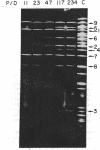
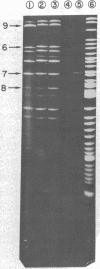
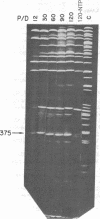
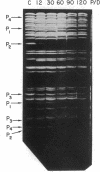
Abstract
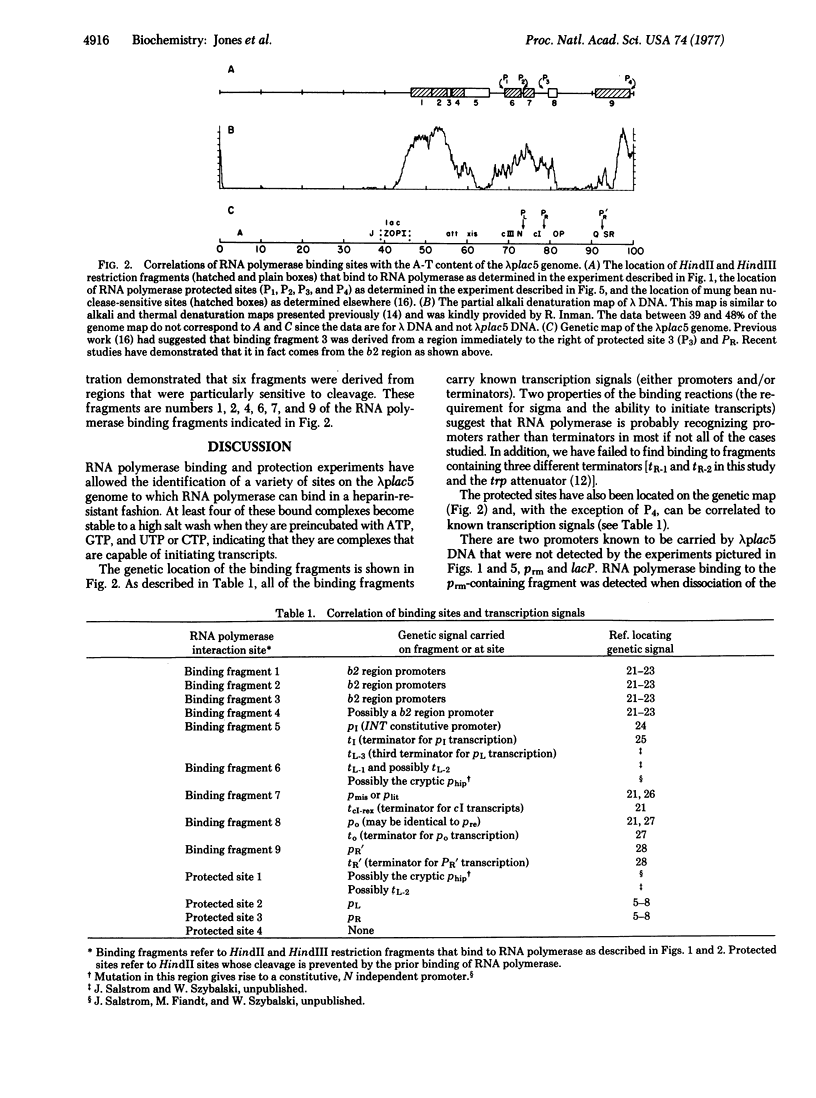
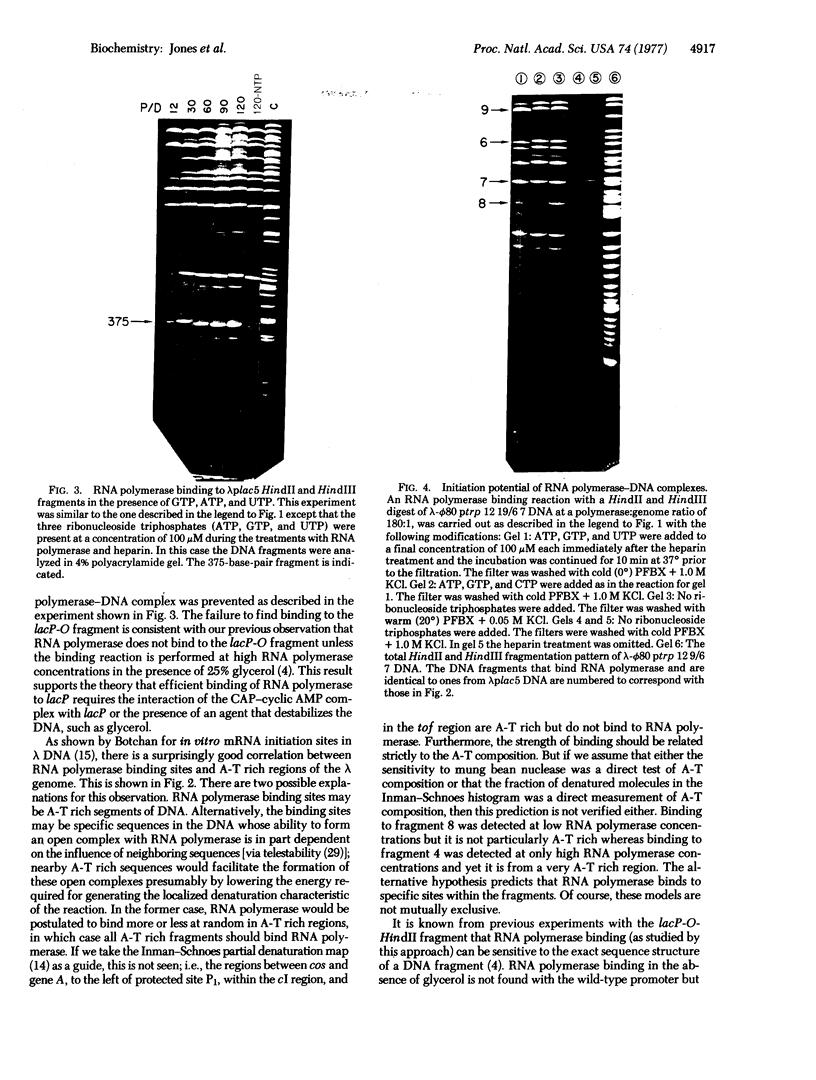
The in vitro binding of the Escherichia coli RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase; EC 2.7.7.6) to fragments of λplac5 DNA generated by restriction endonucleases HindII and HindIII has been studied by a filter binding technique. The results are consistent with RNA polymerase binding at pR′, the INT promoter (pI), several sites in the b2 region, the mis promoter, the oop promoter (or pO), and prm. Binding was also observed on some fragments that are not known to contain active promoters, including the fragment from the cIII-tL region. Some of these binding reactions might also be explained by interaction of RNA polymerase with termination sites. Additional polymerase binding sites have been detected by examining which HindII and HindIII sites were not cleaved when digestion was performed after RNA polymerase had been bound to the DNA. This technique revealed polymerase binding at pL, at pR, at a site between R and cos, and at a site at the junction of the γ and cIII-tL fragments. A comparison of the location of polymerase binding fragments with the partial denaturation map of the λ genome indicates that RNA polymerase binding sites are located within A-T rich regions. It is suggested that RNA polymerase binding is a function both of specific sequences (where recognition occurs) and of the base composition of the surrounding regions (which affects the stability of the helix at the specific site).
Keywords: bacteriophage λ, RNA nucleotidyltransferase, promoters
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Bukhari A. I. Analysis of bacteriophage mu and lambda-mu hybrid DNAs by specific endonucleases. J Mol Biol. 1975 Mar 15;92(4):529–540. doi: 10.1016/0022-2836(75)90307-1. [DOI] [PubMed] [Google Scholar]
- Allet B., Katagiri K. J., Gesteland R. F. Characterization of polypeptides made in vitro from bacteriophage lambda DNA. J Mol Biol. 1973 Aug 25;78(4):589–600. doi: 10.1016/0022-2836(73)90281-7. [DOI] [PubMed] [Google Scholar]
- Allet B., Roberts R. J., Gesteland R. F., Solem R. Class of promotor sites for Escherichia coli DNA-dependent RNA polymerase. Nature. 1974 May 17;249(454):217–221. doi: 10.1038/249217a0. [DOI] [PubMed] [Google Scholar]
- Allet B., Solem R. Separation and analysis of promoter sites in bacteriophage lambda DNA by specific endonucleases. J Mol Biol. 1974 Jan 5;85(4):475–484. doi: 10.1016/0022-2836(74)90310-6. [DOI] [PubMed] [Google Scholar]
- Bennett G. N., Schweingruber M. E., Brown K. D., Squires C., Yanofsky C. Nucleotide sequence of region preceding trp mRNA initiation site and its role in promoter and operator function. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2351–2355. doi: 10.1073/pnas.73.7.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan P. An electron microscopic comparison of transcription on linear and superhelical DNA. J Mol Biol. 1976 Jul 25;105(1):161–176. doi: 10.1016/0022-2836(76)90201-1. [DOI] [PubMed] [Google Scholar]
- Burd J. F., Wartell R. M., Dodgson J. B., Wells R. D. Transmission of stability (telestability) in deoxyribonucleic acid. Physical and enzymatic studies on the duplex block polymer d(C15A15) - d(T15G15). J Biol Chem. 1975 Jul 10;250(13):5109–5113. [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Chan H. W., Dodgson J. B., Wells R. D. Influence of DNA structure on the lactose operator-repressor interaction. Biochemistry. 1977 May 31;16(11):2356–2366. doi: 10.1021/bi00630a008. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Hutchison C. A., 3rd, Edgell M. H. Isolation and genetic localization of three phi-X174 promoter regions. Nat New Biol. 1973 Jun 20;243(129):233–236. doi: 10.1038/newbio243233a0. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Allison D. P., Snyder C. E., Mitra S. Base-unpaired regions in supercoiled replicative form DNA of coliphage M13. J Biol Chem. 1977 Aug 25;252(16):5916–5923. [PubMed] [Google Scholar]
- Dhar R., Weissman S. M., Zain B. S., Pan J., Lewis A. M., Jr The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 2. The sequence of the early strand transcript. Nucleic Acids Res. 1974 Apr;1(4):595–611. doi: 10.1093/nar/1.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Hayes S., Szybalski W. Control of short leftward transcripts from the immunity and ori regions in induced coliphage lambda. Mol Gen Genet. 1973 Nov 22;126(4):275–290. doi: 10.1007/BF00269438. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Partial denaturation of thymine- and 5-bromouracil-containing lambda DNA in alkali. J Mol Biol. 1970 Apr 14;49(1):93–98. doi: 10.1016/0022-2836(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Jones B. B., Reznikoff W. S. Tryptophan-transducing bacteriophages: in vitro studies with restriction endonucleases HindII + III and Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1977 Oct;132(1):270–281. doi: 10.1128/jb.132.1.270-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., Chamberlin M. J. Studies of ribonucleic acid chain initiation by Escherichia coli ribonucleic acid polymerase bound to T7 deoxyribonucleic acid. II. The effect of alterations in ionic strength of chain initiation and on the conformation of binary complexes. J Biol Chem. 1974 May 25;249(10):3002–3006. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B. J., Kleid D. G., Ptashne M. Lambda repressor turns off transcription of its own gene. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4785–4789. doi: 10.1073/pnas.72.12.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W., Mosharrafa E., Edmundson R., Zissler J., Fiandt M., Szybalski W. Insertion sequence IS2 associated with int-constitutive mutants of bacteriophage lambda. Gene. 1977;2(2):61–74. doi: 10.1016/0378-1119(77)90073-7. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Nüsslein C., Schaller H. Interaction of RNA polymerase with promoters from bacteriophage fd. Eur J Biochem. 1977 Mar 15;74(1):107–113. doi: 10.1111/j.1432-1033.1977.tb11372.x. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Schaller H. Mapping and characterization of promoters in bacteriophages fd, f1 and m13. J Mol Biol. 1975 Feb 25;92(2):261–277. doi: 10.1016/0022-2836(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Shimada K., Campbell A. Int-constitutive mutants of bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):237–241. doi: 10.1073/pnas.71.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar J., Yot P., Weissman S. M. Determination of genes, restriction sites, and DNA sequences surrounding the 6S RNA template of bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 May;72(5):1817–1821. doi: 10.1073/pnas.72.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A., Pirrotta V. Sequence of the PR promoter of phage lambda. Nature. 1975 Mar 13;254(5496):118–121. doi: 10.1038/254118a0. [DOI] [PubMed] [Google Scholar]
- Zain B. S., Weissman S. M., Dhar R., Pan J. The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 1. The sequence of the late strand transcript. Nucleic Acids Res. 1974 Apr;1(4):577–594. doi: 10.1093/nar/1.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]