Abstract
It has been known for decades that the immune system has a tremendous impact on behavior. Most work has described the negative role of immune cells on the central nervous system. However, we and others have demonstrated over the last decade that a well-regulated immune system is needed for proper brain function. Here we discuss several neuro-immune interactions, using examples from brain homeostasis and disease states. We will highlight our understanding of the consequences of malfunctioning immunity on neurodevelopment and will discuss the roles of the innate and adaptive immune system in neurodevelopment and how T cells maintain a proper innate immune balance in the brain surroundings and within its parenchyma. Also, we describe how immune imbalance impairs higher order brain functioning, possibly leading to behavioral and cognitive impairment. Lastly, we propose our hypothesis that some behavioral deficits in neurodevelopmental disorders, such as in autism spectrum disorder, are the consequence of malfunctioning immunity.
Keywords: Neuroimmunology, Microglia, Innate and adaptive immunity, Autism spectrum disorder, Rett, Syndrome
Introduction
The immune and nervous systems are complex systems that rely heavily on the interactions of multiple cell types for normal development and function. In the immune system, host-defense and long-term memory immunity are accomplished by the coordinated efforts of the innate and adaptive arms. The innate arm of the immune system provides a general first line of defense against pathogens, and includes phagocytes such as macrophages (brain resident macrophages are referred to as microglia) and granulocytes such as neutrophils. The adaptive arm, which includes T and B cells, is used to provide memory for subsequent pathogen challenges. In the nervous system, behaviors are output of integrated neural circuits that are balanced by excitatory and inhibitory neurons, supporting glia, and constant dialogue between cells. In both systems inter-cellular communications yield astounding complexity, and it is perhaps more astonishing then to consider that the systems do not exist in isolation but actually relies upon each other for normal function
The immune and nervous systems are intimately connected and each can directly influence the behavior of the other. The brain can influence immune function via glucocorticoids (Besedovsky et al., 1986) and catecholamines (Kipnis et al., 2004a, Flierl et al., 2008), as well as direct stimulation of lymphoid organs (Nance and Sanders, 2007, Rosas-Ballina et al., 2011). The immune system can likewise influence brain function by multiple mechanisms; this is quite obvious to anyone experiencing sickness behavior from infection (Hart, 1988, Dantzer and Kelley, 1989). It is thought that sickness behavior, although uncomfortable, is beneficial for clearing infections (Dantzer et al., 2008). Changes in behavior including social withdrawal, loss of appetite, lethargy, amongst others, result from elevated levels of pro-inflammatory molecules, most notably: tumor necrosis factor (TNF), interlukin-1β (IL-1β), and IL-6 (Dantzer, 2001). These molecules are produced by circulating immune cells and macrophages and affect neuronal function and behavior (Bluthe et al., 2000, Balschun et al., 2004). In addition to soluble molecules, microglia directly interact with neurons and maintain a proper excitatory/inhibitory balance (Pascual et al., 2012, Zhan et al., 2014). Disrupting these tightly controlled interactions or the imbalance in immune molecules can cause a pro-inflammatory skew and produce life-long changes in neuronal function and behavior (Hsiao and Patterson, 2012, Zhan et al., 2014). Neurodevelopment continues well after birth (Lebel and Beaulieu, 2011) and offers a large time window for immune influence. Although the mechanisms of how the immune system shapes neuronal function and behavior are just beginning to be uncovered (Kipnis et al., 2012), immune dysregulation is most evident in common neurodevelopmental disorders.
Pro-inflammatory Skew in Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a common clinical diagnosis for a heterogeneous group of neurodevelopmental disorders that is estimated to affect 1 in every 68 children (Baio, 2014). The diagnosis is clinically described by dysfunction in social behavior and language, abnormal response to sensory input, and often include repetitive behavior and cognitive disabilities (American Psychiatry Association, 2013). Direct genetic alterations can account for only approximately 10% of all ASD (Abrahams and Geschwind, 2008). Therefore, it is likely that a combination of genetic and alternative variables, such as environmental factors and immunity, contribute to the etiology of ASD.
A pro-inflammatory phenotype can be measured in numerous tissues from patients with ASD. For example, microglia have an activated morphology in numerous brain regions, most notably the frontal cortex and cerebellum, by postmortem analysis of brains from ASD patients (Vargas et al., 2005, Morgan et al., 2010). The age range of these cohorts spanned 40 years suggesting an early and prolonged chronic state of inflammation in ASD (Vargas et al., 2005). This pro-inflammatory phenotype is not limited to the CNS and was observed in peripheral immune cells. T cells isolated from autism patients were hyperexcitable, having an exaggerated response when stimulated with the mitogen phytohaemagglutinin ex vivo (Ashwood et al., 2011b). Several clinical studies also measured elevated pro-inflammatory molecules, such as IL-6, in the plasma (Ashwood et al., 2011a, Ashwood et al., 2011c, Brown et al., 2014), as well as decreased anti-inflammatory molecules, such as transforming growth factor (TGF)–β (Okada et al., 2007, Ashwood et al., 2008) suggesting an overall pro-inflammatory skew.
As it stands now, pro-inflammatory profiles and ASD remain correlative and no causation has been proven. Several studies determined a correlation with diseases of the immune system and ASD (Ashwood et al., 2011a, Ashwood et al., 2011c, Brown et al., 2014). It has yet to be determined if a pro-inflammatory skew can cause ASD, however, there was an increased risk for ASD in families with maternal history for autoimmune disease (Atladottir et al., 2009); also see McDougle et al. in this issue for a more comprehensive review of studies linking familial autoimmune disorders and ASD (McDougle et al., 2014)) and evidence of increased gut permeability and gastrointestinal disorders in ASD patients (Buie et al., 2010, de Magistris et al., 2010, Kohane et al., 2012, Hsiao, 2014, McElhanon et al., 2014). Possibly related to these gut phenotypes, disturbances in normal gut microbiota, or dysbiosis, have been described in patients with ASD (Finegold et al., 2010, Kang et al., 2013). The healthy gut hosts a symbiotic microbiota that has been shown to be necessary for the proper development of the immune system (Kamada et al., 2013). Although neonates are more susceptible to infection at this critical developmental point, establishment of a normal microbiota is important and neonates actively suppress inflammation via CD71+ erythroid cells to assure proper gut colonization (Elahi et al., 2013). The colonization of symbiotic bacteria is necessary for healthy intestinal homeostasis and can contribute to diseases of the gut as well as the CNS (Maloy and Powrie, 2011, Mortha et al., 2014, Wang and Kasper, 2014). The role of the gut microbiota in a gut-brain-behavior axis is only beginning to emerge (Rook et al., 2014). Dysbiosis in mice alone can lead to similar neurodevelopmental behavioral dysfunction observed in ASD. Germ-free mice had social deficits that can be corrected by colonization of the germ-free gut (Desbonnet et al., 2014). Increasing the pro-inflammatory skew of the fetal environment (discussed below) also caused dysbiosis and probiotic treatment with B. fragilis was sufficient to correct the leaky gut, elevated IL-6 levels, and behavioral deficits (Hsiao et al., 2013).
Pro-inflammatory skew in utero: Maternal Immune Activation
It is possible that these inflammatory conditions are hereditable but also that maternal conditions may affect development in utero (Abdallah et al., 2012, Brown et al., 2014). In fact, there was a strong association between an ASD diagnosis and maternal infections occurring in the first 2 trimesters (Atladottir et al., 2010). Also, as high as 23% of mothers with ASD children had circulating antibodies with specificity to fetal brain antigens and this specificity correlated with the offspring’s repetitive behaviors (Braunschweig et al., 2008, Goines et al., 2011, Wills et al., 2011, Braunschweig et al., 2013). Pregnant mice and monkeys exposed to antibodies isolated from ASD mothers led to hyperactivity and repetitive behaviors in their offspring (Martin et al., 2008, Singer et al., 2009). Although numerous studies have correlated maternal infection with the onset of neurodevelopmental disorders, the damage is likely due to a pro-inflammatory fetal environment rather than direct effects from the infectious agent itself (Patterson, 2002, Shi et al., 2005).
These associations between infection during pregnancy and autism risk led to the development of maternal immune activation (MIA) as a disease model, which can mimic behavioral and histological dysfunctions observed in ASD in multiple mammalian systems. Treatment of pregnant rodents or monkeys with bacterial (LPS) or viral (Poly(I:C)) mimetics resulted in autistic-like behavioral dysfunction in the offspring (Zuckerman et al., 2003, Fortier et al., 2004, Shi et al., 2005, Ozawa et al., 2006, Malkova et al., 2012, Bauman et al., 2014). Behavioral abnormalities include deficits in social and exploratory behavior, sensorimotor gating, and increased compulsive behavior (Meyer et al., 2006, Smith et al., 2007, Hsiao et al., 2013). The mechanism of MIA-induced pathology, however, remains unclear, but seems to be dependent on specific T cell populations (i.e. decreased T regulatory cells and the malfunction of CD4+ T cells; (Hsiao et al., 2012). Some behavioral abnormalities were corrected when the immune systems of MIA offspring were replaced by transplanting them with wild-type bone marrow after irradiation (Hsiao et al., 2012). IL-6 is also likely involved (Smith et al., 2007). The behavioral deficits observed by MIA can be mimicked by injecting IL-6 into pregnant dams, and administration of an IL-6 neutralizing antibody or use of IL-6 deficient mice was sufficient to prevent the deficits caused by poly(I:C) injections (Smith et al., 2007).
Taken together, it appears that the immune profile in ASD is skewed to a pro-inflammatory phenotype and that a pro-inflammatory skew may interact with brain development. Although the cause for ASD is currently unknown, several large studies have revealed an association with genes involved in synaptic dysfunction (Zoghbi and Bear, 2012, Ebert and Greenberg, 2013). This begs the question; can a pro-inflammatory skew in the fetal environment hinder synaptic function?
The behavioral dysfunction after MIA is likely due to a number of underlying changes in normal brain function. Pathological changes in brain histology, chemistry, and electrophysiology after MIA highlight the importance of the fetal environment on the development of proper brain circuitry. MIA caused crude anatomical changes, such as enlarged ventricles and small hippocampal volumes, a region critically important for cognition (Li et al., 2009, Piontkewitz et al., 2009). Whether these changes are a consequence of neuronal cell death is questionable and mixed results have been published (Zuckerman et al., 2003, Meyer et al., 2006, Oh-Nishi et al., 2010). However, measurements of neuronal function suggest synaptic and overall network dysfunction in MIA offspring. Multiple neurotransmitter systems, including the dopaminergic, GABAergic, glutamatergic, and serotonergic systems, are imbalanced (Dickerson and Bilkey, 2013). Although heterogeneity exists in the data, MIA seems to cause an overall decrease in neuronal activity; in the hippocampus, excitatory input to pyramidal cells in CA1 were decreased (Zuckerman et al., 2003) as well as decreased baseline synaptic transmission and LTP in the Schafer collaterals (Ito et al., 2010). In the cortex, markers for glutamatergic synapses were decreased (Elmer et al., 2013). It is likely that MIA affects multiple brain regions and leads to an imbalance in overall network integration. Decreased synchrony between the prefrontal cortex and the hippocampus suggests long-range network dysfunction (Dickerson et al., 2010). This is particularly interesting as the prefrontal cortex plays a role in social behavior of humans and mice (Wang et al., 2011, Gariepy et al., 2014) and has be implicated in ASD (Itahashi et al., 2014, Jung et al., 2014, Stoner et al., 2014). These data demonstrate the lasting consequences of an in utero pro-inflammatory environment on neuronal function and behavior in the rodent.
Overall, dysregulation of the immune system in development, most notably a skew to a more pro-inflammatory phenotype, can adversely affect normal brain development. Furthermore, at least a subset of ASD cases are linked to maternal infections, but the exact detrimental components of these infections, the genetic traits that confer vulnerability, and the percent of autism cases associated with them remains unknown. It seems likely that ASD is an umbrella term for diseases of multiple etiologies, maternal infection being one. How a pro-inflammatory skew actually perturbs neuronal development is unclear, but many immune factors can affect neuronal function. Several immune-related molecules (such as complement proteins (Stevens et al., 2007) and MHC-I (Huh et al., 2000)) have pleotropic functions in the brain where they can directly affect synaptic function in the CNS (Boulanger, 2009, Garay and McAllister, 2010). Moreover, it has also been shown that circulating immune cells themselves can alter neuronal function and behavior.
T cell Deficiency and Brain Function
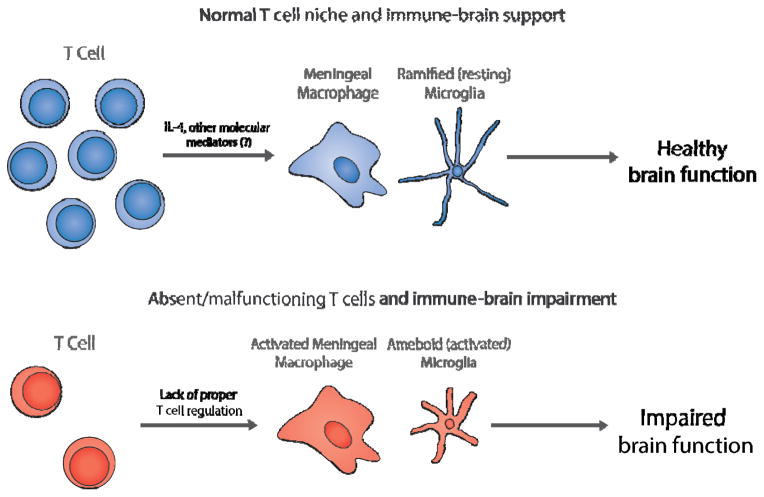
Numerous techniques, both genetic and pharmacological, have been used to investigate the role of the adaptive immune system in normal brain physiology. Our lab (Kipnis et al., 2012) was among the first to assign a beneficial role for T cells in cognition and behavior (Kipnis et al., 2004b, Cohen et al., 2006, Ziv et al., 2006, Brynskikh et al., 2008, Wolf et al., 2009). After training for a cognitive task, CD4+ T cell numbers increased in the meninges surrounding the brain, and blocking this increase was sufficient to cause cognitive impairment (Derecki et al., 2010). Moreover, surgical excision of CNS-draining deep cervical lymph nodes also resulted in abnormal immune repertoire in the meningeal spaces and correlated with impaired learning behavior (Radjavi et al., 2014). Along these lines, immune compromised mice have impaired cognitive and emotional behaviors that have been attributed specifically to CD4+ T cells. Severe combined immunodeficiency (SCID), Rag1−/−, and Rag2−/− mice (which lack T cells and B cells), nude mice (which lack mature T cells), and OTII mice (which lack T cells recognizing self-antigens) all demonstrated impaired learning and memory (Kipnis et al., 2004b, Ziv et al., 2006, Brynskikh et al., 2008, Derecki et al., 2010, Radjavi et al., 2014). Replacing CD4+ T cells was sufficient to prevent this cognitive deficit, demonstrating that a functional T cell pool is necessary for normal brain function (Derecki et al., 2010). Interestingly, repopulation of T cells from IL-4−/− mice did not improve cognition in T cell deficient mice, revealing the role of IL-4 as a major mediator of pro-cognitive immunity (Derecki et al., 2010). The deficits caused by CD4+ T cell depletion are not limited to cognitive deficits. RAG-1−/− mice, which lack T and B cells, had compulsive behavior and deficits in nest building that was fixed by repopulating CD4+ T cell pools (Rattazzi et al., 2013). Further experiments showed that antigen specific T cells, most likely effector-memory T cells, were needed for proper cognition (Baruch et al., 2013, Radjavi et al., 2014). These data demonstrate that not only are T cells needed for normal brain function, but specific populations of T cells in a distinct compartment are needed. How is it that peripheral immune cells communicate with the brain? A functional blood-brain barrier exists early in development (Ek et al., 2012) and no peripheral immune cells are detected within the normal brain parenchyma. Our data suggest that the communication between immune cells and the CNS occurs at the barriers between the brain and the periphery, i.e. the meningeal spaces (Kipnis et al., 2012). Taken together that IL-4 producing T cells can benefit cognition, it is possible that IL-4 produced by T cells in the meninges and cerebrospinal fluid prevents a pro-inflammatory skew of CNS myeloid cells, which may impact normal neuronal function (Figure 1). A lack or malfunction of T cells could, therefore, result in a lack of regulation of meningeal myeloid cells allowing their pro-inflammatory skew. In line with this hypothesis, injecting macrophages treated with IL-4 ex vivo into SCID mice ameliorated the pro-inflammatory skew of their meningeal myeloid cells and improved cognitive function, even in the absence of functional T cells (Derecki et al., 2011). Interestingly, both decreased T cell numbers (Warren et al., 1990, Yonk et al., 1990, Saresella et al., 2009) and dysfunction (Ashwood et al., 2011b) have been associated with some cases of autism spectrum disorders. Thus, if T cell malfunction results in a pro-inflammatory skew of meningeal myeloid cells that lead to impaired cognitive function, it is plausible that T cell malfunction or scarcity in autism may underlie some of the cognitive impairment associated with autism spectrum disorders.
Figure 1. T cells influence brain function by regulating the activation status of local myeloid cells.
Top, In the T cell niche surrounding a healthy brain, T cells regulate meningeal macrophages, and possibly microglia, by the release of IL-4 and other pro-cognitive molecules. Resting microglia survey the brain parenchyma and support a healthy environment for brain development. Bottom, When T cells are absent, or malfunctioning, meningeal macrophages and microglia become activated and unleash pro-inflammatory molecules. This pro-inflammatory milieu is detrimental to normal brain function and may contribute to the developmental abnormalities in ASD.
Besides meningeal macrophages, microglia and astrocytes may also respond to meningeal cytokine contents and acquire a pro-inflammatory skew in the absence of T cells. For instance, astrocytes express the IL-4 receptor and astrocyte cultures respond to IL-4 by producing BDNF and NGF (Brodie et al., 1998, Derecki et al., 2010). Likewise, it is not surprising that microglia express the IL-4 receptor and control local inflammation through IL-4 signaling under multiple circumstances (Suzumura et al., 1994, Ponomarev et al., 2007, Fenn et al., 2014).
Microglia in Development and Disease
Microglia are the professional phagocytes of the CNS. They are of myeloid origin, populate the CNS early in development (~E9.5) (Ginhoux et al., 2010, Schulz et al., 2012) and are most probably maintained by local proliferation (Ajami et al., 2007). They are similar to other tissue resident macrophages but have evolved to maintain local neurons and circuits (for a more in depth review on microglial function see Bilimoria et al. is this issue (Bilimoria and Stevens, 2014)). In the healthy brain, microglia are classified as resting but their processes are very active and they continuously survey their surroundings (Nimmerjahn et al., 2005). It has recently been shown that microglia processes actually contact and engulf synaptic elements (Wake et al., 2009, Tremblay et al., 2010, Paolicelli et al., 2011, Schafer et al., 2012), suggesting that microglia may play a role in synaptic plasticity. In development, deletion of the microglial fractalkine receptor (CX3CR1−/−) transiently decreased microglia in the hippocampus and aberrantly increased synaptic activity and susceptibility to induced seizures (Paolicelli et al., 2011). By 40 days after birth, most of these readouts were normalized suggesting a delay in synaptic pruning. Conversely, another study showed increased microglia in layer V of the cortex in mice deficient for the fractalkine receptor (Ueno et al., 2013). They also observed an increase in layer V apoptotic neurons. Microglia deficient for CX3CR1 were still able to engulf apoptotic neurons but were unable to offer neurotrophic support to surrounding neurons. Both of these studies highlight the important role of microglia on neuronal function. This idea was most elegantly extended by looking at functional connectivity in CX3CR1−/− mice (Zhan et al., 2014). These mice had reduced connectivity between prefrontal cortex and hippocampus (by resting-state fMRI and EEG) and ultimately decreased social behavior and repetitive grooming. The direct effect of adult microglia on neuronal function was determined in a recent study, where conditional elimination of microglia in adulthood decreased the frequency of both NMDA and AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) and resulted in cognitive impairments (Parkhurst et al., 2013). These and other studies hint at a role for microglia in homeostasis, sculpting or “pruning” circuits in the developing and mature brain by targeted phagocytosis. Despite these data that microglia affect pruning and behavior, acute depletion of microglia in adulthood with PLX3397 (which targets Csf-1R, KIT, and FLT3; Plexxikon, Inc.) had minimal effects on behavior (Elmore et al., 2014). In fact, mice treated with PLX3397 for 3 weeks showed improved learning curves in the Barnes maze (a measure of learning and memory). This is contradictory when a more abrupt technique was used to deplete microglia (i.e. CX3CR1 CreER:R26iDTR; (Parkhurst et al., 2013)) These mice had deficit in novel object recognition and classical fear conditioning. Perhaps this contradiction is due to the time course of microglia cell death and the ability of astrocytes (or other non professional phagocytes) to clean up cell debris.
The context in which microglia choose and engulf synaptic elements is under active investigation. Data suggests microglia engulf synaptic elements in an activity dependent manner. In the developing visual cortex, microglia tended to contact smaller spines (which are considered to be relatively transient than their larger more stable counterparts) (Tremblay et al., 2010). Over time, the small spines that were contacted by microglia were often eliminated. During dark adaptation and re-introduction to light, microglia tended to contact active spines and contain more synaptic-like inclusions, suggesting a role of microglial phagocytosis in synaptic remodeling (Tremblay et al., 2010). Another model even earlier in visual development, the establishment of ocular dominance at post-natal day 5, offers a unique system to investigate microglial phagocytosis in synaptic remodeling. In the lateral geniculate nucleus (a relay point in the visual system), microglia engulfed presynaptic terminals from retinal ganglion cells (Schafer et al., 2012). Manipulating neuronal activity with tetrodotoxin (decreasing) or forskolin (increasing) demonstrated that microglia preferentially engulfed inputs originating from the less active neurons. Microglial engulfment depended on the complement protein C3 and its microglial receptor CR3. Deleting either decreased engulfment by ~50% and impaired the formation of normal eye dominance patterns. C3 localized at synapses and this was dependent on upstream complement protein C1q and the cytokine transforming growth factor beta (TGF-β) (Bialas and Stevens, 2013). Whether C3 can selectively target a synapse for phagocytosis or whether many synapses are targeted and stronger synapses are able to offer “don’t eat me” signals have yet to be determined.
In addition to the engulfment of synapses, microglia also engulf apoptotic neurons. Apoptotic cell death of neuronal stem cells is needed for proper brain development. Genetically blocking apoptosis altered a delicate proliferation/death balance and leads to an abnormally expanded nervous system and is often embryonic lethal (reviewed in (Boya and de la Rosa, 2005)). Neurogenesis continues throughout life and most of our current knowledge of how the immune system can affect development stems from the studies looking at postnatal neurogenesis. Microglia make contact with apoptotic neurons and promote cell apoptosis during development (Marin-Teva et al., 2004, Peri and Nusslein-Volhard, 2008, Wakselman et al., 2008). Apoptotic puncta were observed in the microglial branches (Sierra et al., 2010) and inhibiting phagocytosis increased apoptotic cells in the hippocampus (Lu et al., 2011). Further, inhibiting phagocytosis, either genetically or pharmacologically, decreased neurogenesis (Lu et al., 2011).
Microglial dysfunction can contribute to numerous neurodevelopmental disorders. As mentioned above, microglia had an activated morphology in ASD by postmortem analysis (Vargas et al., 2005, Morgan et al., 2010). While our understanding of the contribution of defective microglial in the early stages of disease pathogenesis is growing, the precise mechanisms are still not fully understood.
Rett syndrome is a neurodevelopmental disorder in which patients are often diagnosed with ASD based on their clinical symptoms. The disorder is caused by mutations in the methyl-CpG binding protein, Mecp2 (Van den Veyver and Zoghbi, 2001). Deletion of Mecp2 in mice led to severe pathology and death at ~8 weeks of age (Guy et al., 2001). Microglia isolated from Mecp2 null mice have defects in activation and phagocytosis suggesting they may contribute to the pathology (Derecki et al., 2012). Repopulating Mecp2-null mice with wild-type microglia (by bone-marrow transplantation or expression of a wild type Mecp2 in myeloid cells (including microglia) by genetic manipulation) was sufficient to attenuate disease progression. Repopulating with wild-type microglia has benefited other pathological behavior associated with ASD, such as repetitive behavior. For example, in the brain, HoxB is expressed by microglia (Chen et al., 2010) and deletion of HoxB in mice caused pathological grooming. This grooming pathology was efficiently rescued by repopulating the mice with wild-type microglia.
Communication Between the Innate and Adaptive Arms of the Immune System
It is becoming evident that both the innate and adaptive immune systems can affect the developing brain. Several clinical studies revealed a strong link between a pro-inflammatory skew and ASD (Ashwood et al., 2011a, Ashwood et al., 2011c, Brown et al., 2014). Elevated levels of pro-inflammatory cytokines and a pro-inflammatory phenotype of microglia were observed in post-mortem ASD patients (Vargas et al., 2005, Morgan et al., 2010). Mouse models, such as MIA, suggest that a pro-inflammatory skew in the fetal environment can cause behavioral changes that last well into adulthood (Hsiao and Patterson, 2012). Therefore, on one hand, immune activation seems to deleteriously affect brain development; on the other hand, presence and normally functioning T cells are is necessary for proper brain function (Derecki et al., 2010, Baruch and Schwartz, 2013, Radjavi et al., 2014). How can these seemingly opposite effects of immune activation be reconciled? It is likely that a delicate balance exist between proper activation needed for beneficial immune maintenance and deleterious over-activation of immune cells that results in neuronal dysfunction.
Just as the immune system and nervous system communicate, the innate and the adaptive arms of the immune system closely interact to maintain proper immune balance. If the cells of the innate and the adaptive arms are not well regulated, brain function and development can be affected. This is exemplified in mice deficient for T cells. SCID mice had increased levels of pro-inflammatory cytokines, TNF and IL-12, in innate myeloid cells (CD11b+) of their meninges. When injected into the circulation, anti-inflammatory macrophages (treated with IL-4) sufficiently recovered the pro-inflammatory skew and cognitive deficits of SCID mice (Derecki et al., 2011). These data suggest that T cells provide beneficial regulation by preventing a pro-inflammatory skew of innate myeloid cells and possibly microglia. Since T cells, residing in the meninges, and microglia, residing in the parenchyma, are physically separated, more work is needed to understand how these two compartmentalized cells communicate.
The behavioral deficits in ASD and other neurodevelopmental disorders are pathological and can be severe but it is intriguing to hypothesize that the immune system can contribute to the heterogeneity of normal human behavior. Neuroanatomical imaging studies on attention-deficit/hyperactivity disorder imply that behavioral abnormalities may be due to deviations in developmental timing and trajectories (Giedd and Rapoport, 2010). These same developmental patterns were linked with the severity of hyperactivity and impulsive behavior of normal developing children. In mice, prenatal exposure to a pro-inflammatory skew causes a delay in cortical development (Soumiya et al., 2011). Perhaps, subtle prenatal changes in the skewing of the fetal environment can play a role in sculpting our unique personalities.
Concluding remarks and take-home message
The immune system has profound effects on brain development and function. A pro-inflammatory skew of immune cells has been linked to many neurodevelopmental disorders, such as ASD, and MIA in animals can cause behavioral deficits in offspring. Although a dysregulated inflammatory environment is detrimental in development, functioning T cells are needed for immune homeostasis and proper brain function. CD4+ T cell numbers are decreased in some ASD patients and T cells from patients and mouse models have exaggerated responses when stimulated. Mice lacking T cells have cognitive deficits and a pro-inflammatory skew of their meningeal myeloid cells, suggesting that T cells actively support normal function of tissue-resident myeloid cells and prevent them from unleashing pro-inflammatory molecules that can disturb normal brain development and function.
We propose that immune dysfunction in ASD and possibly other neurodevelopmental disorders, is not simply a manifestation of the pathology, but contributes to the development of the disorder. A healthy immune system is known to support brain function, and an altered one known to result in cognitive impairment. Furthermore, without the normal homeostatic functions of brain resident microglia the ability to cope with pathology is likely hindered. A gap in brain maintenance resulting from this failing support mechanism (the immune system) and an increased demand for this support (due to an ongoing disease), further fuels the pathology.
We further suggest that a malfunctioning T cell compartment in development leads to dysregulation and a pro-inflammatory skew of meningeal myeloid cells and microglia. This leads to an increase in pro-inflammatory molecules surrounding the parenchyma resulting in altered synapses, neural circuits, and ultimately behavior. It is plausible that decreased numbers or malfunction of T cells can contribute to the etiology of ASD and this link needs to be further scrutinized. Drug accessibility of the immune system and its feasible replacement make the immune system an intriguing new therapeutic target in neurodevelopmental disorders.
Highlights.
Well-regulated immune system is needed for proper brain function
Immune imbalance impairs higher order brain functioning
Immune deficiency leads to cognitive impairment
Behavioral deficits in autism might be the consequence of malfunctioning immunity
Acknowledgments
We thank the members of the Kipnis lab as well as the members of the Center for Brain Immunology and Glia (BIG) for their valuable comments during multiple discussions of this work. A.J.F. is supported by T32-AI007496 NIH training grant in immunology and a fellowship from the Hartwell Foundation. This work was supported by grants from the National Institutes on Aging (AG034113), SFARI and RSRT (awards to J.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, Hougaard DM. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a Danish Historic Birth Cohort. Brain, behavior, and immunity. 2012;26:170–176. doi: 10.1016/j.bbi.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature reviews Genetics. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature neuroscience. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN, Van de Water J. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. Journal of neuroimmunology. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, behavior, and immunity. 2011a;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain, behavior, and immunity. 2011b;25:840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. Journal of neuroimmunology. 2011c;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, Parner ET. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of autism and developmental disorders. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Baio J. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–12. [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, Mirlas-Neisberg N, Cardon M, Vaknin I, Cahalon L, Berkutzki T, Mattson MP, Gomez-Pinilla F, Friedman N, Schwartz M. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Schwartz M. CNS-specific T cells shape brain function via the choroid plexus. Brain, behavior, and immunity. 2013;34:11–16. doi: 10.1016/j.bbi.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biological psychiatry. 2014;75:332–341. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nature neuroscience. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bilimoria PM, Stevens B. Microglia function during brain development: new insights from animal models. Brain research. 2014 doi: 10.1016/j.brainres.2014.11.032. In press. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Poli V, Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiology & behavior. 2000;70:367–373. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Boya P, de la Rosa EJ. Cell death in early neural life. Birth defects research Part C, Embryo today: reviews. 2005;75:281–293. doi: 10.1002/bdrc.20054. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, Hertz-Picciotto I, Pessah IN, Van de Water J. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Translational psychiatry. 2013;3:e277. doi: 10.1038/tp.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie C, Goldreich N, Haiman T, Kazimirsky G. Functional IL-4 receptors on mouse astrocytes: IL-4 inhibits astrocyte activation and induces NGF secretion. Journal of neuroimmunology. 1998;81:20–30. doi: 10.1016/s0165-5728(97)00154-9. [DOI] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Molecular psychiatry. 2014;19:259–264. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain, behavior, and immunity. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ, 3rd, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, Carr EG, Gershon MD, Hyman SL, Jirapinyo P, Jyonouchi H, Kooros K, Kushak R, Levitt P, Levy SE, Lewis JD, Murray KF, Natowicz MR, Sabra A, Wershil BK, Weston SC, Zeltzer L, Winter H. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. Journal of neurobiology. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain, behavior, and immunity. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life sciences. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, Militerni R, Bravaccio C. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. Journal of pediatric gastroenterology and nutrition. 2010;51:418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. The Journal of experimental medicine. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain, behavior, and immunity. 2011;25:379–385. doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Molecular psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DD, Bilkey DK. Aberrant neural synchrony in the maternal immune activation model: using translatable measures to explore targeted interventions. Frontiers in behavioral neuroscience. 2013;7:217. doi: 10.3389/fnbeh.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493:327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR. Barriers in the developing brain and Neurotoxicology. Neurotoxicology. 2012;33:586–604. doi: 10.1016/j.neuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer BM, Estes ML, Barrow SL, McAllister AK. MHCI requires MEF2 transcription factors to negatively regulate synapse density during development and in disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:13791–13804. doi: 10.1523/JNEUROSCI.2366-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP. IL-4 Signaling Drives a Unique Arginase+/IL-1beta+ Microglia Phenotype and Recruits Macrophages to the Inflammatory CNS: Consequences of Age-Related Deficits in IL-4Ralpha after Traumatic Spinal Cord Injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA., 3rd Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora’s box? Molecular medicine. 2008;14:195–204. doi: 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. American journal of physiology Regulatory, integrative and comparative physiology. 2004;287:R759–766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Frontiers in synaptic neuroscience. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy JF, Watson KK, Du E, Xie DL, Erb J, Amasino D, Platt ML. Social learning in humans and other animals. Frontiers in neuroscience. 2014;8:58. doi: 10.3389/fnins.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, Hansen R, Hertz-Picciotto I, Ashwood P, Van de Water J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain, behavior, and immunity. 2011;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature genetics. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience and biobehavioral reviews. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hsiao EY. Gastrointestinal issues in autism spectrum disorder. Harvard review of psychiatry. 2014;22:104–111. doi: 10.1097/HRP.0000000000000029. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Developmental neurobiology. 2012;72:1317–1326. doi: 10.1002/dneu.22045. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahashi T, Yamada T, Watanabe H, Nakamura M, Jimbo D, Shioda S, Toriizuka K, Kato N, Hashimoto R. Altered network topologies and hub organization in adults with autism: a resting-state fMRI study. PloS one. 2014;9:e94115. doi: 10.1371/journal.pone.0094115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain, behavior, and immunity. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Kosaka H, Saito DN, Ishitobi M, Morita T, Inohara K, Asano M, Arai S, Munesue T, Tomoda A, Wada Y, Sadato N, Okazawa H, Iidaka T. Default mode network in young male adults with autism spectrum disorder: relationship with autism spectrum traits. Molecular autism. 2014;5:35. doi: 10.1186/2040-2392-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nature reviews Immunology. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PloS one. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cardon M, Avidan H, Lewitus GM, Mordechay S, Rolls A, Shani Y, Schwartz M. Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004a;24:6133–6143. doi: 10.1523/JNEUROSCI.0600-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. PNAS. 2004b;102:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nature reviews Immunology. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, Bickel J, Wattanasin N, Spence S, Murphy S, Churchill S. The co-morbidity burden of children and young adults with autism spectrum disorders. PloS one. 2012;7:e33224. doi: 10.1371/journal.pone.0033224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, Chung S, Chua SE, Sham PC, Wu EX, McAlonan GM. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PloS one. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, Kipnis J. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nature cell biology. 2011;13:1076–1083. doi: 10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain, behavior, and immunity. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart CC, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain, behavior, and immunity. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle CJ, Landino SM, Vahabzadeh A, O’Rourke J, Zurcher NR, Finger BC, Palumbo ML, Helt J, Mullett JE, Hooker, Carlezon JWA. Toward an immune-mediated subtype of autism spectrum disorder. Brain research. 2014 doi: 10.1016/j.brainres.2014.09.048. In press. [DOI] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-analysis. Pediatrics. 2014 doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, behavior, and immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Oh-Nishi A, Obayashi S, Sugihara I, Minamimoto T, Suhara T. Maternal immune activation by polyriboinosinic-polyribocytidilic acid injection produces synaptic dysfunction but not neuronal loss in the hippocampus of juvenile rat offspring. Brain research. 2010;1363:170–179. doi: 10.1016/j.brainres.2010.09.054. [DOI] [PubMed] [Google Scholar]
- Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Sugiyama T, Kawai M, Minabe Y, Takei N, Mori N. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31:187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biological psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Current opinion in neurobiology. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- Peri F, Nusslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biological psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjavi A, Smirnov I, Kipnis J. Brain antigen-reactive CD4(+) T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain, behavior, and immunity. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D’Acquisto F. CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Translational psychiatry. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Lowry CA, Raison CL. Hygiene and other early childhood influences on the subsequent function of the immune system. Brain research. 2014 doi: 10.1016/j.brainres.2014.04.004. In press. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saresella M, Marventano I, Guerini FR, Mancuso R, Ceresa L, Zanzottera M, Rusconi B, Maggioni E, Tinelli C, Clerici M. An autistic endophenotype results in complex immune dysfunction in healthy siblings of autistic children. Biological psychiatry. 2009;66:978–984. doi: 10.1016/j.biopsych.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell stem cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. Journal of neuroimmunology. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumiya H, Fukumitsu H, Furukawa S. Prenatal immune challenge compromises the normal course of neurogenesis during development of the mouse cerebral cortex. Journal of neuroscience research. 2011;89:1575–1585. doi: 10.1002/jnr.22704. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. The New England journal of medicine. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Itoh Y, Marunouchi T. Interleukin-4 induces proliferation and activation of microglia but suppresses their induction of class II major histocompatibility complex antigen expression. Journal of neuroimmunology. 1994;53:209–218. doi: 10.1016/0165-5728(94)90031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nature neuroscience. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- Van den Veyver IB, Zoghbi HY. Mutations in the gene encoding methyl-CpG-binding protein 2 cause Rett syndrome. Brain & development. 2001;23(Suppl 1):S147–151. doi: 10.1016/s0387-7604(01)00376-x. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of neurology. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain, behavior, and immunity. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RP, Yonk LJ, Burger RA, Cole P, Odell JD, Warren WL, White E, Singh VK. Deficiency of suppressor-inducer (CD4+CD45RA+) T cells in autism. Immunological investigations. 1990;19:245–251. doi: 10.3109/08820139009041839. [DOI] [PubMed] [Google Scholar]
- Wills S, Rossi CC, Bennett J, Martinez Cerdeno V, Ashwood P, Amaral DG, Van de Water J. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Molecular autism. 2011;2:5. doi: 10.1186/2040-2392-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. Journal of immunology. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Yonk LJ, Warren RP, Burger RA, Cole P, Odell JD, Warren WL, White E, Singh VK. CD4+ helper T cell depression in autism. Immunology letters. 1990;25:341–345. doi: 10.1016/0165-2478(90)90205-5. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature neuroscience. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature neuroscience. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]