiosis, Frederick, MD).
Portions of tissues were fixed in formalin for histological analysis. Tissue sections were stained with hematoxylin and eosin and blindly examined by a pathologist.
Statistical Analysis
Values are reported as mean + standard error of the mean (SEM). Student’s t test and Log-rank test for differences in Kaplan-Meier survival curves were performed using GraphPad Prism version 5.02 for Windows.
Results
DT-mediated depletion of platelets has no adverse effect on phenotype
To develop a mouse strain capable of conditional platelet depletion, we utilized the Cre recombinase-mediated cell ablation system. To do this, iDTR transgenic C57BL/6 mice containing a loxP-flanked stop sequence were mated with C57BL/6 mice expressing Cre recombinase under the control of PF4 promoter (PF4-Cre). Therefore, in cells expressing PF4, the loxP-flanked stop sequence is removed resulting in expression of the DTR (PF4-DTR). Offspring were tested for both DTR and PF4-Cre alleles (Figure 1A). Since PF4 expression is limited to megakaryocytes and platelets [29], we expected that administration of DT would result in decreased platelet numbers. To test this, WT and PF4-DTR mice received a single systemic injection of either PBS or DT (400ng/mouse), a dose shown to be non-toxic to WT mice in previous reports [27, 35]. WT mice treated with PBS or 400ng DT showed no change in platelet numbers over a 25 day period (Figure 1B) as determined by CBC. As expected, PF4-DTR mice treated with PBS also displayed no change in platelet numbers over the same period. Importantly, PF4-DTR mice treated with 400ng DT showed a time-dependent reduction in in platelet numbers, reaching the lowest numbers 6 days post-DT treatment (Figure 1B). When DT treatment is stopped, platelet numbers began to increase 14 days after the DT injection and platelet numbers recovered to normal by day 19 (Figure 1B). To further quantify this decrease, three 400ng DT treatments were administered at 48h intervals and blood was obtained on day 6 and assessed for CD41+ platelets by flow cytometry. The CD41+ population was nearly undetectable (>99% reduced) in the DT-treated PF4-DTR mice (Figure 1C). It should be noted that platelet-depleted mice maintained body weight similar to control mice (Figure 1D) and no hemorrhage was observed in the DT-treated mice when a veterinarian performed gross necropsy at the conclusion of the experiments. Additionally, no obvious bleeding phenotype was observed during bleeds from facial vein and we failed to detect any difference in bleeding time between PF4-DTR mice and WT mice using tail-snip technique.
Expression of iDTR in bone marrow but not platelets
The time-kinetics of platelet depletion suggests that DT induces apoptosis of DTR-expressing megakaryocytes and that the resulting depletion of platelets takes place as senescent platelets are cleared naturally system without replenishment. This is consistent with the estimated 5-day lifespan of platelets [36, 37]. To investigate the mechanism(s) involved in platelet depletion, we sought to detect iDTR expression in PF4-DTR mice in platelets and megakaryocytes. Western blot and flow cytometry experiments were inconclusive due to the inability of commercially-available antibodies to discriminate between mouse and simian DTR (data not shown). Therefore, we designed primers to discriminate between mouse and simian DTR and performed PCR on mRNA collected from bone marrow and purified platelets. Figure 2A shows iDTR expression in PC3 cells (DTR positive) as well as bone marrow from PF4-DTR mice but not in bone marrow from WT mice or in Raji cells (DTR negative). Interestingly, no expression of DTR was detected in platelets collected from either WT or PF4-DTR mice. To confirm that mRNA could be amplified in platelet samples, we used PCR to assess CD41 which was detectible in bone marrow and platelets from both WT and PF4-DTR mice.
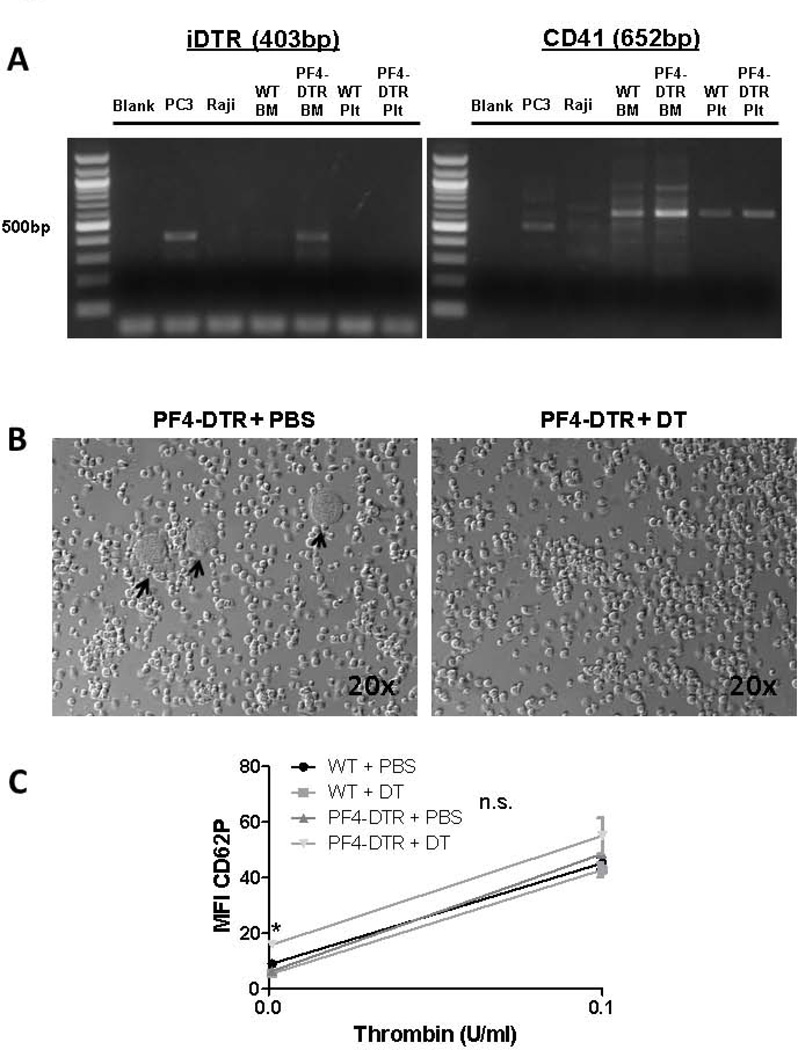
Figure 2. DTR expression, megakaryocyte enumeration and platelet function in PF4-DTR mice.
A) Expression of DTR and CD41 mRNA in control cell lines (PC3=DTR+,Raji=DTR-), bone marrow and platelets from PF4-DTR and WT mice. B) Representative images of bone marrow cell suspensions from PF4-DTR mice treated with PBS or DT. C) Platelets were obtained from facial vein of PF4-DTR or WT mice treated with two doses of PBS or DT. Platelets were purified, washed and treated with thrombin (0 or 0.1U/ml) for 15 minutes at 37°C and levels of CD62P (P-selectin) were measured via flow cytometry. (N=3/group)
To investigate whether bone marrow megakaryocytes are being depleted by DT administration, and that megakaryocyte depletion is causative of the decrease in platelet numbers, bone marrow was isolated from both depleted and control mice and megakaryocytes enumerated. Megakaryocyte numbers were decreased by 76% in DT treated PF4-DTR mice (0.08%) compared to PBS treated PF4-DTR mice (0.32%) and we believe that megakaryocyte depletion is responsible for the reduced platelet numbers in the periphery (Figure 2B, representative images). To investigate if platelet function was affected by DT administration, we administered 2 doses of PBS or DT (to ensure some platelets remained in the circulation) to WT and PF4-DTR mice and isolated platelets. Washed platelets were incubated with either 0 U/ml thrombin or 0.1 U/ml thrombin and surface P-selectin levels were measured. Platelets from all mice responded normally to thrombin treatment (Figure 2C). Platelets from PF4-DTR mice receiving DT showed a statistically significant albeit modest increase in baseline P-selectin expression. One possible explanation is that the remaining platelets (four days old) are approaching the end of their circulating life and may be showing signs of senescence.
DT does not induce changes in blood cell populations in PF4-DTR mice
To investigate whether DT induced changes in other hematopoietic populations, complete blood counts were obtained on blood from WT and PF4-DTR mice before (Table 1) and after administration of DT or PBS (Table 2). As revealed in Tables 1 and 2, platelets are the only parameter significantly changed in PF4-DTR mice treated with DT. These data illustrate that DT-mediated platelet depletion in PF4-DTR mice does not affect other blood cell subtypes.
Table 1.
Complete blood analysis in WT and PF4-DTR mice before DT administeration
| 0 Injections | ||
|---|---|---|
| Parameter | WT (N=15) | PF4-DTR (N=15) |
| RBC (×1012/L) | 8.3 + 0.15 | 8.0 + 0.09 |
| WBC (×109/L) | 9.8 + 0.74 | 10.5 + 1.1 |
| LYM (×109/L) | 9.0 + 0.70 | 9.6 + 1.0 |
| MON (×109/L) | 0.27 + 0.13 | 0.21 + 0.10 |
| NEU (×109/L) | 0.56 + 0.07 | 0.78 + 0.14 |
| PLT (×109/L) | 562 + 35 | 620 + 38 |
| PCT (%) | 0.36 + 0.03 | 0.42 + 0.02 |
| MPV (fL) | 6.5 + 0.07 | 6.7 + 0.15 |
| PDWc (%) | 29.6 + 0.37 | 30.7 + 0.47 |
| HGB (g/dL) | 13.6 + 0.26 | 13.0 + 0.26 |
| HCT (%) | 35.7 + 0.51 | 34.7 + 0.60 |
| MCV (fL) | 43.2 + 0.26 | 43.6 + 0.51 |
| MCH (pg) | 16.3 + 0.08 | 16.3 + 0.24 |
| MCHC (g/dL) | 37.9 + 0.26 | 37.4 + 0.33 |
| RDWc (%) | 18.9 + 0.29 | 19.6 + 0.5 |
WBC-white blood cells, LYM-lymphocytes, MON-monocytes, NEU-neutrophils, RBC-red blood cell, HGB-hemoglobin, HCT-hematocrit, MCV-mean corpuscular volume, MCH-mean corpuscular hemoglobulin, MCHC-mean corpuscular hemoglobulin concentration, RDWc-red blood cell distribution width, PLT-platelet, PCT-platelet crit, MPV-mean platelet volume, PDWc-platelet distribution width.
Table 2.
Complete blood analysis in WT and PF4-DTR mice after 10 PBS or DT injections
| 10 Injections | ||||
|---|---|---|---|---|
| Parameter | WT + PBS (N=5) |
WT + DT (N=5) |
PF4-DTR + PBS (N=4) |
PF4-DTR + DT (N=6) |
| RBC (×1012/L) | 9.2 + 0.2 | 9.1 + 0.2 | 8.6 + 0.3 | 8.7 + 0.3 |
| WBC (×109/L) | 12.5 + 0.8 | 10.9 + 0.7 | 12.5 + 1.5 | 12.4 + 1.8 |
| LYM (×109/L) | 10.7 + 0.9 | 9.7 + 0.5 | 11.3 + 1.6 | 10.6 + 1.5 |
| MON (×109/L) | 0.22 + 0.1 | 0.18 + 0.02 | 0.65 + 0.3 | 0.22 + 0.1 |
| NEU (×109/L) | 1.2 + 0.2 | 1.0 + 0.2 | 1.4 + 0.2 | 1.6 + 0.3 |
| PLT (×109/L) | 605 + 27 | 655 + 12 | 620 + 32 | 45 + 14§ |
| PCT (%) | 0.4 + 0.03 | 0.42 + 0.01 | 0.41 + 0.03 | 0.03 + 0.01§ |
| MPV (fL) | 6.6 + 0.2 | 6.5 + 0.04 | 6.6 + 0.07 | 6.5 + 0.1 |
| PDWc (%) | 30.7 + 0.6 | 29.5 + 0.2 | 30.6 + 0.4 | 30.8 + 0.4 |
| HGB (g/dL) | 14.2 + 0.1 | 14.4 + 0.1 | 13.3 + 0.4 | 13.6 + 0.7 |
| HCT (%) | 39.4 + 0.5 | 39.1 + 0.5 | 37.5 + 1.2 | 38.3 + 1.5 |
| MCV (fL) | 42.6 + 0.3 | 43 + 0.5 | 43.6 + 0.8 | 43.8 + 0.7 |
| MCH (pg) | 15.4 + 0.2 | 15.9 + 0.2 | 15.4 + 0.2 | 15.6 + 0.3 |
| MCHC (g/dL) | 36.6 + 0.6 | 36.8 + 0.2 | 35.4 + 0.3 | 35.5 + 0.5 |
| RDWc (%) | 20.4 + 0.4 | 18.9 + 0.2 | 19.4 + 0.2 | 19.9 + 0.6 |
p<0.005 vs. WT PBS, WT DT and PF4-DTR PBS
Loss of platelets significantly decreases survival in a S. aureus model of sepsis due to increased bacterial burden and kidney damage
To evaluate the role of platelets during infection in vivo, we proceeded to determine the role of platelets during S. aureus USA300 bacteremia using our newly developed model. The S. aureus USA300 strain was chosen for the sepsis model due to its frequency in human sepsis, ability to manipulate host hemostatic components by binding various coagulation factors, associating with platelets, and inducing thrombocytopenia [38, 39]. Based on data in Figure 1B, WT or PF4-DTR mice were administered DT every 48h beginning on day −6 (Figure 3A, arrowheads). On day 0 (Figure 3A, arrow), mice were infected with 108 CFU S. aureus USA300 in 100µl PBS retro-orbitally. Administration of DT was continued every 48 hours until mice succumbed to the infection or the experiment was terminated on day 14 (Figure 3A). PF4-DTR mice treated with DT exhibited a marked decrease in survival (p<0.001) compared to DT treated WT mice (Figure 3A), suggesting that platelets provide protection from S. aureus bacteremia. However, both PF4-DTR and WT mice treated with DT lost similar amounts of body weight at a comparable rate (Figure 3B).
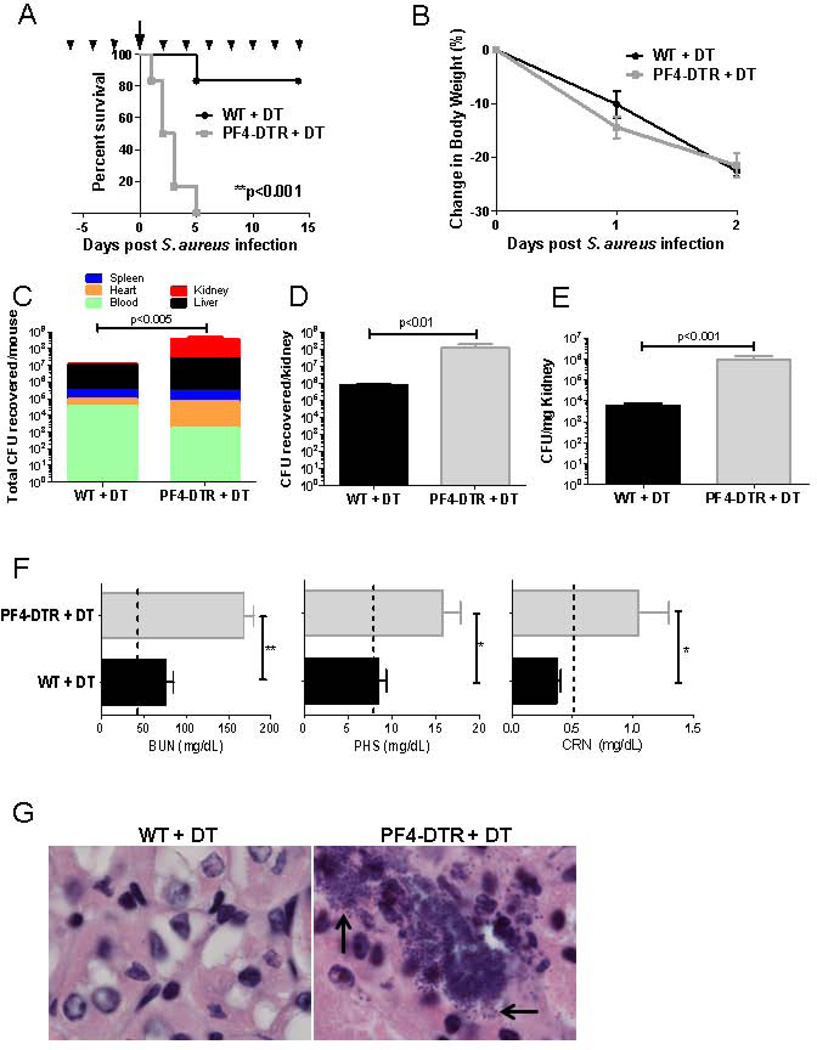
Figure 3. Platelet-depleted mice rapidly die from S. aureus sepsis and display signs of severe organ damage.
A) WT and PF4-DTR mice were treated with DT every 2 days (arrow heads) beginning on day −6 then infected with 1×108 CFUs S. aureus on day 0 (arrow). DT treatment continued every 2 days until the experiment was terminated and survival analysis of WT (N=7) and PF4-DTR (N=6) was analyzed. B) Change in body weight was assessed as a measure of disease development in WT and PF4-DTR mice treated as in A (N=10/group). C–E) WT (N=6) and PF4-DTR (N=4) mice received DT every 2 days beginning on day −6 then infected with 1×108 CFUs S. aureus on day 0. Organs (kidney, liver, spleen, heart) and blood were collected 48 hours after infection and CFUs were enumerated and expressed as total CFUs recovered/animal (C), CFUs recovered per kidney (D) and CFUs per mg of kidney tissue (E). All measures are presented as means ± S.E.M. *p<0.05, **p<0.01. F) Blood urea nitrogen (BUN), phosphorus (PHS) and creatinine (CRN) levels were measured 48h post-infection in WT (N=3) and PF4-DTR mice (N=4). Dashed lines represent levels of each metabolite in non-infected, DT-treated WT and PF4-DTR mice. All measures are presented as means ± S.E.M. *p<0.05**p<0.005. G) Histological analysis of kidney sections stained with hematoxylin and eosin and assessed for bacterial colonization (black arrows).
To elucidate the mechanisms behind the accelerated expiry of platelet-depleted mice, bacterial burdens in blood, heart, liver, spleen and kidney were assessed. Organs were harvested 48h after infection, homogenized, and CFUs were enumerated. The total number of CFUs recovered from PF4-DTR mice (3.95×108) was 10-fold greater than from WT mice (2.26×107) (Figure 3C). The kidney is known to be the major reservoir for S. aureus after intravenous infection and we observed that total CFUs recovered from kidney were significantly higher in PF4-DTR mice than WT mice (Figure 3D) and represented ~50% of the total CFU recovered from PF4-DTR mice. When CFUs were expressed per weight, we observed a >100-fold increase in bacterial burden in PF4-DTR mice compared to WT (Figure 3E).
To determine if increased bacterial burden in kidney results in tissue damage, blood chemistry was examined for markers of kidney dysfunction 48 hours after S. aureus infection. Interestingly, both WT and PF4-DTR mice infected with S. aureus showed a significant increase in blood urea nitrogen (BUN) and phosphate (PHS) compared to uninfected mice (dashed line) indicative of kidney damage (Figure 3F). However, BUN was elevated >3-fold and both PHS and CRN levels in PF4-DTR mice were elevated 2-fold, compared to WT mice suggesting more severe kidney damage.
We next performed histological analysis on kidneys from DT-treated WT and PF4-DTR mice harvested 48 hours after infection. Consistent with bacterial burden data, substantial numbers of bacterial colonies were observed in kidney sections of platelet-depleted PF4-DTR mice but not in WT mice under high-magnification (black arrows, Figure 3G). Overall, these findings reveal that platelet-depleted mice have increased bacterial loads in kidney and elevated markers of kidney failure. These results indicate that PF4-DTR mice suffer a greater degree of kidney damage than WT mice and suggests that the mechanism behind the observed increased mortality may be kidney failure; a main cause of death in sepsis [40, 41].
Platelet-depleted mice display elevated plasma cytokines
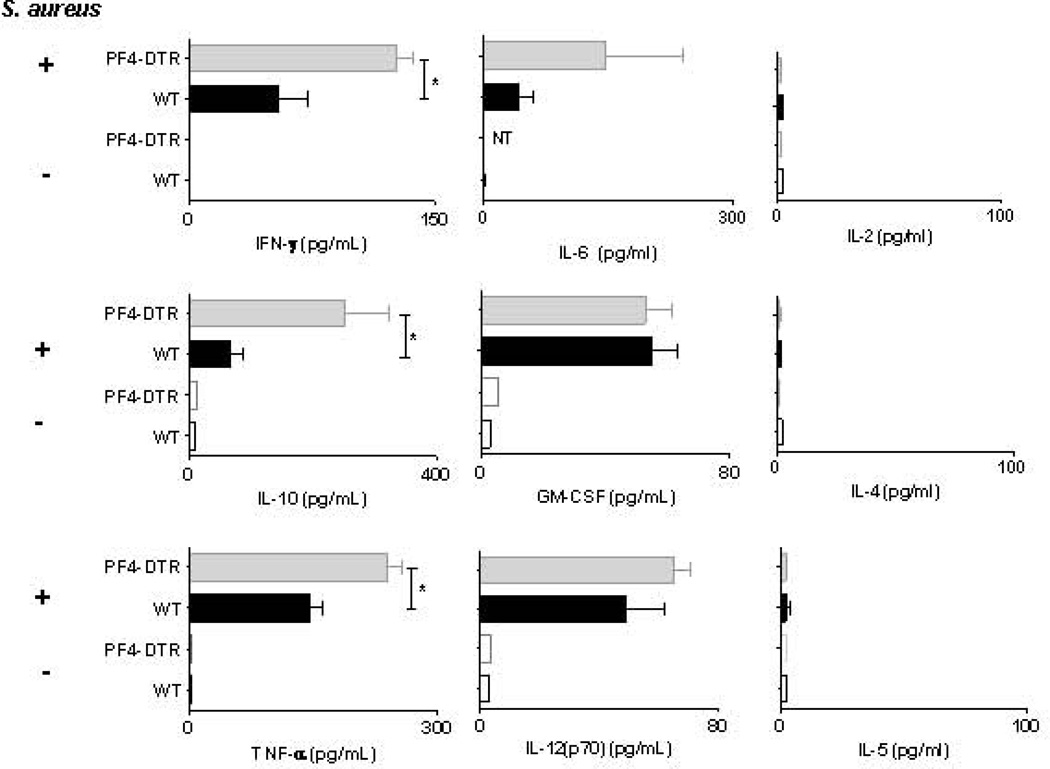
To examine the inflammatory response to S. aureus bactermia in the presence and absence of platelets, plasma from DT-treated WT and PF4-DTR mice was assessed for a number of cytokines associated with the “cytokine storm” [23, 42]. It is well established that IFN-γ, TNF-α and IL-10 are indicators of poor prognosis in sepsis patients and mouse models [43–45]. We observed a striking increase in IFN-γ (>2-fold) and IL-10 (nearly 3-fold) in infected PF4-DTR mice compared to WT (Figure 4A). TNF-α was also significantly elevated in PF4-DTR mice compared to WT (Figure 4A). Furthermore, no differences were observed in IL-6, IL-12, GM-CSF, IL-5, IL-4 or IL-2 (Figure 4). These data suggest that a lack of circulating platelets results in an exacerbated septic shock during S. aureus blood infection leading to increased mortality.
Figure 4. Plasma cytokines in WT mice and PF4-DTR mice 48h post S. aureus infection.
WT (N=8) and PF4-DTR (N=5) mice were administered DT every 48 hours for 6 days then either mock infected or infected with S. aureus. Forty-eight hours after infection, blood was collected and circulating levels of plasma IFN-γ, TNF-α, IL-10, IL-6, IL-12(p70), GM-CSF, IL-5, IL-4 and IL-2 were measured using luminex bead multiplex assay or ELISA (IL-6). All measures are presented as means ± S.E.M. *p<0.05, **<0.005
Discussion
Platelets are classically described as essential components for maintaining vascular hemostasis and blood clotting. However, platelets have more recently been shown to exhibit immune function in the context of both viral and bacterial infections [11, 13, 19, 46]. Platelets can phagocytose and directly kill pathogens as assessed in vitro [14, 19]. However, little is known if these properties are important in vivo. Although antibody mediated depletion of platelets has been effective in short term studies of platelet contributions to regulation of pathogenesis, it has inherent problems when it comes to long term infection or chronic disease. For instance, the αIIbβ3 antibody, along with depleting platelets, has been reported to induce an anaphylactic response in mice [47]. Another antibody depletion method commonly used in vivo targets GP1bα in which significant recovery of platelets is observed by day 5 post injection with a dose as high as 4µg/g (80–100µg of antibody in one dose based on average weight of a mouse) and reported studies have not exceeded 7 days with two injections of antibody [20, 21, 48]. Genetic approaches have also been undertaken to chronically deplete platelets, such as the c-Mpl knockout mouse strain. These mice do not express the receptor for thrombopoietin (TPO) and maintain a thrombocytopenic state (~90% platelet depletion), but the platelets that remain are functional [49, 50]. Since the knockout c-Mpl mouse is not a cell-targeted approach, there are also defects in other hematopoietic progenitor cells [50, 51]. Additionally, one study of the c-Mpl knockout mice reported increased mean platelet volume (MPV) [49], and another reported increased tail bleeding times along with anemia [52] neither of which was observed in the PF4-DTR mice.
In our PF4-DTR transgenic model, platelets are undetectable only after DT administration after six days. Because murine platelets circulate for 4–5 days before clearance, this time course is consistent with the rate of depletion of platelets in our transgenic mice. To determine the mechanism(s) of platelet depletion, we attempted to detect DTR protein on platelets and megakaryocytes but were unsuccessful by either flow cytometry or western blot using commercially-available antibodies from several sources. This is consistent with previous publications using the inducible DTR-mediated cell ablation system which have not shown expression of DTR on the target cell population. However, we successfully detected simian DTR mRNA in the bone marrow of PF4-DTR mice but not WT mice. Interestingly, simian DTR mRNA was not expressed in platelets from either WT or DTR mice, suggesting a lack of expression in platelets (Figure 2A). Thus, we believe that DT treatment depleted platelets by reducing megakaryocyte numbers (Figure 2B) [32, 33] without affecting other hematopoietic populations, illustrating the specificity of this model (Tables 1 and 2). Through this method of platelet depletion, we have achieved a >99% depletion of platelets (Figure 1C) that does not affect platelet function (Figure 2C) and can persist for an extended period of time with repeated doses of DT.
Using this new system, we have shown that platelets contribute to the host response against S. aureus USA300 bacteremia. Although both WT and PF4-DTR mice displayed signs of disease development (weight loss), PF4-DTR mice succumbed to infection much more rapidly than WT mice. It is apparent through blood chemistry analysis that PF4-DTR mice suffered from more extensive kidney damage than WT mice and this correlated with increased bacterial burden in kidney. Since acute kidney injury/failure contributes to the mortality of sepsis and septic shock [53, 54], we found it interesting that PF4-DTR had significantly more bacterial colonization compared to WT. It has been published that platelets can encapsulate bacteria and facilitates their transport to immune effector cells [11, 20], and that platelets can internalize and kill bacteria [14]. Although these mechanisms would eventually lead to clearance of the bacteria, our data suggest alternative mechanisms including sequestering bacteria to the bloodstream not allowing passage into tissue. This hypothesis is supported by our data showing significantly lower CFUs in the blood of DT-treated PF4-DTR mice compared to WT mice (Figure 3C). However, to our knowledge, there has been no direct evidence to date on how platelets contribute to bacterial dissemination during septic infection.
Another consequence of sepsis is the ensuing “cytokine storm”, which is caused via dysregulation of the immune response to pathogens [42]. We observed a significant increase in IFN-γ, TNFα, and IL-10; cytokines involved in sepsis lethality both in humans and mice [44, 45]. Unexpectedly, other pro-inflammatory cytokines IL-12,GM-CSF, and IL-6 were elevated to similar levels in WT and PF4-DTR mice suggesting that only certain cytokine pathways may be regulated by platelets. Consistent with these findings, it has been shown that platelets can secrete different cytokines in response to different types of LPS in vitro which causes differential activation of peripheral blood mononuclear cells (PBMCs) [13]. In gram negative sepsis, platelets are important in regulation of macrophage responses and cytokine release [21]. Our data, along with previous reports suggest that platelets modulate plasma cytokines during sepsis by at least two mechanisms including direct platelet secretion of cytokines and modulation of cytokine production by leukocytes. These results indicate that platelets not only control bacterial localization, but that they also contribute to regulation of the host immune response to bacterial infection. Moreover, our results demonstrate the usefulness of this new research tool that can be applied to study the role of platelets in various disease conditions.
Acknowledgements
The authors would like to acknowledge R. H. Lee, B. Chojnacki, and Dr. J.Y. Tano for technical assistance, Dr. Victor Torres for advice designing the bacterial infection studies, and Drs. Stanislaw Stepkowski and Mark Wooten for assistance with Bioplex experiments. The authors also thank Drs. Wooten and R.M. Blumenthal for critical reading of the manuscript.
Funding Sources
This work was supported by NIH grant HL122401 (to R.G.W.).
Footnotes
Addendum
L. M. Wuescher performed experiments, analyzed data and wrote the manuscript. R. G. Worth designed the research, performed experiments, analyzed data and edited the manuscript. A. Takashima designed the research, analyzed data and edited the manuscript.
Disclosure of Conflict of Interests
The authors have no conflicts of interest to declare.
References
- 1.Clemetson KJ. Platelets and primary haemostasis. Thromb Res. 2012;129:220–224. doi: 10.1016/j.thromres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nature reviews Immunology. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 3.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 4.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 5.Yeaman MR, Bayer AS, Koo SP, Foss W, Sullam PM. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Invest. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeaman MR, Tang YQ, Shen AJ, Bayer AS, Selsted ME. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 8.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC For The Platelet Colloquium P. Platelet functions beyond hemostasis. Journal of Thrombosis and Haemostasis. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 9.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nature medicine. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 12.Antczak AJ, Vieth JA, Singh N, Worth RG. Internalization of IgG-coated targets results in activation and secretion of soluble CD40 ligand and RANTES by human platelets. Clinical and vaccine immunology : CVI. 2011;18:210–216. doi: 10.1128/CVI.00296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berthet J, Damien P, Hamzeh-Cognasse H, Arthaud C-A, Eyraud M-A, Zéni F, Pozzetto B, McNicol A, Garraud O, Cognasse F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clinical Immunology. 2012;145:189–200. doi: 10.1016/j.clim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Riaz AH, Tasma BE, Woodman ME, Wooten RM, Worth RG. Human platelets efficiently kill IgG-opsonized E. coli. FEMS immunology and medical microbiology. 2012;65:78–83. doi: 10.1111/j.1574-695X.2012.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Li J, Li Y, Lang S, Yougbare I, Zhu G, Chen P, Ni H. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Advances in hematology. 2012;2012:384685. doi: 10.1155/2012/384685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. The Journal of experimental medicine. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: Linking proinflammatory responses to procoagulant state. Thromb Res. 2013;131:191–197. doi: 10.1016/j.thromres.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 18.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell host & microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer BF, Campbell RA, Schwertz H, Cody MJ, Franks Z, Tolley ND, Kahr WH, Lindemann S, Seizer P, Yost CC, Zimmerman GA, Weyrich AS. Novel anti-bacterial activities of beta-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nature immunology. 2013;14:785–792. doi: 10.1038/ni.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang B, Zhang G, Guo L, Li XA, Morris AJ, Daugherty A, Whiteheart SW, Smyth SS, Li Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nature communications. 2013;4:2657. doi: 10.1038/ncomms3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowy FD. Staphylococcus aureus Infections. New England Journal of Medicine. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 23.Gordon RJ, Lowy FD. Pathogenesis of Methicillin-Resistant Staphylococcus aureus Infection. Clinical Infectious Diseases. 2008;46:S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreillon P, Que YA, Bayer AS. Pathogenesis of streptococcal and staphylococcal endocarditis. Infectious disease clinics of North America. 2002;16:297–318. doi: 10.1016/s0891-5520(01)00009-5. [DOI] [PubMed] [Google Scholar]
- 25.Parimon T, Li Z, Bolz DD, McIndoo ER, Bayer CR, Stevens DL, Bryant AE. Staphylococcus aureus alpha-hemolysin promotes platelet-neutrophil aggregate formation. J Infect Dis. 2013;208:761–770. doi: 10.1093/infdis/jit235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 27.Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nature methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 28.Bergmeier W, Boulaftali Y. Adoptive transfer method to study platelet function in mouse models of disease. Thrombosis research. 2014;133(Suppl 1):S3–S5. doi: 10.1016/j.thromres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 30.Worth RG, Chien CD, Chien P, Reilly MP, McKenzie SE, Schreiber AD. Platelet FcgammaRIIA binds and internalizes IgG-containing complexes. Experimental hematology. 2006;34:1490–1495. doi: 10.1016/j.exphem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Antczak AJ, Singh N, Gay SR, Worth RG. IgG-complex stimulated platelets: a source of sCD40L and RANTES in initiation of inflammatory cascade. Cellular immunology. 2010;263:129–133. doi: 10.1016/j.cellimm.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berlacher MD, Vieth JA, Heflin BC, Gay SR, Antczak AJ, Tasma BE, Boardman HJ, Singh N, Montel AH, Kahaleh MB, Worth RG. FcgammaRIIa Ligation Induces Platelet Hypersensitivity to Thrombotic Stimuli. The American journal of pathology. 2013;182:244–254. doi: 10.1016/j.ajpath.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 34.McCullough AC, Seifried M, Zhao X, Haase J, Kabat WJ, Yogev R, Blumenthal RM, Mukundan D. Higher incidence of perineal community acquired MRSA infections among toddlers. BMC pediatrics. 2011;11:96. doi: 10.1186/1471-2431-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nature biotechnology. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 36.Berger G, Hartwell DW, Wagner DD. P-Selectin and platelet clearance. Blood. 1998;92:4446–4452. [PubMed] [Google Scholar]
- 37.Machlus KR, Italiano JE., Jr The incredible journey: From megakaryocyte development to platelet formation. The Journal of cell biology. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flick MJ, Du X, Prasad JM, Raghu H, Palumbo JS, Smeds E, Hook M, Degen JL. Genetic elimination of the binding motif on fibrinogen for the S. aureus virulence factor ClfA improves host survival in septicemia. Blood. 2013;121:1783–1794. doi: 10.1182/blood-2012-09-453894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011;7:e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lerolle N, Nochy D, Guerot E, Bruneval P, Fagon JY, Diehl JL, Hill G. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 41.De Vriese AS. Prevention and treatment of acute renal failure in sepsis. Journal of the American Society of Nephrology : JASN. 2003;14:792–805. doi: 10.1097/01.asn.0000055652.37763.f7. [DOI] [PubMed] [Google Scholar]
- 42.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiology and molecular biology reviews : MMBR. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Poll T, de Waal Malefyt R, Coyle SM, Lowry SF. Antiinflammatory Cytokine Responses during Clinical Sepsis and Experimental Endotoxemia: Sequential Measurements of Plasma Soluble Interleukin (IL)-1 Receptor Type II, IL-10, and IL-13. Journal of Infectious Diseases. 1997;175:118–122. doi: 10.1093/infdis/175.1.118. [DOI] [PubMed] [Google Scholar]
- 44.Wunder C, Eichelbrönner O, Roewer N. Are IL-6, IL-10 and PCT plasma concentrations reliable for outcome prediction in severe sepsis? A comparison with APACHE III and SAPS II. Inflamm res. 2004;53:158–163. doi: 10.1007/s00011-003-1239-3. [DOI] [PubMed] [Google Scholar]
- 45.van den Berg S, Laman JD, Boon L, ten Kate MT, de Knegt GJ, Verdijk RM, Verbrugh HA, Nouwen JL, Bakker-Woudenberg IA. Distinctive cytokines as biomarkers predicting fatal outcome of severe Staphylococcus aureus bacteremia in mice. PloS one. 2013;8:e59107. doi: 10.1371/journal.pone.0059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeaman MR. Platelets in defense against bacterial pathogens. Cellular and molecular life sciences : CMLS. 2010;67:525–544. doi: 10.1007/s00018-009-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, Mackman N, Owens AP, 3rd, Ware J, Kahn ML, Bergmeier W. Platelet ITAM signaling is critical for vascular integrity in inflammation. The Journal of clinical investigation. 2013;123:908–916. doi: 10.1172/JCI65154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Stoppelaar SF, van 't Veer C, Claushuis TA, Albersen BJ, Roelofs JJ, van der Poll T. Thrombocytopenia impairs host defense in gram-negative pneumonia derived sepsis. Blood. 2014 doi: 10.1182/blood-2014-05-573915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- 50.Alexander WS, Roberts AW, Maurer AB, Nicola NA, Dunn AR, Metcalf D. Studies of the c-Mpl Thrombopoietin Receptor through Gene Disruption and Activation. STEM CELLS. 1996;14:124–132. doi: 10.1002/stem.5530140716. [DOI] [PubMed] [Google Scholar]
- 51.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 52.Chan ER, Lavender H, Li G, Haviernik P, Bunting KD, Adams MD. An ENU-induced recessive mutation in Mpl leads to thrombocytopenia with overdominance. Experimental hematology. 2009;37:276–284. doi: 10.1016/j.exphem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayeur N, Rostaing L, Nogier MB, Jaafar A, Cointault O, Kamar N, Conil JM, Fourcade O, Lavayssiere L. Kinetics of plasmatic cytokines and cystatin C during and after hemodialysis in septic shock-related acute renal failure. Critical care (London, England) 2010;14:R115. doi: 10.1186/cc9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murugan R, Kellum JA. Acute kidney injury: what's the prognosis? Nature reviews Nephrology. 2011;7:209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]