Abstract
Phosphatidylinositide 3′ (PI3′)-lipid signaling cooperates with oncogenic BRAFV600E to promote melanomagenesis. Sustained PI3′-lipid production commonly occurs via silencing of the PI3′-lipid phosphatase PTEN or, less commonly, through mutational activation of PIK3CA, encoding the 110kDa catalytic subunit of PI3′-kinase-α (PI3Kα). To define the PI3K catalytic isoform dependency of BRAF-mutated melanoma, we utilized pharmacologic, isoform-selective PI3K inhibitors in conjunction with melanoma-derived cell lines and genetically engineered mouse (GEM) models. While BRAFV600E/PIK3CAH1047R melanomas were sensitive to the anti-proliferative effects of selective PI3Kα blockade, inhibition of BRAFV600E/PTENNull melanoma proliferation required combined blockade of PI3Kα, δ and γ, and was insensitive to PI3Kβ blockade. In GEM models, isoform-selective PI3K inhibition elicited cytostatic effects, but significantly potentiated melanoma regression in response to BRAFV600E pathway-targeted inhibition. Interestingly, PI3K inhibition forestalled the onset of MEK inhibitor resistance in two independent GEM models of BRAFV600E-driven melanoma. These results suggest that combination therapy with PI3K inhibitors may be a useful strategy to extend the duration of clinical response of BRAF-mutated melanoma patients to BRAFV600E pathway-targeted therapies. (Words: 165)
Keywords: Melanoma, BRAFV600E, PI3′-kinase, Genetically engineered mouse model, Pre-clinical therapeutics
INTRODUCTION
Over the past fifteen years, key genetic lesions that initiate melanomagenesis, promote disease progression and remain necessary for melanoma maintenance have been identified (1, 2). Approximately 50% of melanomas express mutationally activated BRAFV600E, leading to constitutive activation of the BRAFV600E→MEK1/2→ERK1/2 mitogen-activated protein (MAP) kinase pathway (3). The importance of this pathway in melanoma maintenance is highlighted by the ability of BRAFV600E pathway-targeted inhibitors to elicit dramatic tumor regression in BRAF-mutated, advanced melanoma patients (4–6). Although the response rate of such patients is high, the depth and durability of response is limited by the onset of drug resistant disease that is largely refractory to additional BRAFV600E pathway-targeted therapy (7, 8). Therefore, it is critical to identify signaling pathways that contribute to de novo or acquired drug resistance, and to determine if pharmacological blockade of these pathways can increase the response rate or the durability of response to BRAFV600E pathway-targeted therapies. Although multiple mechanisms of acquired drug resistance have been documented, it remains unclear the extent to which parallel inhibition of signaling pathways will enhance melanoma patient responses (9, 10).
Using genetically engineered mouse (GEM) models, we previously demonstrated that either PTEN silencing or mutationally activated PIK3CAH1047R cooperates with BRAFV600E to elicit metastatic melanoma. However, BRAFV600E/PIK3CAH1047R melanomas grew more slowly than similarly elicited BRAFV600E/PTENNull melanomas (11). In addition, although a pan-class I PI3K inhibitor (BKM120) significantly potentiated the ability of a BRAFV600E inhibitor (LGX818) to induce regression of autochthonous BRAFV600E/PTENNull melanomas, BKM120 was largely ineffective as a single agent (11). Given the frequency of alterations in PI3′-lipid signaling in BRAF-mutated melanoma (12–15), we wished to explore the role of PI3K signaling in melanoma progression and maintenance, as well as the therapeutic implications of targeting this pathway using isoform-selective inhibitors. Our studies reveal that the difference in growth rate between BRAFV600E/PIK3CAH1047R and BRAFV600E/PTENNull melanomas is likely due to the strength of PI3K pathway activation. However, potent blockade of PI3K signaling in either BRAFV600E/PIK3CAH1047R or BRAFV600E/PTENNull melanomas elicited largely cytostatic effects. Finally, and most interestingly, combined blockade of BRAFV600E and PI3K signaling significantly enhanced the depth and durability of the response of BRAFV600E/PIK3CAH1047R or BRAFV600E/PTENNull melanoma to the MEK1/2 inhibitor GDC-0973. These data provide a scientific rationale for the clinical deployment of such regimens for BRAF-mutated melanoma patients in which the PI3K pathway is activated either by PTEN silencing or PIK3CA mutation.
RESULTS
PTEN is reported to have both phosphatase-dependent and -independent tumor suppressor activities (16–18). To address whether differences in growth rate between BRAFV600E/PIK3CAH1047R and BRAFV600E/PTENNull melanoma reflect a role for phosphatase-independent tumor suppressor activities of PTEN, we compared the growth rate of BRAF-mutated melanomas in Tyr::CreER; BRafCA mice that were homozygous for the Ptenlox4-5 allele or either heterozygous or homozygous for the conditional Cre-activated Pik3calat-1047R (Pik3calat hereafter) allele (Fig. 1A). As shown previously (11), BRAFV600E/PTENNull melanomas grew more rapidly than BRAFV600E/PIK3CAH1047R melanomas arising in heterozygous Pik3calat/+ mice (Fig. 1A). However, BRAFV600E/PIK3CAH1047R melanomas arising in homozygous Pik3calat/lat mice grew significantly more rapidly than BRAFV600E/PTENNull melanomas, suggesting that differences in melanoma growth rate between BRAFV600E/PIK3CAH1047R and BRAFV600E/PTENNull melanoma are likely due to the magnitude of PI3K pathway activation. In addition, cell lines derived from BRAFV600E/PTENNull/CDKN2ANull (B10C) or BRAFV600E/PIK3CAH1047R/H1047R/CDKN2ANull (BP2C) melanomas grew more rapidly in vitro than did a cell line derived from a BRAFV600E/PIK3CAH1047R/+/CDKN2ANull (BPC) melanoma (unpublished observation).
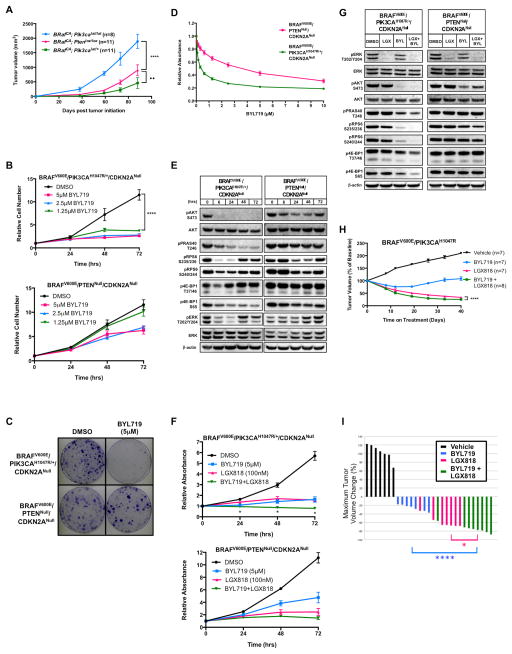
Figure 1. Autochthonous BRAFV600E/PIK3CAH1047R melanomas and cell lines are sensitive to PI3Kα-selective inhibition.
(A) Melanoma was initiated in Tyr::CreER; BRafCA mice carrying Ptenlox/lox or either heterozygous or homozygous for Pik3calat by topical application of 4-hydroxytamoxifen (4-HT), with melanoma growth assessed for 88 days. Average tumor volumes (mm3 ± SEM) were measured starting at day 38 days p.i. Asterisks indicate significant difference in melanoma growth between Tyr::CreER; BRafCA; Pik3calat/lat and Tyr::CreER; BRafCA; Ptenlox/lox mice and filled circles indicate significant difference between Tyr::CreER; BRafCA; Pik3calat/+ and Tyr::CreER; BRafCA; Ptenlox/lox mice (2-way ANOVA, ••, p<0.005, ****, p<0.0001).
(B) BPC or B10C melanoma cells were cultured in the presence of the indicated concentrations of BYL719 with cells counted every 24 hours for 72 hours. Cell counts are indicated as change in cell number relative to the number of cells at the initiation of drug treatment with error bars representing standard error of the mean (SEM). Asterisks indicate significant difference between DMSO and 1.25μM BYL719 (2-way ANOVA, p<0.0001).
(C) BPC or B10C melanoma cells were cultured in the presence of BYL719 (5μM) for a period of nine days before being fixed and stained with Crystal Violet.
(D) BPC or B10C melanoma cells were cultured in the presence of the indicated concentrations of BYL719 for 72 hours before being fixed and stained with Crystal Violet. Crystal Violet staining was quantified by solubilizing the fixed dye and assessing the absorbance at 562nm. Values are normalized to DMSO control and error bars represent SEM.
(E) Lysates of BPC or B10C melanoma cells, treated for the indicated period of time with BYL719 (5μM), were analyzed by immunoblotting with the indicated antisera.
(F) BPC or B10C melanoma cells were cultured in the presence of BYL719 (5μM), LGX818 (100nM) or the combination of both agents with cells fixed and stained with Crystal Violet every 24 hours for a total period of 72 hours. Crystal Violet staining was quantified as described above. Error bars represent SEM. Asterisks indicate significant difference between combination drug treatment and LGX818 treatment (multiple t-tests, p<0.05).
(G) Lysates of BPC or B10C melanoma cells treated for 6 hours with DMSO, LGX818 (100nM), BYL719 (5μM) or the combination of both agents were analyzed by immunoblotting.
(H) BRAFV600E/PIK3CAH1047R melanomas were initiated in suitably manipulated adult Tyr::CreER, BRafCA; Pik3calat mice. Following randomization, mice were treated with vehicle, BYL719 (50mg/kg, b.i.d.), LGX818 (30mg/kg, q.d.) or the combination of both agents. Melanoma growth or regression was measured weekly with digital calipers over the course of 40 days of continuous drug treatment. Tumor sizes are displayed as the average percent change in tumor size from the start of treatment, with error bars indicating SEM. Asterisks indicate significant difference between combination drug treatment and LGX818 drug treatment (2-way ANOVA, p<0.0001).
(I) A waterfall plot of the best tumor response for each of the 29 mice that received vehicle versus drug treatment in (H). The percent change in tumor size from the start of treatment is shown on the y-axis. Negative values indicate tumor shrinkage. Asterisks indicate significant difference between combination drug treatment and BYL719 (blue) or LGX818 (pink) (Unpaired t-test, *, p<0.05, ****, p<0.0001).
To determine the PI3K isoform dependence of BRAF-mutated melanoma, we treated BPC and B10C melanoma cell lines with pharmacological inhibitors of PI3K (Supp. Table 1). BPC melanoma cells treated with BYL719, a selective inhibitor of PI3′-kinase-α (PI3Kα) (19), displayed a robust reduction in cell proliferation over a 72-hour time period and colony forming ability over a ten-day period (Figs. 1B & 1C). By contrast, BYL719 treatment of B10C melanoma cells elicited only a modest reduction in short-term cell proliferation and had no effect on long-term colony formation (Figs. 1B & 1C). Indeed, there was a greater than 10-fold difference between the concentration of BYL719 required for 50% inhibition of proliferation (GI50) of BPC versus B10C cells (Fig. 1D). In addition, the BP2C melanoma cell line derived from homozygous Pik3calat/lat mice displayed similar sensitivity to BYL719 as did the BPC cells (Figs. S1A & S1B). Thus, BRAFV600E/PIK3CAH1047R melanoma cells display the predicted genotype-drug response phenotype relationship. By contrast, BRAFV600E/PTENNull melanoma cells appear not to depend solely on PI3Kα for their proliferation.
To examine the effects of PI3Kα blockade on signal pathway activity, extracts of BPC or B10C melanoma cells treated with BYL719 (5μM) were subjected to immunoblot analysis (Fig. 1E). In BPC cells, BYL719 elicited a complete and sustained inhibition of pAKT (pS473) over 72 hours. We also noted diminished phosphorylation of downstream pathway components of PI3K→AKT signaling including PRAS40 and 4E-BP1 (Fig. 1E). By contrast, BYL719-treated B10C cells displayed only a partial and transient inhibition of pAKT with almost no effect on pPRAS40 or p4E-BP1.
Since BRAFV600E and PI3K signal cooperatively through mTORC to regulate melanoma cell proliferation (20), we investigated whether PI3Kα inhibition would enhance the effects of BRAFV600E inhibition in BPC or B10C melanoma cells. While single agent BRAFV600E (LGX818) (21) or PI3Kα (BYL719) inhibition potently suppressed BPC melanoma cell proliferation, combined treatment elicited a significantly greater inhibition of cell proliferation at 24, 48, and 72 hours (Fig. 1F). Further, while inhibition of PI3Kα suppressed pPRAS40, pRPS6 and p4EB-P1 in BPC melanoma cells, combined inhibition of both BRAFV600E and PIK3CAH1047R signaling elicited a more robust inhibition of these phosphorylation events (Fig. 1G). Similar observations were made in the independently derived BP2C melanoma cell line (Fig. S1C). By contrast, while BRAFV600E inhibition (LGX818) potently suppressed B10C cell proliferation, addition of BYL719 did not significantly enhance the anti-proliferative effects of BRAFV600E inhibition at any time point (Fig. 1F). In B10C cells, LGX818 inhibited pERK but had little effect on pRPS6 or p4E-BP1 (Fig. 1G). Although treatment of B10C cells with BYL719 elicited a modest decrease in pAKT there was no effect on pPRAS40, pRPS6 or p4E-BP1. Most importantly, combined treatment of B10C cells with LGX818 plus BYL719 displayed no cooperative effects on pPRAS40, pRPS6 or p4E-BP1. Together, these data demonstrate that inhibition of PI3Kα enhanced the effects of BRAFV600E inhibition in BRAFV600E/PIK3CAH1047R but not in BRAFV600E/PTENNull melanoma cells.
The anti-proliferative activity of PI3Kα selective inhibition on BRAFV600E/PIK3CAH1047R cells in vitro prompted us to design a preclinical trial in mice to test the ability of BYL719, either alone or in combination with LGX818, to elicit regression of autochthonous BRAFV600E/PIK3CAH1047R melanomas. To that end, BRAFV600E/PIK3CAH1047R melanomas were initiated on the back skin of adult Tyr::CreER; BRafCA; Pik3calat/+ mice. In this scenario, melanomas are elicited by the cooperative action of two dominantly acting oncogenes: BRAFV600E and PIK3CAH1047R (11, 22). Seven weeks post-initiation (p.i), mice were randomized to receive vehicle, LGX818, BYL719, or combined LGX818 plus BYL719 treatment, with melanoma size measured weekly. Pharmacodynamic analysis of pAKT inhibition in BRAFV600E/PIK3CAH1047R melanomas indicated the need to dose BYL719 twice daily to achieve maximal target inhibition (Fig. S1D).
Single agent BYL719 initially elicited modest melanoma regression (<30%), followed by a prolonged cytostatic effect (Fig. 1H). By contrast, single agent LGX818 elicited profound melanoma regression. Importantly, the combination of BYL719 plus LGX818 promoted significantly more potent melanoma regression than that observed with LGX818 monotherapy (Fig. 1H). Analysis of the best overall response by waterfall plot indicated that only 2/8 mice treated with BYL719 displayed >30% melanoma regression, which qualifies as a partial response (PR) by modified RECIST 1.1 guidelines (Fig. 1I) (23). By contrast, 7/7 mice treated with LGX818 exceeded the 30% regression threshold, as did 8/8 mice receiving BYL719+LGX818. Finally, combined treatment with LGX818 plus BYL719 provided significantly superior melanoma regression compared to single agent LGX818 therapy (Fig. 1I).
Analysis of glioblastoma, breast or prostate cancer models suggests that PI3′-kinase-β (PI3Kβ/PIK3CB) is the predominant driver of PI3′-lipid production in PTENNull tumors (24, 25). Consequently, we tested the effects of PI3Kβ selective inhibition on the proliferation of human BRAFV600E/PTENNull melanoma-derived cells. Two structurally distinct PI3Kβ selective inhibitors were used: GSK2636771 (GSK771) and KIN193 (26–28). Perhaps surprisingly, even at the highest concentration tested (10μM), GSK771 failed to reach the GI50 of BRAFV600E/PTENNull human melanoma cells (Fig. 2A and Fig. S2A). Further, even at 5μM, GSK771 elicited only a minor reduction of pAKT in BRAFV600E/PTENNull melanoma cells, with no effects on the phosphorylation of downstream PI3K pathway components (Fig. 2B and Fig. S2B). While KIN193 displayed enhanced—but still modest—anti-proliferative activity compared to GSK771, the anti-proliferative activity of KIN193 on PI3Kα-dependent BPC cells (Fig. S2A) suggests that at higher concentrations, the anti-proliferative activity of KIN193 is likely due to inhibition of PI3Kα. Additionally, although 5μM KIN193 modestly suppressed pAKT in some human-derived BRAFV600E/PTENNull melanoma cells (Fig. 2B and S2B), the ability of 5μM KIN193 treatment to robustly suppress pAKT in PI3Kα-dependent BPC cells (Fig. S2B) further underscores the fact that this activity is likely due to inhibition of PI3Kα. B10C melanoma cells also displayed proliferative and biochemical resistance to PI3Kβ inhibition (Figs. S2A & S2B). Interestingly, while both KIN193 and GSK771 treatment suppressed pAKT activation in M249 BRAFV600E/PTENNull melanoma cells, this inhibition did not result in reduced phosphorylation of PRAS40 (Fig. S2B). Moreover, PI3Kβ inhibition had only modest anti-proliferative effects on M249 cells, suggesting that residual PI3Kβ-independent PI3K signaling was sufficient to sustain cell proliferation.
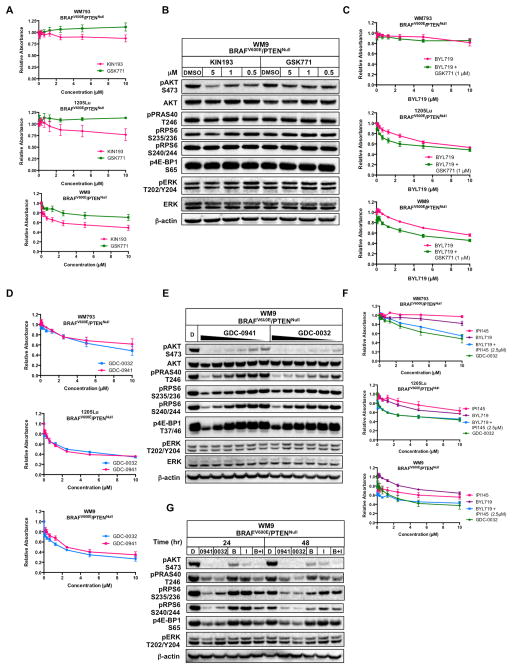
Figure 2. BRAFV600E/PTENNull human melanoma-derived cells are insensitive to PI3Kβ-selective inhibition but sensitive to PI3Kβ-sparing class I PI3K inhibition.
(A) WM793, 1205Lu or WM9 melanoma cells were cultured in the presence of the indicated concentrations of KIN193 (pink) or GSK771 (green) for 72 hours before being fixed and stained with Crystal Violet. Crystal Violet staining was quantified as described above. Values indicated are normalized to DMSO control and error bars represent SEM.
(B) Lysates of WM9 melanoma cells treated for 24 hours with the indicated concentrations of KIN193 or GSK771 were analyzed by immunoblotting.
(C) WM793, 1205Lu or WM9 melanoma cells were cultured in the presence of the indicated concentrations of BYL719 (pink) or BYL719 plus GSK771 (1μM, green) for 72 hours prior to fixation and staining with Crystal Violet. Crystal Violet staining was quantified as described above. Values indicated are normalized to DMSO control and error bars represent SEM.
(D) WM793, 1205Lu or WM9 melanoma cells were cultured in the presence of the indicated concentrations of GDC-0032 (blue) or GDC-0941 (pink) for 72 hours prior to fixation and staining with Crystal Violet. Crystal Violet staining was quantified as described above. Values indicated are normalized to DMSO control and error bars represent SEM.
(E) Lysates of WM9 melanoma cells, treated for 24 hours with DMSO (D), GDC-0941 or GDC-0032, with drug treatment applied in a serial two-fold dilution series from 10μM to 31.25 nM as indicated by gradient, were analyzed by immunoblotting.
(F) WM793, 1205Lu or WM9 melanoma cells were cultured in the presence of the indicated concentrations of IPI145 (pink), BYL719 (purple), BYL719 + 2.5μM IPI145 (blue) or GDC-0032 (green) for 72 hours prior to fixation and staining with Crystal Violet. Crystal Violet staining was quantified as described above. Values indicated are normalized to DMSO control and error bars represent SEM.
(G) Lysates of WM9 melanoma cells treated for the indicated time period with DMSO (D), GDC-0941 (0941), GDC-0032 (0032), BYL719 (B), IPI145 (I) or BYL719+IPI145 (B+I) (all at 5μM) were analyzed by immunoblotting.
The lack of robust single agent activity on BRAFV600E/PTENNull melanoma cells observed with PI3Kβ selective inhibition led us to hypothesize that BRAFV600E/PTENNull human melanoma cells might require the combined activity of PI3Kα and PI3Kβ for sustained proliferation. To test this, we assessed cell proliferation of BRAFV600E/PTENNull cells treated with BYL719 in the presence or absence of a fixed concentration of GSK771 (Fig. 2C). Although PI3Kα inhibition had a modest inhibitory effect on melanoma cell proliferation, the addition of a PI3Kβ inhibitor did not dramatically enhance that effect, suggesting that BRAFV600E/PTENNull melanoma cells do not rely exclusively on the combined activity of PI3Kα and PI3Kβ for their proliferation.
To test if BRAFV600E/PTENNull melanoma cells require PI3Kβ to promote PI3′-lipid signaling with downstream effects on cell proliferation we employed GDC-0032, a PI3Kβ-sparing class I PI3K inhibitor that inhibits PI3Kα, δ and γ (29). Initially, we compared the anti-proliferative activity of GDC-0032 to that of GDC-0941, a pan-class I PI3K inhibitor (30). Both GDC-0032 and GDC-0941 displayed equivalent GI50 values in all BRAFV600E/PTENNull melanoma cells tested (Figs. 2D & S2C). Further, treatment with GDC-0032 elicited a dose-dependent reduction in pAKT and its downstream effectors with modestly enhanced potency compared to GDC-0941 (Figs. 2E & S2D). Taken together, these data indicate that PI3Kβ contributes little or nothing to PI3′-lipid signaling or proliferation of BRAFV600E/PTENNull melanoma cells.
To confirm that BRAFV600E/PTENNull melanoma cells rely on the combined activity of PI3Kα plus PI3Kδ and/or PI3Kγ for sustained proliferation, we investigated whether the effects of GDC-0032 could be mimicked by combined use of PI3Kα (BYL719) and PI3Kδ/γ (IPI145) selective inhibitors (31). Treatment of BRAFV600E/PTENNull WM793, 1205Lu or WM9 human melanoma cells with single agent BYL719 or IPI145 failed to reach the GI50, even at 10μM (Fig. 2F). However, when the cells were treated with a fixed concentration of IPI145 (2.5μM) in the presence of BYL719, the combination elicited a more robust anti-proliferative response similar to the effects of GDC-0032 (Fig. 2F). Furthermore, while single agent treatment of WM9 cells with either BYL719 or IPI145 elicited only a modest reduction in pAKT with little or no effect on downstream pathway components, the combination of BYL719 plus IPI145 elicited a complete and sustained inhibition of pAKT that mirrored the effects of GDC-0032 (Fig. 2G). Similar results were obtained with 1205Lu BRAFV600E/PTENNull melanoma cells (Fig. S2E). Together, these results suggest that PI3Kβ activity is dispensable for the proliferation and PI3K pathway activation of BRAFV600E/PTENNull melanoma cells and that these cells instead rely upon the combined activities of PI3Kα, δ and/or γ.
To determine if a PI3Kβ-sparing PI3K inhibitor might augment the effects of BRAFV600E pathway-targeted inhibition, we treated BRAFV600E/PTENNull human or mouse melanoma cells with GDC-0973 (an inhibitor of MEK1/2), GDC-0032, or GDC-0973 plus GDC-0032 (Figs. 3A & S3A) (32). Importantly, the combined use of these agents elicited robust suppression of pAKT and pERK, as well as more robust suppression of pRPS6 and p4EB-P1 than that achieved with either single agent alone. Additionally, whereas single agent GDC-0973 or GDC-0032 suppressed BRAFV600E/PTENNull human or mouse melanoma cell proliferation, combined treatment elicited a significant reduction in proliferation compared to either single agent alone (Figs. 3B & S3B).
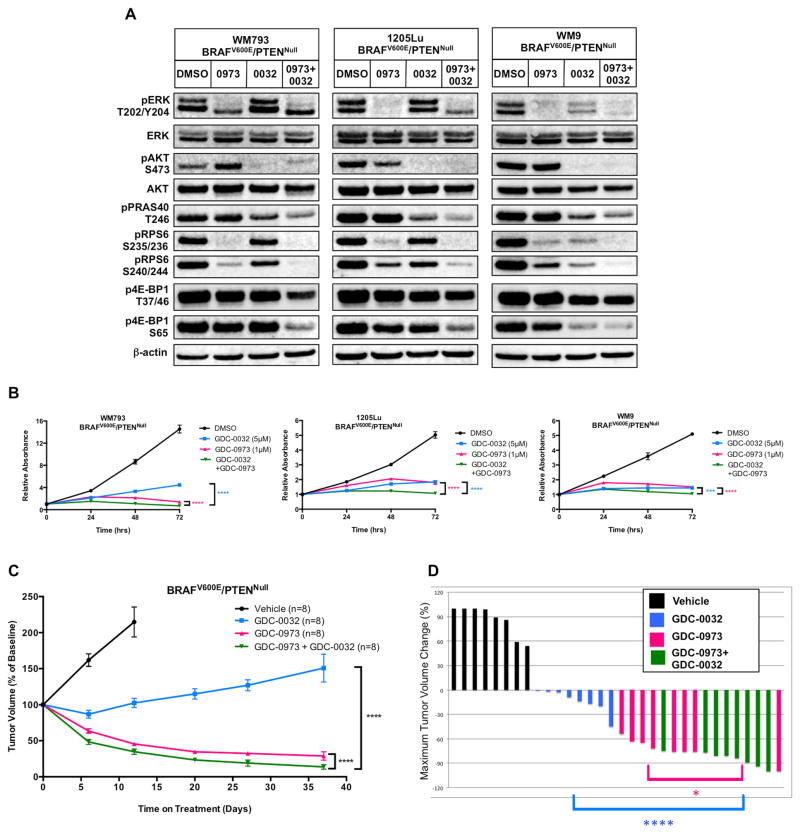
Figure 3. A PI3Kβ-sparing inhibitor enhances the effects of MEK1/2 inhibition on both BRAFV600E/PTENNull human melanoma cells and autochthonous mouse melanomas.
(A) Lysates of WM793, 1205Lu or WM9 melanoma cells treated for 6 hours with DSMO, GDC-0973 (1μM, 0973), GDC-0032 (5μM, 0032) or GDC-0973 plus GDC-0032 (0973+0032) were analyzed by immunoblotting.
(B) WM793, 1205Lu or WM9 melanoma cells were cultured in the presence of GDC-0032 (5μM, blue), GDC-0973 (1μM, pink) or GDC-0973 plus GDC-0032 (green) with cells fixed and stained with Crystal Violet every 24 hours for of 72 hours. Crystal Violet staining was quantified as described above. Error bars represent SEM. Asterisks indicate significant difference between combination drug treatment and single agent drug treatment (2-way ANOVA, ***, p<0.0005, ****, p<0.0001).
(C) BRAFV600E/PTENNull melanomas were initiated in suitably manipulated adult Tyr::CreER, BRafCA; Ptenlox/lox mice. Following randomization, mice were treated with vehicle, single agent or combination GDC-0032 (22.5mg/kg) and GDC-0973 (4.5mg/kg, q.d.). Melanoma growth or regression was measured weekly with digital calipers over the course of 37 days of continuous drug treatment. Mice received GDC-0032 (as single agent or combination therapy) on a b.i.d. regimen for the first 12 days of treatment, but due to apparent toxicity, mice were dosed q.d starting on day 13. Tumor sizes are displayed as the average percent change in tumor size from the start of treatment, with error bars indicating SEM. Asterisks indicate significant difference between combination drug treatment and single agent drug treatment (2-way ANOVA, p<0.0001).
(D) A waterfall plot of the best tumor response for each of the 32 mice that received vehicle versus drug treatment in (C). The percent change in tumor size from the start of treatment is shown on the y-axis. Negative values indicate tumor shrinkage. Asterisks indicate significant difference between combination drug treatment and GDC-0032 (blue) or GDC-0973 (pink) (Unpaired t-test, *, p<0.05, ****, p<0.0001).
The in vitro activity of GDC-0032 against BRAFV600E/PTENNull melanoma cells prompted us to conduct a preclinical trial to test the ability of GDC-0032 to elicit regression of BRAFV600E/PTENNull melanomas in vivo, either alone or in combination with GDC-0973. To that end, BRAFV600E/PTENNull melanoma was initiated in adult Tyr::CreER; BRafCA; Ptenlox/lox mice and, seven weeks p.i., mice were randomized to receive vehicle, GDC-0973, GDC-0032 or combination therapy with melanoma size measured weekly (Fig. 3C). As with BRAFV600E/PIK3CAH1047R melanomas treated with BYL719, inhibition of PI3K signaling with GDC-0032 had largely cytostatic effects on BRAFV600E/PTENNull melanomas (Figs. 3C & 3D). By contrast, MEK1/2 inhibition with GDC-0973 elicited substantial regression of BRAFV600E/PTENNull melanomas, which was significantly enhanced by combined treatment with GDC-0032 (Figs. 3C & 3D).
To investigate the potential role of PI3Kβ in BRAF-mutated melanoma cells in which there is no documented genetic alteration in components of PI3K signaling, we treated SK-MEL-239 human melanoma cells (BRAFV600E/PTENWT) with inhibitors of PI3Kβ, and characterized the anti-proliferative response (Fig. S4A). While we observed modestly enhanced potency for KIN193 as compared to GSK771, the GI50 for both PI3Kβ inhibitors on SK-MEL-239 melanoma cells was >10μM. While SK-MEL-239 cells exhibit low basal levels of pAKT, inhibition of PI3Kβ did not suppress activation of downstream pathway components pPRAS40, pRPS6, or p4E-BP1 (Fig. S4B). Moreover, both GDC-0032 and GDC-0941 displayed equivalent GI50 values in SK-MEL-239 cells (Fig. S4C). The combined use of GDC-0032 plus GDC-0973 elicited robust suppression of pAKT and pERK, as well as an even more robust suppression of pRPS6 and p4E-BP1 than that achieved with either single agent (Fig. S4D). Finally, whereas single agent GDC-0973 or GDC-0032 suppressed SK-MEL-239 cell proliferation, combined treatment elicited a significant reduction in proliferation compared to either single agent alone (Figs. S4E). Collectively these results indicate that, in at least one BRAFV600E/PTENWT human melanoma cell line, PI3Kβ activity is dispensable for proliferation and that these cells rely upon the combined activities of PI3Kα, δ and/or γ.
Although the majority of BRAF-mutated melanoma patients experience initial tumor regression in response to BRAFV600E pathway-targeted therapies, the durability of response is limited by the onset of drug resistant disease (6). Therefore, we wished to test if inhibition of class I PI3K isoform(s) would influence the development of resistance to inhibitors that target BRAFV600E signaling. We initially tested this question using vehicle-treated BRAFV600E/PTENNull melanoma-bearing mice enrolled in the study described in Fig. 3C. When these mice (n=8) were near to end-stage, they were randomly re-assigned to receive extended treatment with either GDC-0973 monotherapy or combined GDC-0973 plus GDC-0032. These mice received a reduced dose of GDC-0973 (2mg/kg) and a full dose GDC-0032 (22.5mg/kg) to minimize the toxicity of full dose combination therapy. As expected, mice in both treatment groups experienced initial tumor regression, which was superior with combined treatment compared to GDC-0973 monotherapy (Figs. 4A and 4B). However, over the course of 113 days of treatment, all of the mice receiving GDC-0973 monotherapy developed drug resistant disease, defined as tumor re-growth ≥100% of the tumor volume at the initiation of therapy. By contrast, none of the mice receiving combination therapy developed drug resistant disease. To further investigate the role of PI3K pathway activity in promoting resistance to GDC-0973 treatment, when the first mouse receiving GDC-0973 monotherapy reached end-stage, the tumor was resected, fragmented, and implanted into a cohort (n=8) of immunocompromised mice. Immediately following implantation, these mice received GDC-0973 monotherapy treatment. As expected, the transplanted tumor displayed resistance to GDC-0973 and grew progressively over 50 days. At this time, half of the mice were randomly re-assigned to receive GDC-0973 plus GDC-0032 combination therapy, while the rest continued to receive GDC-0973 monotherapy (Fig. 4C). The mice receiving GDC-0973 plus GDC-0032 combination therapy experienced potent tumor cytostasis, suggesting that PI3K pathway activity is necessary for the sustained growth of a melanoma that has developed resistance to a MEK1/2 inhibitor.
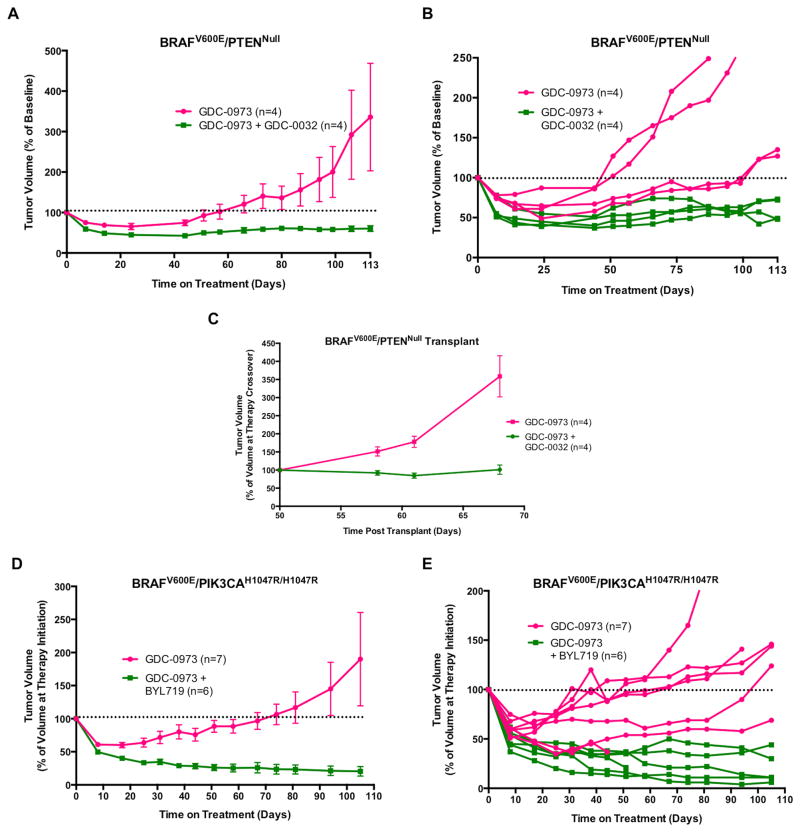
Figure 4. PI3K inhibition forestalls the development of MEK1/2 inhibitor resistance in two different GEM models of melanoma.
(A) After two weeks of vehicle treatment, BRAFV600E/PTENNull melanoma-bearing mice from the experiment described in Fig. 3C were crossed over to receive either GDC-0973 (2mg/kg) or GDC-0973 (2mg/kg) plus GDC-0032 (22.5mg/kg). Tumors were measured weekly with digital calipers with tumor size displayed as the average percent change in tumor size from the initiation of drug treatment, with error bars indicating SEM.
(B) A spider plot of the individual tumor response for each of the eight mice treated in (E). Horizontal dotted line indicates progressive disease when tumor volume was ≥100% of tumor volume prior to drug treatment. Tumor sizes are displayed as the average percent change in tumor size from the crossover point.
(C) Fragments of a single GDC-0973 resistant tumor from (A) were implanted into eight immunocompromised mice and allowed to grow into measurable tumors over 50 days with daily administration of GDC-0973 (2mg/kg). At that time, mice were randomized to receive either combination GDC-0973 (2mg/kg) plus GDC-0032 (22.5mg/kg) (green) or continuation of single agent GDC-0973 (2mg/kg) (pink). Tumor sizes were measured weekly and plotted as described previously. Tumor sizes are displayed as the average percent change in tumor size from the crossover point, with error bars indicating SEM.
(D) BRAFV600E/PIK3CAH1047R melanomas were initiated in 13 adult Tyr::CreER; BRafCA; Pik3calat/lat mice and 8 weeks later they were randomized by tumor size and sex for treatment with GDC-0973 (2mg/kg, q.d., n=7) or GDC-0973 plus BYL719 (50mg/kg, b.i.d., n=6). Melanoma growth or regression was measured weekly over the course of 106 days of drug treatment. Tumor sizes were measured weekly and plotted as described previously, with error bars indicating SEM.
(E) A spider plot of the individual tumor response for each of the 13 mice treated in (D). Horizontal dotted line indicates progressive disease when tumor volume was ≥100% of tumor volume prior drug treatment. Tumor sizes are displayed as the average percent change in tumor size from the start of treatment.
To further validate the ability of PI3K inhibitors to forestall the onset of resistance to targeted blockade of BRAFV600E signaling, BRAFV600E/PIK3CAH1047R/H1047R melanoma was initiated in 13 adult Tyr::CreER; BRafCA; Pik3calat/lat mice and, 61 days p.i., mice were randomized to receive GDC-0973 (2mg/kg) monotherapy or GDC-0973 plus BYL719 (50mg/kg) combination therapy. As observed with BRAFV600E/PTENNull driven melanomas, mice in both treatment groups experienced initial tumor regression, which was superior with combined treatment compared to GDC-0973 monotherapy (Figs. 4D and 4E). However, over the course of 106 days, 6/7 mice receiving GDC-0973 monotherapy developed drug resistant disease. By contrast, none of the mice receiving combination therapy developed drug resistance.
DISCUSSION
At the initiation of these studies, the high rate of PTEN silencing compared to mutational activation of PIK3CA suggested that PTEN might exert PI3′-lipid phosphatase-independent tumor suppressor functions to restrain progression of BRAF-mutated melanoma (14). We previously noted that, while heterozygous mutational activation of Pik3calat was sufficient to promote melanoma progression, BRAFV600E/PIK3CAH1047R melanomas grew significantly more slowly than did BRAFV600E/PTENNull melanomas (11). However, BRAFV600E-driven melanomas homozygous for PIK3CAH1047R expression grew even more rapidly than BRAFV600E/PTENNull melanomas. Although we cannot formally exclude a role for PI3′-lipid phosphatase-independent PTEN tumor suppressor functions to restrain progression of BRAF-mutated melanoma, there is no compelling rationale to invoke such mechanisms. Importantly, the correlation between PI3K pathway activation and melanoma growth rate indicates that PI3K catalytic isoforms are relevant drug targets in the treatment of BRAFV600E/PTENNull melanoma.
The “oncogene addiction” hypothesis posits that, despite a high burden of genetic damage, tumors remain dependent on the sustained activity of one or a small number of oncogenes for maintenance of the malignant phenotype (33). As a corollary, inhibition of the oncogene(s) to which a tumor is addicted can elicit profound tumor regression (34). Accordingly, our model of BRAFV600E/PIK3CAH1047R melanoma is driven by two oncogene activation events one of which, BRAFV600E, drives tumor initiation and the other of which, PIK3CAH1047R, promotes melanoma progression (22). Potent inhibitors of either BRAFV600E or PIK3CAH1047R allowed us to characterize to which of these two oncoproteins are BRAFV600E/PIK3CAH1047R melanomas most addicted in vivo. While inhibition of BRAFV600E elicited profound melanoma regression, selective inhibition of PIK3CAH1047R elicited largely cytostatic effects: only 2/8 mice displayed a ≥30% reduction in melanoma size in response to BYL719 monotherapy and that response was largely transient (23). These results may illustrate a fundamental difference between the effects of oncogenic BRAFV600E and PIK3CAH1047R on the sustained survival of melanoma cells, carrying potential clinical implications.
Previous studies of PTEN deficient tumor cells have indicated that PI3Kβ is an essential contributor to PI3′-lipid signaling and aberrant cell proliferation (24, 25). However, our work suggests that PI3Kβ, either alone or in combination with PI3Kα, does not contribute to PI3′-lipid signaling or to the proliferation of human or mouse BRAFV600E/PTENNull melanoma cells. Although PI3Kβ inhibitors modestly attenuated pAKT in M249 cells, this did not translate into suppression of cell proliferation. Furthermore, a PI3Kβ–sparing inhibitor and a combination of agents that inhibits PI3Kα, δ and γ had potent inhibitory effects on PI3′-lipid signaling and proliferation of BRAFV600E/PTENNull melanoma cells. Interestingly, SK-MEL-239 melanoma cells, which express BRAFV600E and normal PTEN, also displayed resistance to PI3Kβ inhibition and equivalent sensitivity to pan class I (GDC-0941) or PI3Kβ-sparing PI3K inhibition (GDC-0032), demonstrating no role for PI3Kβ in these cells. It is tempting to speculate that PI3Kβ may play a role in the proliferation of melanomas in which RAC1 is mutated or amplified, as PI3Kβ is a direct target of activated RAC1-GTP (1, 35).
Despite the potent biochemical and anti-proliferative effects of the PI3Kβ-sparing inhibitor GDC-0032 in vitro, this agent elicited largely cytostatic effects in our BRAFV600E/PTENNull GEM melanoma model and showed modest, but significant, cooperation with MEK1/2 inhibition to promote melanoma regression. Perplexingly, although both human and mouse cancer genetics indicate an important role for PI3K signaling in disease progression, the limited activity of PI3K inhibitors in solid tumor clinical trials does not correlate with PI3K pathway activation (36). This may be due to a role for PI3K signaling predominantly in promoting cell cycle progression and not for suppression of apoptosis. However, more promisingly, treatment with GDC-0032 forestalled the onset of resistance to a MEK1/2 inhibitor (GDC-0973) in our GEM model of BRAFV600E/PTENNull melanoma. Importantly, upon serial transplantation, MEK1/2 inhibitor resistant melanomas retained sensitivity to combined GDC-0973 plus GDC-0032 treatment, highlighting the importance of PI3K pathway signaling in maintenance of the MEK1/2 inhibitor-resistant phenotype. Further emphasizing the importance of PI3K signaling in MEK1/2 inhibitor resistance, the emergence of GDC-0973 resistant melanomas was forestalled by combined treatment with BYL719 in our BRAFV600E/PIK3CAH1047R GEM melanoma model. Since a major limitation in single agent treatment of BRAF-mutated melanoma is the onset of drug resistant disease, the observation that PI3K inhibition enhances the depth and durability of response to BRAFV600E pathway-targeted inhibition may illuminate an arena in which PI3K inhibitors will offer substantial clinical benefit.
MATERIALS AND METHODS
Cell Culture and Drug Treatments
Human melanoma cell lines WM793, WM9, and 1205Lu were kindly provided from the well-curated cell line repository established by Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA), and genomic sequencing of these cells was performed in the laboratory of Dr. Katherine Nathanson (University of Pennsylvania, Philadelphia, PA). Human melanoma cell lines M249 and M233 were kindly provided by Dr. Antoni Ribas (University of California, Los Angeles, Los Angeles, CA) and authenticated by genomic sequencing as previously described (37). Human melanoma cell line SK-MEL-239 was kindly provided by Dr. David B. Solit (Memorial Sloan-Kettering Cancer Center, New York, NY) authenticated by genomic sequencing as previously described (38). Mouse melanoma cell lines B10C, BPC, and BP2C were established as described previously and authenticated by PCR and immunoblot analyses (11). Efficient generation of melanoma cell lines from our various GEM models requires the silencing of the Cdkn2a locus encoding INK4A and ARF. Mouse or human melanoma cell lines were cultured as described previously (11). Pathway-targeted pharmacological agents were obtained from various colleagues in the public or private sector or from commercial sources (see Supplementary Table 1 for provenance).
Proliferation and Growth Assays
Melanoma cell proliferation was assessed over 72-hours by seeding 5 × 104 cells in 12-well dishes. Cells were treated with the various pharmacological agents as described with viable cells enumerated using a Countess® cell counter (Invitrogen). In addition, melanoma cells were seeded and treated with pharmacological agents as described for 72 hours at which time viable cells were stained with crystal violet and quantified by solubilization in 33% acetic acid with A562 absorbance assessed. GI50 assays were performed by seeding 8.0 × 103 cells in a 96-well plate and treating cells with pharmacological agents as described for 72 hours at which time viable cells were stained with Crystal Violet and quantified by solubilization in 33%(v/v) acetic acid with A562 absorbance assessed. At least three independent experiments, performed in biological triplicate, were completed for all 72-hour assays. Long-term colony formation assays were performed by culturing 500–2000 cells in a 10cm dish for 6–11 days in the absence or presence of various agents with cell colonies fixed and stained with Crystal Violet.
Immunoblot Analysis
Cell lysates were generated for analysis of 50μg aliquots by immunoblotting as described previously (20). Membranes were stained with primary antibodies with antigen-antibody complexes detected using fluorescent goat anti-Rabbit IRDye 800 or goat anti-Mouse IRDye 680 secondary antibodies (LI-COR Biosciences) and visualized with a LI-COR infrared imaging system (Odyssey Fc). Immunoblot data were analyzed using Image Studio v2.0 software (LI-COR Biosciences).
Experimental Animals
The UCSF Institutional Animal Care and Use Committee (IACUC) reviewed and approved all animal procedures. Tyr::CreER, BRafCA, Ptenlox4-5 or Pik3caLat-1047R mice, maintained on an outbred background, were intercrossed to generate experimental mice which were genotyped as previously described (11, 12). Melanocyte-specific Cre activity was induced in adult mice by topical application of 1.5μl of 5mM 4-hydroxytamoxifen (4-HT, 70% Z-isomer, in 100% Ethanol, Sigma Aldrich) to shaved back skin. Animals were euthanized based on a body conditioning score (39) or when tumor volume ≥2cm3, whichever occurred first. At necropsy, tissue was snap frozen in liquid nitrogen. Tissue was homogenized in RIPA buffer using the Tissue Lyser II (Qiagen®) for immunoblotting as described previously (11).
Treatment of Mice with Pathway-Targeted Inhibitors
Melanoma-bearing mice were divided among treatment arms to give equal distribution of tumor volume and gender when the mean tumor volume of the cohort exceeded 500mm3. Mice were assigned to receive LGX818 (30mg/kg q.d.) or BYL719 (50mg/kg b.i.d.) formulated in 0.5%(w/v) carboxymethylcellulose/0.5%(v/v) Tween-80 (Sigma Aldrich) or GDC-0973 (2.0 or 4.5mg/kg q.d.) or GDC-0032 (22.5mg/kg q.d.) formulated in 0.5%(w/v) methylcellulose/0.2%(v/v) Tween-80 (Sigma Aldrich) and administered via oral gavage six days per week. Melanoma growth was measured weekly using digital calipers with relative tumor volume (RTV) estimated using the ellipsoid volume formula as described previously (11).
Statistical Analysis
All quantitative data is represented as means ± SEM. GraphPad Prism 6 statistical software was used to determine p values for the proliferation graphs by performing two-way ANOVA analysis and t-tests as indicated.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Although BRAFV600E pathway-targeted therapies elicit melanoma regression, the onset of drug resistance limits the durability of response. Here we show that combined treatment with PI3K inhibitors significantly forestalled the onset of MEK1/2 inhibitor resistant disease in BRAF-mutated GEM melanoma models. These results provide a conceptual framework for the combined deployment of BRAFV600E plus PI3K pathway-targeted inhibitors in the treatment of a subset of BRAF-mutated melanoma patients.
Acknowledgments
We thank all of the members of the McMahon lab for advice and guidance on this project, with special thanks to Jillian Silva for stimulating discussions on melanoma cell signaling and immunoblotting. We thank Adil Daud (UCSF) for invigorating discussions on melanoma therapy, Meenhard Herlyn (Wistar Institute) and Antoni Ribas (UCLA) for melanoma cell lines and Marcus Bosenberg (Yale School of Medicine) for Tyr::CreER mice. We thank colleagues for providing access to the following compounds and information on their use: Emmanuelle Di Tomaso, Janet Lyle, and Darrin Stuart (Novartis) for BYL719 and LGX818 and Leisa Johnson, Deepak Sampath and Lori Freedman (Genentech) for GDC-0941, GDC-0032 and GDC-0973 (Genentech). We thank Byron Hann and the Helen Diller Family Comprehensive Cancer Center’s Preclinical Therapeutics Core for advice and the Laboratory Animal Resource Center for animal husbandry. We thank the mice for their participation. This research was supported by grants from the National Health and Medical Research Council of Australia (to WAP) and the National Cancer Institute (CA176839), Melanoma Research Alliance & National Comprehensive Cancer Network (to MM).
Footnotes
AUTHOR CONTRIBUTIONS
MMD, VMD and MM designed the study. MMD collected and analyzed data. WAP provided mouse strains. MMD and MM drafted the manuscript. MM supervised the research and assisted in data analysis and interpretation. All authors critically appraised the manuscript and approved its submission.
Conflict of interest statement: Dr. McMahon has received grant support from Novartis Inc.
References
- 1.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–71. doi: 10.1146/annurev-pathol-012513-104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 4.McArthur GC, Robert PBC, et al. Improved survival with vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma. Lancet Oncol. 2014 In Press. [Google Scholar]
- 5.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 6.Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nature reviews Cancer. 2014;14:455–67. doi: 10.1038/nrc3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lito P, Rosen N, Solit DB. Tumor adaptation and resistance to RAF inhibitors. Nat Med. 2013;19:1401–9. doi: 10.1038/nm.3392. [DOI] [PubMed] [Google Scholar]
- 8.Bucheit AD, Davies MA. Emerging insights into resistance to BRAF inhibitors in melanoma. Biochem Pharmacol. 2014;87:381–9. doi: 10.1016/j.bcp.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh Durban V, Deuker MM, Bosenberg MW, Phillips W, McMahon M. Differential AKT dependency displayed by mouse models of BRAFV600E-initiated melanoma. J Clin Invest. 2013;123:5104–18. doi: 10.1172/JCI69619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–41. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shull AY, Latham-Schwark A, Ramasamy P, Leskoske K, Oroian D, Birtwistle MR, et al. Novel somatic mutations to PI3K pathway genes in metastatic melanoma. PLoS One. 2012;7:e43369. doi: 10.1371/journal.pone.0043369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JE, Stones C, Joseph WR, Leung E, Finlay GJ, Shelling AN, et al. Comparison of growth factor signalling pathway utilisation in cultured normal melanocytes and melanoma cell lines. BMC Cancer. 2012;12:141. doi: 10.1186/1471-2407-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–70. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 17.Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, et al. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–99. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–22. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- 19.Furet P, Guagnano V, Fairhurst RA, Imbach-Weese P, Bruce I, Knapp M, et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett. 2013;23:3741–8. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Silva JM, Bulman C, McMahon M. BRAFV600E cooperates with PI3K signaling, independent of AKT, to regulate melanoma cell proliferation. Mol Cancer Res. 2014;12:447–63. doi: 10.1158/1541-7786.MCR-13-0224-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darrin D, Stuart NL, Poon Daniel J, Aardalen Kimberly, Kaufman Susan, Merritt Hanne, Salangsang Fernando, Lorenzana Edward, Li Allen, Ghoddusi Majid, Caponigro Giordano, Sun Frank, Kulkarni Swarupa, Kakar Shefali, Turner Nancy, Zang Richard, Tellew John, Pryer Nancy. Preclinical profile of LGX818: A potent and selective RAF kinase inhibitor. Cancer Res. 2012:72. [Google Scholar]
- 22.Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–69. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci U S A. 2008;105:13057–62. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, et al. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–14. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 27.Ralph A, Rivero MAH. Identification of GSK2636771, a potent and selective, orally bioavailable inhibitor of phosphatidylinositol 3-kinase-beta (PI3Kα) for the treatment of PTEN deficient tumors. Cancer Res. 2012:72. [Google Scholar]
- 28.Ni J, Liu Q, Xie S, Carlson C, Von T, Vogel K, et al. Functional characterization of an isoform-selective inhibitor of PI3K-p110beta as a potential anticancer agent. Cancer Discov. 2012;2:425–33. doi: 10.1158/2159-8290.CD-12-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem. 2013;56:4597–610. doi: 10.1021/jm4003632. [DOI] [PubMed] [Google Scholar]
- 30.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–32. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 31.Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20:1364–74. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Rice KD, Aay N, Anand NK, Blazey CM, Bowles OJ, Bussenius J, et al. Novel Carboxamide-Based Allosteric MEK Inhibitors: Discovery and Optimization Efforts toward XL518 (GDC-0973) ACS Med Chem Lett. 2012;3:416–21. doi: 10.1021/ml300049d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–80. doi: 10.1158/0008-5472.CAN-07-3293. discussion 80. [DOI] [PubMed] [Google Scholar]
- 35.Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153:1050–63. doi: 10.1016/j.cell.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodon J, Brana I, Siu LL, De Jonge MJ, Homji N, Mills D, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32:670–81. doi: 10.1007/s10637-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 37.Sondergaard JN, Nazarian R, Wang Q, Guo D, Hsueh T, Mok S, et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. Journal of translational medicine. 2010;8:39. doi: 10.1186/1479-5876-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–90. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci. 1999;49:319–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.